Abstract
We treated Norway spruce (Picea abies) stems with methyl jasmonate (MeJA) to determine possible quantitative and qualitative effects of induced tree defenses on pheromone emission by the spruce bark beetle Ips typographus. We measured the amounts of 2-methyl-3-buten-2-ol and (S)-cis-verbenol, the two main components of the beetle’s aggregation pheromone, released from beetle entrance holes, along with phloem terpene content and beetle performance in MeJA-treated and untreated Norway spruce logs. As expected, phloem terpene levels were higher and beetle tunnel length was shorter (an indication of poor performance) in MeJA-treated logs relative to untreated logs. Parallel to the higher phloem terpene content and poorer beetle performance, beetles in MeJA-treated logs released significantly less 2-methyl-3-buten-2-ol and (S)-cis-verbenol, and the ratio between the two pheromone components was significantly altered. These results suggest that host resistance elicited by MeJA application reduces pheromone emission by I. typographus and alters the critical ratio between the two main pheromone components needed to elicit aggregation. The results also provide a mechanistic explanation for the reduced performance and attractivity observed in earlier studies when bark beetles colonize trees with elicited host defenses, and extend our understanding of the ecological functions of conifer resistance against bark beetles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conifers have evolved elaborate constitutive and inducible defense mechanisms that are effective against a variety of attacking organisms (Phillips and Croteau 1999; Franceschi et al. 2005; Keeling and Bohlmann 2006). Constitutive defenses include the secretion of chemicals such as terpenes and phenolics from preformed reservoirs, as well as anatomical structures which inhibit incursion, tissue degradation and ingestion by invaders (Krekling et al. 2000; Hudgins et al. 2003; Franceschi et al. 2005). The inducible defenses are characterized by increased production of defensive chemicals and related changes in tree anatomy, and include mass oleoresin secretion, increased phenolic synthesis and a hypersensitive-like response in the phloem surrounding the attack site (Berryman 1972; Viiri et al. 2001; Krokene et al. 2003). These temporally and spatially overlapping defense systems are chemically toxic or repellent, and may physically trap or deter insects and microbes (Krekling et al. 2000; Hudgins et al. 2003; Franceschi et al. 2005; Raffa et al. 2008).
Bark beetles (Coleoptera: Curculionidae) are among the most important insect pests of coniferous trees (Raffa et al. 2008). Most bark beetles feed and reproduce in recently dead and dying trees, but a few aggressive species, primarily in the genera Dendroctonus and Ips, can attack and kill healthy trees. Pheromones are an essential component of the tree-killing strategies of aggressive bark beetles (Wood 1982; Raffa 2001) and regulate many aspects of the bark beetle life cycle and its interaction with host trees, including mass aggregation, mate finding, reproduction and niche partitioning. Many bark beetle species use oxygenated terpenoids as aggregation pheromones (Wood 1982; Raffa 2001; Bearfield et al. 2009). These pheromones are produced de novo following stimulation by feeding or host compounds, metabolized from host terpenoids, converted from host terpenoids by beetle-associated microorganisms, or are generated by various combinations of these mechanisms (Leufvén et al. 1984; Lanne et al. 1989; Ivarsson et al. 1993; Blomquist et al. 2010).
Several studies have reported possible interference of bark beetle aggregation and colonization of host tissues by host defenses (Raffa and Berryman 1983; Christiansen and Krokene 1999; Erbilgin et al. 2006; Zeneli et al. 2006). For example, Raffa and Berryman (1983) reported that Dendroctonus ponderosae Hopkins were unable to initiate aggregation on vigorous lodgepole pine trees. It has been speculated that this failed aggregation can be attributed to impaired pheromone production by beetles exposed to sticky resin and other tree defenses (Raffa and Berryman 1983; Erbilgin et al. 2006). However, very few studies have investigated the actual mechanisms of failed aggregation, particularly the way induced tree defenses affect the quality and quantity of pheromone production (Birgersson and Leufvén 1988; Birgersson 1989).
One potential problem in designing experiments targeting the precise effects of host defenses on bark beetle pheromone production is the difficulties in manipulating host defenses. Jasmonates, endogenous plant phytohormones involved in defense signaling (Creelman and Mullet 1997), have been intensively studied in the context of induced defense against herbivores (Creelman and Mullet 1997; Thaler 1999) and has proven to be an effective tool for manipulating conifer defenses (Franceschi et al. 2002; Martin et al. 2002, 2003; Erbilgin et al. 2006; Zeneli et al. 2006; Zhao et al. 2010). For example, Erbilgin et al. (2006) used methyl jasmonate (MeJA), a methyl ester of jasmonate, to manipulate defenses of mature Norway spruce and investigate how induced defenses interfere with attraction and colonization by the spruce bark beetle Ips typographus (L.). They found that spruce trees treated with MeJA had significantly less beetle colonization than untreated control trees and exhibited shorter beetle galleries with reduced oviposition, beetle emergence, and offspring quality. Notably, they also found that fewer beetles were attracted to cues emitted by conspecifics tunneling in MeJA-treated spruce trees. They concluded that induced terpene production in MeJA-treated tissues could explain the observed decrease in beetle colonization and reproduction and speculated that induced tree defenses might also interfere with beetle pheromone production and communication, although they did not investigate the actual mechanisms involved.
This study is a continuation of Erbilgin et al. (2006), with the objective to test how pheromone production by I. typographus is influenced by induced tree defenses. Ips typographus is the most aggressive insect pest of Norway spruce in Europe, and has killed more than 50 million m3 of spruce trees in several outbreaks since the late 1940s (Worrell 1983; Christiansen and Bakke 1988; Wermelinger 2004). Like many other tree-killing bark beetle species, I. typographus uses pheromones to initiate mass attacks. We quantified the beetle’s key aggregation pheromone components, 2-methyl-3-buten-2-ol (MB) and (S)-cis-verbenol (cV) (Birgersson et al. 1984; Schlyter et al. 1987a) released from entrance holes in MeJA-treated or untreated Norway spruce logs, as well as phloem terpene content and beetle performance in treated and untreated logs.
Materials and methods
Experimental procedure
In early May 2009, two trees from the same clone were selected from a plantation of 48-year-old Norway spruce at Hogsmark, Ås, SE Norway. On one randomly selected tree, a stem section between 0.8 and 3.8 m above ground was sprayed with 100 mM MeJA in water and 0.1% Tween 20, using a small spray gun. The bark was kept wet for a minimum of 5 min by repeated application of MeJA. The other tree, standing 2 m away, received no treatment. Trees were felled on 3 June, and six 50-cm-long logs were cut from the lower 0.8–3.8 m of each tree and taken to the laboratory at the Royal Institute of Technology, Sweden, the same day. One 40-cm-long section from the center of each of three treated and untreated logs was covered by mesh net and 20 recently trapped I. typographus beetles were introduced into each net bag on 4 June. The logs were checked for new beetle entrance holes for 5 days, and logs with new attacks were targeted for volatile extraction. All logs were kept at room temperature for the duration of the experiment. Two months after the beetles were introduced to the logs, the beetle galleries in the bark were exposed by removing the outer bark. The number of maternal galleries with or without a mating chamber was recorded and the length of each maternal gallery was measured.
Collection of aggregation pheromone components
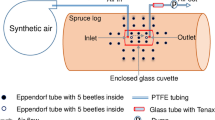
A total of 20 entrance holes (7 on control and 13 on MeJA treated logs) on three different logs per treatment were used for chemical analyses. A 200-μl pipette tip (Fisher Scientific, UK) was fixed above each entrance hole to collect volatiles. The gap between the cork bark and the base of the tip was sealed by aluminum foil, and a 65-μm polydimethylsiloxane/divinylbenzene (PDMS/DVD) SPME fiber (Supecol, PA, USA) was placed inside the pipette tip for 2 h to collect/trap volatiles. The same collection protocol was repeated with a conditioned SPME fiber and a new pipette tip from the same entrance hole for 6 days or until neither MB nor cV was detected for two consecutive days. The SPME fiber with trapped volatiles was injected into the GC–MS for chemical analyses.
Extraction of phloem terpenes
Three samples of intact phloem, each consisting of two bark plugs, were collected using a 5-mm cork borer from the remaining three MeJA-treated and untreated logs on 3 June 2009. The samples were extracted in 1.0 ml hexane containing 0.08 mg pentadecane as an internal standard for 48 h at room temperature. To quantify the chemical response to mechanical wounding, we wounded a 6 × 6 mm bark area 15–20 times with a push pin on five MeJA-treated and three untreated logs. Twenty-four hours after wounding, we removed the wounded phloem and extracted the samples individually as described above. The extracts were placed in 2-ml glass vials and stored at −25°C until chemical analyses. After extraction, the bark plugs were dried at 60°C for 48 h and weighed by a Sartorius electronic balance for absolute amount calculation.
Chemical analyses
All samples were separated, identified and quantified using a Varian 3400 Gas Chromatography (GC) equipped with a DB-wax capillary column (30 m × 0.25 mm × 0.25 μm; J&W Scientific, CA, USA) and connected to a Finnigan SSQ 7000 Mass Spectrometer (MS). A split/splitless injector was used with a 30-s splitless injection at 225°C. The SPME samples were injected into the GC–MS manually, and the fiber was kept in the injector for 5 min to fully desorb the compounds. Compounds were analyzed using the following temperature program: 40°C for 3 min, increased at 4°C min−1 to 160°C, then at 20°C min−1 to 230°C, and kept constant at 230°C for 3 min. The phloem hexane extracts were introduced to the injector by a Finnigan A200S autosampler and analyzed by the following temperature program: 40°C for 3 min, increased to 230°C at 4°C min−1, and kept constant at 230°C for 19 min. The enantiomeric composition of α-pinene and limonene in phloem extraction was analyzed in the same GC–MS with a Varian CP-Chirasil-DEX CB column (25 m × 0.25 mm × 0.25 μm) using a temperature program of 60°C for 1 min, increased to 90°C at 0.5°C min−1, and then to 180°C at 20°C min−1. The compounds were identified by comparing retention times and mass spectra with available authentic standards, or by comparing retention indexes and mass spectra with Massfinder 3.0 and the NIST MS library. The absolute amounts of terpenes in hexane extracts were calculated relative to internal standards and expressed as mg g−1 dry weight equivalent to pentadecane. The relative proportions of terpenes were calculated as the ratio of the area of each peak to the sum of the total area of all terpene hydrocarbons in a defined GC fraction, and expressed as percentage.
Quantification of MB and cV was done by comparison with response curves derived from collection of volatiles from synthetic reference compounds (MB: Aldrich, 98% purity; cV: KTH, 99.5% purity). A hole was made through the bark of a fresh log by removing a bark plug using a 5-mm cork borer, and a similar sized piece of paper foil was placed carefully at the bottom of the hole to prevent chemicals from being absorbed into the sapwood. A pipette tip was fixed above the hole, sealed to the cork bark by foil paper as described above, and 1 μl of MB or cV diluted in pentane to different concentrations was carefully applied to the foil paper using a 5-μl syringe (Hamilton, Switzerland). The same type of SPME fiber described above was placed inside the pipette tip for 2 h to extract the mixture of host volatiles and synthetic chemicals. The calibration curve for MB was made using seven concentrations ranging from 0.0001 to 20 μg μl−1, and that for cV using six concentrations ranging from 0.0001 to 0.1 μg μl−1.
Data analyses
The relative proportions (normalized to 100%) of individual terpenes in intact and wounded phloem from MeJA-treated and untreated logs were subjected to principal components analysis (PCA) using the multivariate data analysis software Canoco 4.5 (Biometris Plant Research International, The Netherlands). One-way ANOVA was used to test for differences in individual terpenes between wounded and intact phloem in MeJA-treated and control logs. If treatments were significantly different (P < 0.05), means were separated using LSD at P = 0.05 (Statistica 6.0; Statsoft, USA). Data were arcsin-transformed before ANOVA to correct for unequal variance and departures from normality. Levels of MB, cV and individual terpenes, the ratio of MB:cV during the study period, and tunnel length were compared in MeJA-treated and untreated logs by Mann–Whitney U test. The frequency of galleries in MeJA-treated and control logs with and without a nuptial chamber was compared by Fisher’s Exact Test.
Results
Methyl jasmonate elicited host defenses in P. abies
To quantify the induced defenses in the experimental trees, we measured terpene concentrations in intact and wounded bark of MeJA-treated and untreated logs, and evaluated the performance of I. typographus in the bark. Beetles performed much better on untreated logs than on MeJA-treated logs. All the seven gallery systems exposed in untreated logs contained a mating chamber, compared to only 38.5% of the gallery systems in MeJA-treated logs (Table 1). In addition, beetles excavated 3.4-fold longer galleries in untreated logs than in MeJA-treated logs (Table 1).
As expected, MeJA treatment also induced terpene accumulation in the bark. The concentration of mono-, sesqui- and diterpenes in intact phloem were 2.4, 3.2 and 2.8-fold higher, respectively, in MeJA-treated bark than in untreated bark (P = 0.07, 0.10 and 0.08, respectively) (Fig. 1). The relative proportions of these compounds did not differ between treatments (Fig. 1).
Absolute and relative amounts (normalized to 100%) of monoterpenes (MT), sesquiterpenes (ST) and diterpenes (DT) in intact (white bars) and wounded (black bars) phloem of control logs and methyl jasmonate (MeJA)-treated Norway spruce logs. Error bars 1SE. Wounded phloem was intensively wounded using a push pin 24 h before sampling. n = 3 logs, except for wounded phloem in MeJA-treated logs where n = 5
Overall, phloem that had been mechanically wounded 24 h previously had higher terpene levels than intact phloem, and MeJA-treated logs showed a much stronger quantitative response to wounding than untreated logs (Fig. 1). In wounded phloem, all terpene classes were significantly more abundant in MeJA-treated than in untreated phloem (U test, P = 0.012–0.023), and diterpenes showed a particularly strong response. Consequently, MeJA-treated logs had higher diterpene (U test, P = 0.031) and lower monoterpene proportions (U test, P = 0.022) than untreated logs (Fig. 1). For individual terpenes, the relative proportion of thunbergol, neoabienol, abienol and methyl dehydroabietate was significantly higher in MeJA-treated phloem after wounding, whereas that of α-pinene and some other monoterpenes was significantly lower (Table 2). The wounded phloem samples were well separated from the other samples in the PCA plot based on relative amounts of the terpenes (Fig. 2).
PCA plot based on the relative proportion of terpenes from intact and wounded Norway spruce phloem in control logs and methyl jasmonate (MeJA)-treated logs. Each symbol represents one sample: intact phloem from control (crosses) and MeJA-treated logs (filled circles); wounded phloem from control (plus symbols) and MeJA-treated logs (filled triangles). The first and second principal components explained 54.9 and 17.7%, respectively, of the sample variation
Induced tree resistance reduced pheromone emission by I. typographus
Volatiles released from 13 I. typographus entrance holes in MeJA-treated Norway spruce logs and 7 entrance holes in untreated logs were extracted and analyzed by SPME–GC–MS. MB and cV were detected from all entrance holes in untreated logs, but from only 46.2 (MB) and 61.5% (cV) of the holes in MeJA-treated logs (Table 1). Furthermore, MeJA treatment significantly reduced the quantity of MB and cV emitted by the beetles. Over the 6-day collection period, beetles tunneling in untreated logs produced 35.9-fold more MB and 13.9-fold more cV than beetles tunneling in MeJA-treated logs (U test, P = 0.023 for MB and P < 0.01 for cV). The strongest reduction in MB and cV emissions from MeJA-treated logs was observed 3 (MB) and 4 (cV) days after beetle entry, when 60.2-fold more MB and 20.7-fold more cV was emitted from untreated logs (Fig. 3).
Induced tree resistance altered the ratio between MB and cV
The ratio of MB to cV was generally higher in untreated than in MeJA-treated logs during the 6-day study period (U test, P = 0.037), except for the first day after gallery initiation (Fig. 4). In MeJA-treated logs, the highest ratio occurred at day 1, while the highest ratio in untreated logs occurred at days 3 and 4. The ratio increased steadily during the first 3–4 days after gallery initiation in control logs, whereas it remained constant or decreased slightly over time in MeJA-treated logs.
Ratio of 2-methyl-3-buten-2-ol (MB) to (S)-cis-verbenol (cV) emitted from Ips typographus entrance holes in control logs (white bars) and methyl jasmonate-treated (black bars) Norway spruce logs the first 6 days after beetle entry. For each day, the MB:cV ratio was calculated as sum of MB/sum of cV from all entrance holes within the same treatment (control, n = 7; methyl jasmonate, n = 13)
Discussion
This study clearly showed that exogenous application of MeJA to Norway spruce stems reduced pheromone emission by I. typographus and altered the ratio between MB and cV, the two major components of its aggregation pheromone. In addition, MeJA-treated stem sections had higher terpene levels in the phloem, much stronger terpene accumulation in response to mechanical wounding, fewer galleries with a mating chamber, and shorter beetle galleries than untreated Norway spruce stem sections. These results confirm that MeJA induces defenses in Norway spruce in a similar way as reported by Erbilgin et al. (2006) and Zeneli et al. (2006), and suggest that the induced defenses negatively affect host colonization and reduce pheromone production by I. typographus. To the best of our knowledge, this is the first report explicitly comparing pheromone emission by bark beetles in trees with or without induced defenses.
Erbilgin et al. (2006) reported that fewer I. typographus were attracted to beetles tunneling in MeJA-treated Norway spruce phloem compared to beetles tunneling in untreated phloem, but did not explain the underlying mechanism of lower beetle attraction. Our results provide a reasonable explanation for their observation. We speculate that increased levels of mono-, sesqui-, and diterpenes in MeJA-treated Norway spruce phloem interfere with host colonization and establishment by I. typographus. Bark beetles that have to deal with induced defences while tunneling in an elicited tree are likely to be under severe physiological stress and this may interfere with their ability to produce sufficient aggregation pheromones. Parallel to this expectation, work on a congeneric Ips species has demonstrated that increased quantities of host allelochemicals reduced beetle establishment (Wallin and Raffa 2000). In addition to the direct impact of induced tree defenses on beetle gallery establishment, increased terpenoids could also reduce beetle colonization success indirectly by inhibiting the growth of the beetle’s phytopathogenic fungal associates which help in overcoming tree resistance during host colonization (Kozlowski et al. 1999; Franceschi et al. 2002; Schmidt et al. 2005; Zeneli et al. 2006).
The reduced pheromone emission from MeJA-treated logs may also be a result of less extensive beetle feeding and/or lower quantities of precursors for pheromone production in induced bark tissues. Ips typographus uses ingested (−)-α-pinene as a precursor to synthesize cV (Klimetzek and Francke 1980). Highly reduced beetle tunneling activity, and lower relative amounts of (−)-α-pinene in MeJA-treated stem bark after mechanical wounding, suggest that male I. typographus entering MeJA-treated logs ingested less (−)-α-pinene and thus produced less cV. MB appears to be synthesized de novo by the beetles under strict hormonal control (Lanne et al. 1989), but MB production is stimulated by tunneling and feeding, and depends on the beetle’s physiological condition (Birgersson et al. 1988). Reduced tunneling and feeding, probably in response to increased quantities of terpenoids (Berryman 1972; Cates and Alexander 1982; Cook and Hain 1988; Raffa 2001), could thus have led to the observed reduction in MB production. A negative effect of reduced beetle feeding on pheromone production is also supported by a pilot study, where I. typographus males placed in pre-made holes in the bark released 87% less MB and cV than males that had excavated their own entrance holes (T. Zhao et al., unpublished data).
Ips typographus releases MB in very high quantities compared to the other pheromone components, and the mass of MB produced by male I. typographus in the first week of an attack may constitute 3.3% of the mass of an individual beetle (Birgersson and Bergström 1989). Thus, MB synthesis represents a huge metabolic load for the beetles and is probably particularly vulnerable to physiological stress. The cytochrome P450 enzyme group provides a likely metabolic link between physiological stress and reduced pheromone synthesis in I. typographus and other bark beetles, as cytochrome P450s among many other functions are involved in insect pheromone biosynthesis (Sandstrom et al. 2006) and detoxification of host plant chemicals (Feyereisen 1999). A large family of cytochrome P450 genes has recently been characterized in Ips paraconfusus (Huber et al. 2007). Most of these genes are strongly upregulated after feeding, particularly in the pheromone-producing males, suggesting that they play a role in male-specific aggregation pheromone production in at least some Ips species. By quantifying transcript patterns of P450s in bark beetles that have been exposed to, e.g., MeJA-treated or untreated Norway spruce phloem, we may determine how host defenses affect the expression of these genes. Improved knowledge about the interaction between tree defenses and bark beetle pheromone biosynthesis may in the long term provide new management tools against these important conifer pests.
MeJA application significantly altered the ratio of the two essential components of I. typographus’ aggregation pheromone. This is an important result since the beetle’s response to MB and cV is concentration-dependant (Dickens 1981; Schlyter et al. 1987a, b). Quantitative and qualitative changes in MB and cV emission after MeJA application may therefore reduce the number of beetles attracted to a tree and could ultimately result in a failed mass attack. In addition, MB and cV have different roles in the aggregation behavior of I. typographus; cV is used for long-range orientation towards an attacked forest stand, whereas MB is as short-range orientation or arrestment substance which concentrates beetles to an attacked tree (Dickens 1981; Birgersson et al. 1984; Schlyter et al. 1987a). The MB:cV ratio emitted by I. typographus colonizing living spruce trees ranges from 25:1 to 125:1 during the first 6 days after attack (Birgersson and Bergström 1989), which is close to that of 22:1–93:1 observed from untreated control logs in the current study. In contrast, we found much lower MB:cV ratios (ranging from 16:1 to 39:1) released by beetles tunneling in MeJA-treated spruce logs. The low MB:cV ratios released from MeJA-treated trees suggests that induced tree defenses may influence beetle landing more than long-range orientation, as speculated by Erbilgin et al. (2006).
Like other Ips bark beetles, I. typographus produces ipsdienol and ipsenol in the gut after mating. Ipsdienol is slightly attractive to I. typographus (Schlyter et al. 1987a) and ipsenol acts as an anti-aggregation pheromone to regulate attack densities and reduce intraspecific competition (Bakke 1981). However, in this study, we only detected ipsenol and ipsdinol from one entrance hole.
MeJA treatment in itself induced only a moderate increase in terpene levels in the bark, but MeJA-treated trees responded much more strongly to mechanical wounding than untreated trees. This appears to be an example of defense priming, i.e. a process in which responses to a challenge (i.e. MeJA treatment, pathogen infection or herbivore infestation) are accelerated, enhanced, or potentiated by prior stimulation (Engelberth 2006). The strong priming response suggests that bark beetles boring into MeJA-treated bark rapidly become exposed to very high terpene concentrations, further explaining the poor performance of I. typographus in MeJA-treated trees. Priming and wounding appeared to activate all terpene biosynthetic pathways, but particularly those enzymes leading to production of diterpenes, such as GGPP (geranylgeranyl diphosphate) synthase and specific diterpene synthases. Although the first molecular and chemical responses in Norway spruce to elicitation by MeJA or other stimuli may occur within hours or days, trees typically need 2–3 weeks to become resistant to beetle or fungus attack under Scandinavian field conditions. Once activated, they may remain in an activated or primed state for at least 1 year following a single induction event (Christiansen et al. 1999; Krokene et al. 2003; Erbilgin et al. 2006; Zhao et al. 2010).
In conclusion, we have demonstrated the potential influence of induced host defenses on pheromone emission by a tree-killing bark beetle. Although we realize that the number of trees per treatment used in the study may limit our conclusions, the similarity of our results with other studies (particularly Erbilgin et al. 2006) suggests that our results are representative for the I. typographus–Norway spruce interaction and thus extend our understanding of the ecological functions of conifer resistance against bark beetles.
References
Bakke A (1981) Inhibition of the response in Ips typographus to the aggregation pheromone: field evaluation of verbenone and ipsenol. Z Angew Entomol 92:172–177
Bearfield JC, Henry AG, Tittiger G, Blomquist GJ, Ginzel MD (2009) Two regulatory mechanisms of monoterpenoid pheromone production in Ips spp. of bark beetles. J Chem Ecol 35:689–697
Berryman AA (1972) Resistance of conifers to invasion by bark beetle–fungi associations. Bioscience 22:598–602
Birgersson G (1989) Host tree resistance influencing pheromone production in Ips typographus (Coleoptera: Scolytidae). Holarctic Ecol 12:451–456
Birgersson G, Bergström G (1989) Volatiles released from individual spruce bark beetle entrance holes: quantitative variations during the first week of attack. J Chem Ecol 15:2465–2484
Birgersson G, Leufvén A (1988) The influence of host tree response to Ips typographus and fungal attack on production of semiochemicals. Insect Biochem 18:761–770
Birgersson G, Schlyter F, Löfqvist J, Bergström G (1984) Quantitative variation of pheromone components in the spruce bark beetle Ips typographus from different attack phases. J Chem Ecol 10:1029–1055
Birgersson G, Schlyter F, Bergström G, Löfqvist J (1988) Individual variation in aggregation pheromone content of the bark beetle, Ips typographus. J Chem Ecol 14:1737–1761
Blomquist GJ, Figueroa-Teran R, Aw M, Song M, Gorzalski A, Abbott NL, Chang E, Tittiger C (2010) Pheromone production in bark beetle. Insect Biochem Mol Biol 40:699–712
Cates RG, Alexander H (1982) Host resistance and susceptibility. In: Mitton JB, Sturgeon KB (eds) Bark beetle in North American conifers: a system for the study of evolutionary ecology. University of Texas Press, Austin, pp 212–263
Christiansen E, Bakke A (1988) The spruce bark beetle of Eurasia. In: Berryman AA (ed) Dynamics of forest insect populations. Plenum, New York, pp 479–503
Christiansen E, Krokene P (1999) Can Norway spruce trees be ‘vaccinated’ against attack by Ips typographus? Agric For Entomol 1:185–187
Christiansen E, Krokene P, Berryman AA, Franceschi VR, Krekling T, Lieutier F, Lönneborg A, Solheim H (1999) Mechanical injury and fungal infection induce acquired resistance in Norway spruce. Tree Physiol 19:399–403
Cook SP, Hain FP (1988) Toxicity of host monoterpenes to Dendroctonus frontalis and Ips calligraphus (Coleoptera: Scolytidae). J Entomol Sci 23:287–292
Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol 48:355–381
Dickens JC (1981) Behavioural and electrophysiological responses to the bark beetle Ips typographus to potential pheromone components. Physiol Entomol 6:251–261
Engelberth J (2006) Essay 13.8: smelling the danger and getting prepared: volatile signals as priming agents in defense response. Plant Physiology, 4th edn Online. http://www.plantphys.net Accessed 02/Feb/2010
Erbilgin N, Krokene P, Christiansen E, Zeneli G, Gershenzon J (2006) Exogenous application of methyl jasmonate elicits defenses in Norway spruce (Picea abies) and reduces host colonization by the bark beetle Ips typographus. Oecologia 148:426–436
Feyereisen R (1999) Insect P450 enzymes. Annu Rev Entomol 44:507–533
Franceschi VR, Krekling T, Christiansen E (2002) Application of methyl jasmonate on Picea abies (Pinaceae) stems induces defense-related responses in phloem and xylem. Am J Bot 89:578–586
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167:353–376
Huber DPW, Erickson ML, Leutenegger CM, Bohlmann J, Seybold SJ (2007) Isolation and extreme sex-specific expression of cytochrome P450 genes in the bark beetle, Ips paraconfusus, following feeding on the phloem of host ponderosa pine, Pinus ponderosa. Insect Mol Biol 16:335–349
Hudgins JW, Christiansen E, Franceschi VR (2003) Methyl jasmonate induces changes mimicking anatomical and chemical defenses in diverse members of the Pinaceae. Tree Physiol 23:361–371
Ivarsson P, Schlyter F, Birgersson G (1993) Demonstration of de novo pheromone biosynthesis in Ips duplicatus (Coleoptera: Scolytidae): inhibition of ipsdienol and Emyrcenol production by compactin. Insect Biochem Mol Biol 23:655–662
Keeling CI, Bohlmann J (2006) Genes, enzymes, and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol 170:657–675
Klimetzek D, Francke W (1980) Relationship between the enantiomeric composition of α-pinene in host trees and the production of verbenols in Ips species. Experentia 36:1343–1344
Kozlowski G, Buchala A, Metraux JP (1999) Methyl jasmonate protects Norway spruce [Picea abies (L.) Karst.] seedlings against Pythium ultimum Trow. Physiol Mol Plant Path 55:53–58
Krekling T, Franceschi VR, Berryman AA, Christiansen E (2000) The structure and development of polyphenolic parenchyma cells in Norway spruce (Picea abies) bark. Flora 195:354–369
Krokene P, Solheim H, Krekling T, Christiansen E (2003) Inducible anatomical defense responses in Norway spruce stems and their possible role in induced resistance. Tree Physiol 23:191–197
Lanne BS, Ivarsson P, Johnsson P, Bergström G, Wassgren AB (1989) Biosynthesis of 2-methyl-3-buten-2-ol, a pheromone component of Ips typographus (Coleoptera: Scolytidae). Insect Biochem 19:163–168
Leufvén A, Bergström G, Falsen E (1984) Interconversion of verbenols and verbenone by identified yeasts isolated from the spruce bark beetle Ips typographus. J Chem Ecol 10:1349–1361
Martin D, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129:1003–1018
Martin D, Gershenzon J, Bohlmann J (2003) Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol 132:1586–1599
Phillips MA, Croteau RB (1999) Resin-based defense in conifers. Trends Plant Sci 4:184–190
Raffa KF (2001) Mixed messages across multiple trophic levels: the ecology of bark beetle chemical communication systems. Chemoecology 11:49–65
Raffa KF, Berryman AA (1983) The role of host plant-resistance in the colonization behavior and ecology of bark beetles (Coleoptera, Scolytidae). Ecol Monog 53:27–49
Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH (2008) Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58:501–517
Sandstrom P, Welch WH, Blomquist GJ, Tittiger C (2006) Functional expression of a bark beetle cytochrome P450 that hydroxylates myrcene to ipsdienol. Insect Biochem Mol Biol 36:835–845
Schlyter F, Birgersson G, Byers J, Löfqvist J, Bergström G (1987a) Field response of spruce bark beetle, Ips typographus, to aggregation pheromone candidates. J Chem Ecol 13:701–716
Schlyter F, Birgersson G, Löfqvist J (1987b) Behavioral sequence in the attraction of the bark beetle pheromone and terpene attraction in the bark beetle Ips typographus to pheromone sources. Physiol Entomol 12:185–196
Schmidt A, Zeneli G, Hietala AM, Fossdal CG, Krokene P, Christiansen E, Gershenzon J (2005) Induced chemical defences in conifers: biochemical and molecular approaches to studying their function. In: Romeo JT (ed) Chemical ecology and phytochemistry in forest ecosystems. Elsevier, Amsterdam, pp 1–28
Thaler JS (1999) Jasmonic acid mediated interactions between plants, herbivores, parasitoids, and pathogens: a review of field experiments in tomato. In: Agrawal AA, Tuzun S, Bent E (eds) Induced plant defenses against pathogens and herbivores: biochemistry, ecology and agriculture. APS, St. Paul, pp 319–334
Viiri H, Annila E, Kitunen V, Niemelä P (2001) Induced responses in stilbenes and terpenes in fertilized Norway spruce after inoculation with blue-stain fungus, Ceratocystis polonica. Trees 15:112–122
Wallin KF, Raffa KF (2000) Influences of host chemicals and internal physiology on the multiple steps of postlanding host acceptance behavior of Ips pini (Coleoptera: Scolytidae). Environ Entomol 29:442–453
Wermelinger B (2004) Ecology and management of the spruce bark beetle Ips typographus—a review of recent research. For Ecol Manag 202:67–82
Wood DL (1982) The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu Rev Entomol 27:411–446
Worrell R (1983) Damage by the spruce bark beetle in south Norway 1970–80: a survey, and factors affecting its occurrence. Medd Nor Inst Skogforsk 38:1–34
Zeneli G, Krokene P, Christiansen E, Krekling T, Gershenzon J (2006) Methyl jasmonate treatment of mature Norway spruce (Picea abies) trees increases the accumulation of terpenoid resin components and protects against infection by Ceratocystis polonica, a bark beetle-associated fungus. Tree Physiol 26:977–988
Zhao T, Krokene P, Björklund N, Långström B, Solheim H, Christiansen E, Borg-Karlson AK (2010) The influence of Ceratocystis polonica inoculation and methyl jasmonate application on terpene chemistry of Norway spruce, Picea abies. Phytochemistry 71:1332–1341
Acknowledgments
This study was supported by FORMAS (The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning), Norwegian Forest and Landscape Institute and University of Alberta, Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Julia Koricheva.
Rights and permissions
About this article
Cite this article
Zhao, T., Borg-Karlson, AK., Erbilgin, N. et al. Host resistance elicited by methyl jasmonate reduces emission of aggregation pheromones by the spruce bark beetle, Ips typographus . Oecologia 167, 691–699 (2011). https://doi.org/10.1007/s00442-011-2017-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2017-x