Abstract
Natural populations of wild cabbage (Brassica oleracea) show significant qualitative diversity in heritable aliphatic glucosinolates, a class of secondary metabolites involved in defence against herbivore attack. One candidate mechanism for the maintenance of this diversity is that differential responses among herbivore species result in a net fitness balance across plant chemotypes. Such top-down differential selection would be promoted by consistent responses of herbivores to glucosinolates, temporal variation in herbivore abundance, and fitness impacts of herbivore attack on plants varying in glucosinolate profile. A 1-year survey across 12 wild cabbage populations demonstrated differential responses of herbivores to glucosinolates. We extended this survey to investigate the temporal consistency of these responses, and the extent of variation in abundance of key herbivores. Within plant populations, the aphid Brevicoryne brassicae consistently preferred plants producing the glucosinolate progoitrin. Among populations, increasing frequencies of sinigrin production correlated positively with herbivory by whitefly Aleyrodes proletella and negatively with herbivory by snails. Two Pieris butterfly species showed no consistent response to glucosinolates among years. Rates of herbivory varied significantly among years within populations, but the frequency of herbivory at the population scale varied only for B. brassicae. B. brassicae emerges as a strong candidate herbivore to impose differential selection on glucosinolates, as it satisfies the key assumptions of consistent preferences and heterogeneity in abundance. We show that variation in plant secondary metabolites structures the local herbivore community and that, for some key species, this structuring is consistent over time. We discuss the implications of these patterns for the maintenance of diversity in plant defence chemistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Populations of plants, across several taxa, harbour high levels of diversity in secondary metabolites involved in herbivore deterrence (Berenbaum et al. 1986; Harvey et al. 2005; Mithen et al. 1995; Moyes et al. 2000; Richards and Fletcher 2002; van Leur et al. 2006). This observation challenges the evolutionary prediction that the optimum compound or chemical profile for defence against herbivore attack should spread to fixation in a population.
There are four mechanisms that could be operating to maintain the observed variation in secondary metabolites in natural populations. First, following the neutral theory of evolution (Kimura 1991), selection on secondary metabolite variants may be negligible and so diversity is maintained by genetic drift and gene flow among populations. The three alternative hypotheses assume that secondary metabolites influence plant fitness, and involve fluctuating or differential selection pressures (Ehrlich and Raven 1964). First, the selection pressures acting on secondary metabolites may be mediated by the abiotic environment, resulting in bottom-up differential selection. Second, as the diversity in secondary metabolites probably evolved through an evolutionary arms race between herbivores and plants (Fahey et al. 2001), top-down differential selection mediated by herbivores may also be involved in the maintenance of this diversity. A key requirement for top-down differential selection is that herbivore species present in the community should show temporally consistent, but possibly different, responses to the plant chemical variants. This allows differential selection pressures on plant chemotypes to arise through variation in herbivore abundance across populations or through time and can provide a mechanism for maintenance of variation in natural populations. In other words, the type and magnitude of herbivory can be considered an unpredictable environment, and different plant genotypes are better suited to different herbivory environments (Karban and Nagasaka 2004). This genotype-by-environment interaction (Gillespie and Turelli 1989; Via and Lande 1985) could maintain genotypic variation in chemical defence against herbivory through time. Fourth, there is the possibility that top-down differential selection could be imposed by consistent levels of abundance of one or more key herbivore species that vary among years in their preference for plant chemical variants. In this case, environmental variation would be imposed by fluctuating preferences of key herbivores, rather than fluctuating densities of herbivores with fixed preferences.
Evidence in support of ecological links between plant chemotypes and herbivory is provided by the fact that variation in plant resource quality can structure herbivore populations; indeed, plant genotype can exert as much influence on herbivore populations as many environmental factors (Karban 1992). Recent common garden experiments on cultivated brassicas (Poelman et al. 2009) and natural Arabidopsis chemotypes (Bidart-Bouzat and Kliebenstein 2008) demonstrate that qualitative and quantitative variation in glucosinolates (a highly variable class of secondary metabolites common to all plants in the order Brassicales) has a significant influence on herbivore diversity and patterns of plant use. While common garden and laboratory experiments provide valuable information that cannot be obtained in natural populations, such studies can suffer from an oversimplification of natural biotic interactions. Recently, however, a study of herbivore responses to glucosinolate variation in natural wild cabbage (Brassica oleracea L. var. oleracea, subsequently referred to as B. oleracea) populations (Newton et al. 2009b) identified species-specific responses to structural variation in glucosinolates. This 1-year study raised important questions regarding the consistency of responses shown by the different herbivore species and inter-annual fluctuations in the intensity of herbivore attack which could only be answered by repeating the study over a longer period of time.
Natural populations of wild cabbage show significant inter- and intra-population variation in heritable aliphatic glucosinolates (Mithen et al. 1995; Moyes et al. 2000; Newton et al. 2009a). We utilised these natural levels of variation in 12 populations of wild cabbage to investigate assumptions of the top-down differential selection hypothesis: first, that different herbivore species consistently prefer or avoid different chemotypes; and second, that rates of herbivory vary significantly through time. A further assumption, which is not critical to the action of top-down differential selection but could strengthen its effects, is that different herbivore species should differ in their response to chemotypes. In order to test these assumptions and the top-down differential selection hypothesis, we surveyed rates of herbivory on wild plants of known chemotype for 3 years, and analysed patterns of herbivory at two ecological scales, within and among plant populations.
Materials and methods
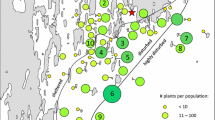
We surveyed 12 spatially distinct populations of B. oleracea across three counties in southwest England: three populations in Cornwall, three in Devon and six in Dorset (Fig. 1). In 2006 a minimum of 50 rosette stage or first flowering plants were selected and marked for glucosinolate analysis and herbivore survey at each population. In 2007 and 2008 additional plants were selected and marked, using the same selection criteria, to replace plants that had died during the first year of the study in order to maintain approximately 40–50 survey plants in each of the 12 populations. This was achieved in all populations except for the three Cornish ones. Here, many plants were used in an experimental manipulation of aphid presence/absence, to investigate genotype-by-herbivory interactions in plant performance (Newton et al. 2009a). Hence, we are unable to provide aphid survey results among years for the Cornish populations. Surveys of other herbivore species were continued on experimental plants in Cornwall, therefore surveys of all non-aphid herbivores were analysed for the full dataset of 40–50 plants/population per year.
Map of natural wild cabbage (Brassica oleracea) populations surveyed for herbivory. Top panel Large-scale map of the coastline of Southwest UK, with circles delineating Cornish, Devon and Dorset populations. Bottom panels County-level maps of populations within counties. Coordinates for the populations are as follows: Prussia Cove 1 (PC1; 50°10′N, 5°42′W), PC2 (50°12′N, 5°42′W), PC3 (50°10′N, 5°41′W), Watcombe (WAT; 50°50′N, 3°51′W), Walls Hill (WH; 50°46′N, 3°49′W), Kingswear (KW; 50°34′N, 3°56′W), Old Harry (OH; 50°64′N, 1°92′W), Durdle Door (DD; 50°62′N, 2°27′W), Aldhelm’s Head (AH; 50°69′N, 2°05′W), Winspit 1 (WS1; 50°59′N, 2°03′W), WS2 (50°58′N, 2°04′W) and Kimmeridge 2 (K2; 50°60′N, 2°13′W)
Plants were surveyed twice per year for herbivore presence, in June and September 2006, 2007 and 2008. The dominant herbivore species, found in sufficient numbers to allow statistical analysis of responses, were the specialists Pieris rapae (butterfly), Pieris brassicae (butterfly), Aleyrodes proletella (whitefly), Brevicoryne brassicae (aphid), and generalist snails (all snail species were analysed collectively). Each marked plant was thoroughly examined for herbivores on the leaves and flowering stems and any evidence of past herbivory (such as exuviae of lepidopteran larvae, and mummified remains of parasitised aphids) was also recorded.
Glucosinolate profiles were determined by high performance liquid chromatography (see Newton et al. 2009b for full description of methods). The variation in glucosinolate profiles was caused by the presence or absence of sinigrin (2-propenyl) and progoitrin (2-hydroxy-3-butenyl). This variation resulted in each plant producing one of four chemotypes: sinigrin, progoitrin, both, or neither. The ‘neither’ chemotype was extremely rare. All new plants marked in 2007 and 2008 showed one of the four previously described chemotypes (Newton et al. 2009b).
Statistical analysis
All data analysis was carried out using mixed effects modelling in R version 2.9.1, with models simplified using likelihood ratio tests of significance. Fixed effects were tested for significance using maximum likelihood versions of the mixed effects models. Standard model checks were used to verify normality and homogeneity of standardised residuals (Crawley 2007).
For the within-population-scale analysis, the consistency of herbivore presence on plants differing in glucosinolate chemotype, and the inter-annual variation in herbivore attack were analysed using generalised linear mixed effects models with a binomial error structure. Analyses of glucosinolate profiles revealed that cabbage populations were nearly monomorphic for the presence of the glucosinolate sinigrin except for in the three Cornish populations, which were instead monomorphic for the presence of progoitrin (Fig. 2). Devon populations were monomorphic for the presence of both glucosinolates. We therefore restricted our analyses of preference for each glucosinolate polymorphism to cabbage populations that showed non-negligible polymorphism (i.e. more than two plants showing the rarest chemotype). In practice, this means that preference for sinigrin-producing plants was analysed in Cornish populations only, while preference for progoitrin-producing plants was analysed in Dorset populations only. The presence or absence of each glucosinolate (sinigrin or progoitrin), year, and season, were included as fixed effects. Plant identity (nested within population) was included as a random effect to account for repeated measures and spatial structuring. We also assessed the interannual and seasonal variation in glucosinolate preference by testing the glucosinolate-by-year and glucosinolate-by-season interactions as fixed effects.
Frequency of chemotypes of wild cabbages surveyed for herbivory among 12 populations. Populations are ranked longitudinally from west to east. a Frequency of plants producing (grey bars) or not producing (black bars) sinigrin. Populations outside Cornwall are nearly monomorphic for sinigrin production. b Frequency of plants producing (grey bars) or not producing (black bars) progoitrin. Populations outside Dorset are monomorphic for progoitrin production. For abbreviations, see Fig. 1
The magnitude of among-year and between-season variation in herbivore infestation rates was tested using year and season as fixed effects in models that included all 12 plant populations (with population as a random effect). Within-population analyses also allowed us to study the relative magnitude of variance components attributable to plants within populations, and populations. We calculated these variance components using the all-population models used to test the fixed effects of season and year.
For analyses of the consistency in herbivore responses and temporal heterogeneity in herbivore attack at the among-population scale, we used similar linear mixed effects models, extended to include the effect of spatial autocorrelation in residuals (Pinheiro and Bates 2000). All 12 cabbage populations were used to assess relationships between frequency of herbivory and frequency of production of sinigrin or progoitrin. The response variable for each model was the proportion of plants attacked by each herbivore, logit transformed to normalise the residuals. The proportion of plants producing each glucosinolate, and season, were tested as fixed effects. Season nested within year were also included as random effects, corrected for heteroscedasticity using the VarPower command in R (Crawley 2007). Recognising the existence of spatial autocorrelation in some of our response and explanatory variables, we used a Gaussian autocorrelation function to describe the influence of geographic distance between populations on each model’s residuals. This absorbing of spatial autocorrelation means that any significant responses of herbivores to population-scale glucosinolate frequencies, revealed by the mixed effects models, were independent of any geographical structuring of plant populations and herbivores. Finally, the magnitude of among-year and between-season variation in herbivore infestation rates was tested using year and season as fixed effects. Since aphid survey data were not available for Cornish populations in 2007 and 2008, we could not analyse the relationship between aphid infestation and frequency of sinigrin production. We analysed the relationship between aphid infestation and progoitrin frequency by restricting our analysis to non-Cornish cabbage populations.
Modelling procedure and multiple testing
We tested significance of fixed effects (glucosinolate profile, year and season) and their interactions using likelihood ratio tests and model simplification of maximum likelihood versions of the mixed effects models (Crawley 2007). In order to reduce type I errors a local false discovery rate (FDR) (Benjamini and Hochberg 1995) adjustment of P values was applied to the intra- and inter-population scale tests using the fdrtool package for R (Strimmer 2008). FDRs were adjusted at the level of herbivore and ecological scale, rather than to the model simplification results within the analysis for each herbivore species. All minimal adequate models (i.e. for each herbivore species at each ecological scale) survived this assessment of FDRs, with the exception of seasonal heterogeneity in population-scale herbivory by P. brassicae (see “Results”).
Key and subsidiary hypotheses
For each herbivore species at each ecological scale, we wished to test two major hypotheses and two subsidiary hypotheses:
Hypothesis 1 (key)
The herbivore species shows a significant and consistent preference for or aversion to a particular plant glucosinolate profile (test of main effect of each glucosinolate on herbivore presence/frequency).
Hypothesis 2 (key)
Herbivore presence/frequency varies significantly between seasons and/or among years.
Hypothesis 3 (subsidiary)
The herbivore species shows among-year variation in the strength of preference for glucosinolate profiles (i.e. a test of inconsistent response, determined by the glucosinolate-by-year interaction).
Hypothesis 4 (subsidiary)
The herbivore species show within-year variation in the strength of preference for glucosinolate profiles (i.e. a test of inconsistent response, determined by the glucosinolate-by-season interaction).
Results
Within-population herbivore responses to glucosinolate polymorphism
Among plants within populations, B. brassicae and A. proletella responded to the presence of progoitrin in some way (all P values for the analysis of herbivore responses to glucosinolates at the within-population scale are presented in Table 1). Snails, P. rapae and P. brassicae showed no response to glucosinolate polymorphism. There was dramatic variation among years in the probability of plants being infested with each of the herbivore species studied. There also existed significant heterogeneity in herbivory between early and late season surveys, for all herbivore species.
B. brassicae was observed consistently more frequently on plants producing, compared to those lacking, progoitrin (Table 1; Fig. 3a). The response of B. brassicae to plants producing sinigrin was not analysed here, but we note an aversion to sinigrin found in 2006 (Newton et al. 2009b), and a negative impact of sinigrin on aphid colony sizes in 2007 and 2008 (Newton et al. 2009a). Aphid infestation rates varied significantly among years within non-Cornish populations.
Temporal consistency of responses to glucosinolate polymorphisms in wild cabbages, for five key herbivores at the within-population scale. Bars represent the probability of finding each herbivore on a plant lacking (black bars) or producing (grey bars) each glucosinolate, in each of 3 years of survey, for an average survey timing in an average population. Probabilities and SEs estimated using generalised linear mixed effects models with year and glucosinolate presence as fixed effects, and plants nested within populations within counties as random effects. a, b, d, f, h Responses to progoitrin; c, e, g, i responses to sinigrin. Brevicoryne brassicae shows a consistent preference for progoitrin-producing plants. Aleyrodes proletella shows significantly inconsistent responses to progoitrin. Pieris rapae, Pieris brassicae and snails lack among-year responses to either glucosinolate
The presence of A. proletella was correlated with progoitrin (Table 1), but this effect was inconsistent among years: plants producing progoitrin were more likely to be infested with whitefly in 2006 and 2007, but this response became negative in 2008 (Fig. 3d). Plants were significantly more likely to be infested with whitefly in late season surveys than in early season. Whitefly infestation rates varied significantly among years (Fig. 3d, e).
Both P. rapae and P. brassicae showed neither consistent nor inconsistent preferences for sinigrin versus non-sinigrin plants, nor for progoitrin versus non-progoitrin plants. Season significantly affected presence of both butterflies: a greater number of plants were observed to be infested with these species in the late season surveys (Table 1). Infestation rates of both herbivores varied significantly among years (Fig. 3b, c, f, g). Snails showed the same patterns as the two butterfly species. There was no response to either progoitrin or sinigrin production. Plants were more likely to be infested by snails in late season surveys. Snail infestation rates varied significantly among years (Fig. 3h, i).
The use of mixed models to analyse within-population rates of herbivory also allowed an assessment of the nested variance structure, scaling up from heterogeneity at the level of plants to populations. Two patterns were observed (Table 1). The herbivores B. brassicae, P. rapae, P. brassicae and snails showed an even split of variance between the two scales. A. proletella, on the other hand, varied most in rates of herbivory among rather than within populations.
Among population herbivore responses to glucosinolate polymorphism
We found no consistent or inconsistent impacts of progoitrin frequency on rates of herbivory among populations (all P values for the analysis of herbivore responses to glucosinolates at the among-population scale are presented in Table 2). However, A. proletella and snails showed consistent responses among years to the frequency of sinigrin. There was little among-year heterogeneity in infestation rate for all herbivore species at this scale, with the exception of B. brassicae. All herbivore species showed between-season heterogeneity in frequency at this scale, with the exception of snails (Table 2).
At the among-population scale, the proportion of plants infested with B. brassicae showed no response to the proportion of plants in a population producing progoitrin (Table 2). In the absence of aphid survey data for Cornish populations in 2007 and 2008, we can only note the significant negative correlation between the frequency of singirin production and the frequency of aphid infestation, found in 2006 (Newton et al. 2009b). B. brassicae infestation rate varied among years and between seasons in non-Cornish populations (Table 2).
The infestation rate of A. proletella was consistently negatively correlated with the proportion of plants in the population producing sinigrin (Table 2; Fig. 4a). The response of whitefly to the proportion of plants producing sinigrin was not affected by year or season, nor was there any significant inter-annual variation in whitefly infestation rate (Table 2). The proportion of plants producing progoitrin had no effect on infestation by A. proletella and showed no interaction with year or season (Table 2). Whitefly infestation rates were higher in late season surveys than in early season.
Among-year consistent responses of two key herbivores to the population-scale frequency of sinigrin production. Observed frequencies of herbivore infestation are shown for surveys in 2006 (open circles), 2007 (open triangles) and 2008 (open squares). Fitted lines estimated from generalised linear mixed effects models relating frequency of herbivory to the frequency of sinigrin production, with survey month nested within year as random effects. Whitefly (A. proletella) infestation rates decrease with increasing frequencies of sinigrin production, while snail infestation rates increase
Neither P. rapae nor P. brassicae was affected (either inconsistently or consistently through time) by sinigrin or progoitrin at the among-population scale. Only season influenced the proportion of plants infested with either butterfly and frequency of herbivory by each species was higher in late season surveys (Table 2). Note that this seasonal effect on P. brassicae was the only minimal adequate model that did not survive correction for FDRs, hence further study would be required to confirm its effect.
The proportion of plants producing sinigrin positively affected the frequency of snail infestation in early season surveys but this response became non-significant in late season (Table 2). Ignoring this seasonal interaction we found a consistent positive correlation between sinigrin frequency and snail infestation rates (Fig. 4b). There was no population-level response to the frequency of plants producing progoitrin. Infestation rates of snails did not vary among years or between seasons (Table 2).
Discussion
We carried out a 3-year survey in order to test key assumptions of the hypothesis that top-down differential selection plays a role in maintaining secondary metabolite variation in natural populations of wild cabbage. In particular we wished to identify key herbivore species that showed consistent responses to the presence or absence of the heritable glucosinolates sinigrin and progoitrin, combined with seasonal and interannual heterogeneity in frequency of herbivory. Therefore, we focus here on the among-year consistency in the responses to glucosinolates shown by the different herbivore species, and temporal heterogeneity of herbivore infestation at the among- and within-population scales.
Overall, the results of the 3-year survey revealed that only B. brassicae, A. proletella and snails showed consistent responses among years to glucosinolate variation. B. brassicae showed a within-population preference for plants producing progoitrin. This effect, coupled with among-year and between-season heterogeneity in aphid infestation rates, could be a candidate driver of top-down differential selection for variation in progoitrin production. A. proletella showed no consistent responses to sinigrin or progoitrin within populations, but at the population level infestation rates of this herbivore declined with increasing proportions of plants producing sinigrin. Snails also did not show consistent responses within populations, but infestation rates increased with increasing proportions of plants producing sinigrin. These opposing effects (with increasing frequency of sinigrin-production, plant populations suffer higher rates of snail herbivory but lower rates of whitefly infestation) could be key drivers of the maintenance of glucosinolate diversity, especially if they are coupled with gene flow between cabbage populations.
Neither of the white butterfly herbivore species, P. rapae and P. brassicae, showed any responses to glucosinolate variation. This fits with previous results of surveys of white butterfly performance in Dorset populations of B. oleracea: variation in concentrations of aliphatic glucosinolates had no impact on the development time of larvae of these species (Gols et al. 2008). In our surveys, abundances of white butterflies were higher in late season surveys, and they showed heterogeneity in attack at the within- (but not among-) population scale.
All herbivore species showed among-year variation in the probability of attacking individual plants (i.e. within-population heterogeneity among years). Variation in herbivory between seasons was also observed in all species. At the population scale, frequency of herbivory varied between seasons for all herbivores except snails, while only the aphid B. brassicae showed among-year heterogeneity at the population scale. The frequency of herbivory was generally greatest in 2006; the summer of this year was much warmer and drier than those of subsequent years, which may explain some of the inter-annual variation in herbivore numbers.
Based on the results obtained in this survey, B. brassicae, A. proletella and snails meet at least some of the important criteria for consistent top-down differential selection. All three herbivores show consistent responses to sinigrin variation at the population scale (the result for B. brassicae is recalled from Newton et al. 2009b). However, snails show no heterogeneity in rates of herbivory between seasons or among years at this scale. A. proletella infestation rates vary between seasons but not among years. Of these herbivores, only B. brassicae shows consistent responses to glucosinolates among years, both among and within populations (a preference for progoitrin-producing plants identified here, and negative impacts of sinigrin on aphid colony dynamics demonstrated in Newton et al. 2009a), coupled with temporal heterogeneity in abundance both among years and between seasons. While further surveys might provide evidence of variation in A. proletella and snail infestation rates, among populations, over a longer time period, for now it appears that B. brassicae is the strongest candidate agent of differential selection for variation in the production of sinigrin and progoitrin.
There are some conflicting results from the longer-term study when compared to results obtained in the first survey year (Newton et al. 2009b). In particular, there was no evidence for aphid preference for progoitrin in 2006, but the statistical power achieved by 3 years of survey reveals a consistent preference among years for this glucosinolate within populations. In 2006 we found no signal of preference of whitefly for either glucosinolate within populations, but a population-scale negative response to the frequency of sinigrin. Three years of survey confirm this population-scale response to be consistent, and further reveal inconsistent among-year response to progoitrin. Significant responses to sinigrin found for P. brassicae in 2006, turned out to be non-significant among years.
The survey technique used is only a snapshot of herbivore presence, and may not be sufficient to give a clear picture about the responses of the less abundant herbivore species. The methodology used is sufficient to provide a strong indication that structural variation in glucosinolates is responsible for structuring the herbivore community across a wide range of species. However, more detailed surveys and experiments are required in order to provide more information on the responses of certain species of herbivores such as Lepidoptera, which are present in lower abundance but cause extensive damage to plant tissues. In addition, the survey methodology used did not control for the genetic background of the herbivores. The pierids are highly mobile and can migrate large distances (Feltwell 1982; Richards 1940). Therefore the insects observed in the populations may come from different genetic stock each year and this may explain the more variable responses to glucosinolates shown by these species compared to herbivores with more limited dispersal mechanisms.
The three herbivores that showed the most consistent responses to glucosinolates across both scales were also the most abundant herbivores: B. brassicae, A. proletella and snails. These herbivore species have passive or limited dispersal mechanisms [wind dispersal in the case of B. brassicae and A. proletella (Compton 2002)]. Such dispersal limitations may explain why responses seen within populations are often different to those observed among populations. The negative relationship between frequency of sinigrin and frequency of whitefly infestation does not scale down to an aversion to progoitrin among plants within populations. Similarly, the higher frequency of snail herbivory in plant populations with higher sinigrin frequency, is not revealed as a preference for this chemotype among plants within populations. Aphids, which have more specialised winged disperser morphs (Hodgson 2001), show no scaling-up of their preference for progoitrin-producing plants to the population scale. The only evidence for upscaling of glucosinolate responses from within to among populations, comes from the link found in 2006 between aphid aversion to plants producing sinigrin, and a negative correlation between frequency of sinigrin production and frequency of aphid infestation (Newton et al. 2009b).
Why do herbivores vary in response to glucosinolates?
Several mechanisms for herbivore species-specific preferences for or aversions to glucosinolate chemotypes were discussed in Newton et al. (2009b). To summarise, herbivores can be classified in terms of their interaction with food plants, in four ways. First, they differ in their exposure to toxic by-products of glucosinolates. Chewing insects such as Lepidoptera and molluscs bring aliphatic glucosinolates into contact with myrosinase, thus causing the breakdown of the glucosinolate into toxins, while sucking insects such as aphids and whitefly are exposed mainly to intact glucosinolates or passively to the by-products produced by damage elsewhere on the plant. Second, they vary in the mechanism of dealing with toxins. For example P. rapae metabolises the toxins into nitriles for excretion; Plutella xylostella excretes a sulphatase to prevent the breakdown of glucosinolates; other herbivores may sequester toxins for their own defence against natural enemies. Third, they vary in the spatial scale of host-plant choice by ovipositing adults. Butterflies visit many plants during oviposition, and lay eggs singly (e.g. P. rapae) or in batches (e.g. P. brassicae); aphids and whitefly show strong natal philopatry during colony development. Fourth, they vary in the evolutionary history of plant-herbivore coevolution. All herbivores surveyed here can be considered Brassica specialists, with the exception of snails. These classifications in terms of choice, exposure, feeding response and coevolution can be expected to scale up to null, unpredictable or extremely divergent patterns of herbivory across plant chemotypes.
Implications for the maintenance of secondary metabolite diversity
The rate of gene flow between these natural B. oleracea populations is low (Raybould et al. 1999), although long distance seed dispersal mediated by humans is possible (Wichmann et al. 2009). Thus, temporal mixing of chemotypes, mediated by differential responses of herbivores together with temporal variation in herbivore attack, may be a more important driver of diversity in genetically determined chemotypes than spatial mixing. For top-down differential selection by herbivores to influence the maintenance of the observed inter- and intra-population variation in glucosinolate chemotypes, herbivore species must demonstrate different responses to genetically determined plant defence chemical variants, and herbivore abundance should also vary through time. We previously detected significant differential responses of herbivore species to qualitative variation in plant defence compounds over a 1-year survey period and surmised from these results that plant chemistry can structure the herbivore community (Newton et al. 2009a, b). The results from an extended survey over 3 years show that three common herbivore species showed significant consistent responses to structural variation in glucosinolates, while two Lepidoptera species showed no detectable responses.
Of the three herbivores showing consistent responses, B. brassicae is perhaps the strongest contender as a predictable agent of differential selection on glucosinolate genotype. This specialist aphid shows a consistent preference for progoitrin-producing plants within populations. Previous results indicate this species has an aversion to sinigrin-producing plants (Newton et al. 2009a, b) and is less frequent in populations harbouring high frequencies of these (Newton et al. 2009b). Furthermore, we observed significant variation in the abundance of this herbivore species among years and seasons. This affirms two of the key assumptions of the differential selection hypothesis: first, that this key herbivore shows consistent preferences for glucosinolate chemotypes, and second that herbivory pressure exerted by this species varies through time.
The final piece of evidence required to demonstrate top-down differential selection on glucosinolates in wild cabbage populations, is evidence that the production of chemical defences against herbivory imposes fitness costs (Bergelson and Purrington 1996; Strauss et al. 2002), and that key herbivore species affect plant fitness. If these herbivores do not influence the fitness of wild cabbages, then glucosinolate variation may simply be maintained by genetic drift, but remains ecologically important thanks to its role in structuring herbivore communities, with applied relevance for the choice of crop chemotypes in integrated horticulture. If, on the other hand, B. brassicae, A. proletella or snails harm plant fitness directly, then they may be responsible for the maintenance of variation of important secondary metabolites in wild cabbage populations. The field survey results presented here have identified candidate herbivore species (particularly the aphid B. brassicae) that show temporally consistent responses to plant chemical variation, coupled with temporal heterogeneity in attack, and provide a basis for further investigation into herbivore-mediated, top-down differential selection.
References
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300
Berenbaum MR, Zangerl AR, Nitao JK (1986) Constraints on chemical coevolution—wild parsnips and the parsnip webworm. Evolution 40:1215–1228
Bergelson J, Purrington CB (1996) Surveying patterns in the cost of resistance in plants. Am Nat 148:536–558
Bidart-Bouzat MG, Kliebenstein DJ (2008) Differential levels of insect herbivory in the field associated with genotypic variation in glucosinolates in Arabidopsis thaliana. J Chem Ecol 34:1026–1037
Compton SGA (2002) Sailing with the wind: dispersal by small flying insects. In: Bullock JM (ed) Dispersal ecology. Forty-second symposium of the British ecological society
Crawley MJ (2007) The R book. Wiley, Chichester
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Fahey JW, Zalcmann AT, Talalay P (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56:5–51
Feltwell J (1982) The large white butterfly. The biology, biochemistry and physiology of Pieris brassicae (Linnaeus). Springer, Berlin
Gillespie JH, Turelli M (1989) Genotype–environment interactions and the maintenance of polygenic variation. Genetics 121:129–138
Gols R, Wagenaar R, Bukovinsky T, van Dam NM, Dicke M, Bullock JM, Harvey JA (2008) Genetic variation in defence chemistry in wild cabbages affects herbivores and their endoparasitoids. Ecology 89:1616–1626
Harvey JA, Van Nouhuys S, Biere A (2005) Effects of quantitative variation in allelochemicals in Plantago lanceolata on development of a generalist and a specialist herbivore and their endoparasitoids. J Chem Ecol 31:287–302
Hodgson DJ (2001) Monoclonal aphid colonies and the measurement of clonal fitness. Ecol Entomol 26:444–448
Karban R (1992) Plant variation: its effects on populations of herbivorous insects. In: Fritz RS, Simms EL (eds) Plant resistance to herbivores and pathogens: ecology, evolution and genetics. University of Chicago Press, Chicago, pp 195–215
Karban R, Nagasaka K (2004) Are defenses of wild radish populations well matched with variability and predictability of herbivory? Evol Ecol 18:283–301
Kimura M (1991) Recent developments of the neutral theory viewed from the Wrightian tradition of theoretical population genetics. Proc Natl Acad Sci USA 88:5969–5971
Mithen R, Raybould AF, Giamoustaris A (1995) Divergent selection for secondary metabolites between wild populations of Brassica oleracea and its implications for plant–herbivore interactions. Heredity 75:472–484
Moyes CL, Collin HA, Britton G, Raybould AF (2000) Glucosinolates and differential herbivory in wild populations of Brassica oleracea. J Chem Ecol 26:2625–2641
Newton E, Bullock JM, Hodgson D (2009a) Bottom-up effects of glucosinolate variation on aphid colony dynamics in wild cabbage populations. Ecol Entomol 34:614–623
Newton E, Bullock JM, Hodgson DJ (2009b) Glucosinolate variation in wild cabbage (Brassica oleracea) influences the structure of herbivore communities. Oecologia 160:63–76
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-plus. Springer, New York
Poelman EH, van Dam NM, van Loon JJA, Vet LEM, Dicke M (2009) Chemical diversity in Brassica oleracea affects biodiversity of insect herbivores. Ecology 90:1863–1877
Raybould AF, Mogg RJ, Clarke RT, Gliddon CJ, Gray AJ (1999) Variation and population structure at microsatellite and isozyme loci in wild cabbage (Brassica oleracea L.) in Dorset (UK). Genet Resour Crop Evol 46:351
Richards OW (1940) The biology of the small white butterfly (Pieris rapae) with special reference to the factors controlling its abundance. J Anim Ecol 9:228–243
Richards AJ, Fletcher A (2002) The effect of altitude, aspect, grazing and time on the proportion of cyanogenics in neighbouring populations of Trifolium repens L. (white clover). Heredity 88:432–436
Strauss SY, Rudgers JA, Lau JA, Irwin RE (2002) Direct and ecological costs of resistance to herbivory. Trends Ecol Evol 17:278–285
Strimmer K (2008) fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics 24:1461–1462
van Leur H, Raaijmakers CE, van Dam NM (2006) A heritable glucosinolate polymorphism within natural populations of Barbarea vulgarise. Phytochemistry 67:1214–1223
Via S, Lande R (1985) Genotype–environment interaction and the evolution of phenotypic plasticity. Evolution 39:505–522
Wichmann MC et al (2009) Human mediated dispersal of seeds over long distances. Proc R Soc B Biol Sci 276:523–532
Acknowledgments
Thanks to J. Blount, A. Barlow and N. van Dam for providing advice on HLPC and techniques for purifying desulphoglucosinolates, Matthias Wichmann for advice about the Dorset sites, Isobel Giblin and James Grecian for fieldwork assistance, and Anne Altringham for mapping. P. Behrens, T. Jones, Jane Hodgson and Torbay Coast and Countryside Trust allowed access to field sites. This work was funded by European Social Fund and NERC grants to D. J. H.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Richard Karban.
Rights and permissions
About this article
Cite this article
Newton, E., Bullock, J.M. & Hodgson, D. Temporal consistency in herbivore responses to glucosinolate polymorphism in populations of wild cabbage (Brassica oleracea). Oecologia 164, 689–699 (2010). https://doi.org/10.1007/s00442-010-1702-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1702-5