Abstract
Vegetative resprouting, soil or canopy-stored seed banks, post-fire seed dispersal and germination are the major strategies by which plants regenerate after fires. Post-fire regeneration modes of plants are commonly based on the presence or absence of post-fire recruitment as well as the presence or absence of post-fire resprouting. High temperatures, smoke and ash are characteristics of fire and the post-fire environment. We hypothesized that heat, smoke, ash and pH will have differential effects on seed germination depending on species’ post-fire regeneration strategies: serotinous vs. nonserotinous (which may have soil seed banks) and resprouters vs. nonresprouters (which may be obligate seeders). Here we examined the effects of these factors on the germination of 27 common east Australian species. Most serotinous species supported our hypothesis by showing no effect or reduced germination in response to heat. However, contrary to our prediction, all nonserotinous nonresprouting species also showed no effect or reduced germination in response to heat. Smoke, contrary to our hypothesis, had a negative or no effect on all serotinous and nonresprouting species, but no clear directional effect on serotinous and resprouting species. Supporting our hypotheses, ash and high pH showed positive or nonsignificant effects on the germination of all serotinous resprouting species, and a negative or no effect on nonserotinous resprouting species. However, contrary to our prediction, it had a negative or no effect on the serotinous nonresprouting species and no clear effect on nonserotinous nonresprouting species. We also discovered large differences in germination responses between conspecific populations that varied in their degree of resprouting. Although our data confirmed several of our predictions, the overall conclusion is that the responses of seeds to heat, smoke, ash and pH are not tightly associated with post-fire regeneration functional types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fire is a major disturbance that shapes plant communities occupying fire-prone habitats worldwide. These communities consist mostly of plant species that are capable of regenerating after fire by vegetative resprouting, or through the germination of seed from banks stored in soil or in plant canopies regulated by fire-related cues (Gill 1981; Trabaud 1987; Whelan 1995; Bond and van Wilgen 1996). Variation between species in the combination of such traits is thought to be important in governing their differential responses across the wide range of fire regimes that they may experience in nature (Noble and Slatyer 1980; Lavorel and Cramer 1999). Consequently, if life history traits influence selective pressures during regeneration, we expect that seed germination responses to fire-related environmental conditions will correspond to regeneration modes (resprouting or nonresprouting) and seed bank types (serotinous or nonserotinous).
Early reviews classified plants by their post-fire regeneration modes or life-history traits (Biswell 1974; Gill 1975; Naveh 1975; Kruger et al. 1977). The main distinction is between resprouters (or sprouters) and nonresprouters (or nonsprouters). Resprouters are plants that recover even after full leaf scorch by resprouting from their roots, basal stems or epicormic buds. Nonresprouters are plants that die after full leaf scorch but regenerate by germination (Gill 1981). Bond and van Wilgen (1996), and subsequently Pausas (1999), proposed a factorial division into four groups, based on the dichotomy between fire-recruiting and non-fire-recruiting as well as resprouting and nonresprouting survival modes.
The level of post-fire seed germination varies between species. Seed from in situ sources may be stored in soil seed banks or in canopy-stored seed banks (bradyspory or serotiny). In the Northern Hemisphere, serotiny occurs exclusively in conifers, mainly in pines (Pausas et al. 2006), but it is more common in the southern hemisphere, where it occurs in the Proteaceae (Myerscough et al. 2000) and several other plant families (Lamont et al. 1991; Bond and van Wilgen 1996). Among serotinous species, rates of seed release in the absence of fire tend to be greater in resprouters than in nonresprouters (Myerscough et al. 2000; Cowling and Lamont 1985; Whelan et al. 1998). This suggests that, for resprouters, a greater proportion of seeds are available for recruitment during intervals between fires than for nonresprouters, in which nearly all seeds are made available for germination during a short period after fire (Keeley 1991). Thus the germination and establishment of serotinous nonresprouters should be better adapted to the post-fire environment when compared with serotinous resprouters, because more seeds and seedlings of the latter group are able to avoid selective pressures associated with the post-fire period.
For a wide range of fire-prone species with soil seed banks, there is abundant evidence that heat (Keeley 1991; Trabaud and Oustric 1989; Auld and O’Connell 1991; Cocks and Stock 1997; Bell 1999) and smoke (Dixon et al. 1995) are important stimuli for germination. While high temperatures and smoke are the characteristics of fire itself, ash—the mineral remains of burned plants—is a typical characteristic of the post-fire environment. Small amounts of ash may provide plants with rich mineral nutrition, but the elevated pH values (>10) that are typical of thick ash layers (Henig-Sever et al. 1996) may inhibit seed germination and growth. For plants with traits that impose greater dependence on post-fire recruitment, we therefore expect positive germination responses to, or at least a greater tolerance of, fire-related stimuli (heat and smoke).
Only a few studies have examined seed responses to fire-related stimuli in relation to other fire-related plant traits. Moreno and Oechel (1991) found seeds of nonresprouters to be more tolerant to heat shock than those of resprouters, while Bell and Williams (1998) found no consistent pattern. In northern hemisphere floras, serotiny and post-fire obligate seeding are linked traits and occur mainly within pines and cypresses, while most resprouters are angiosperms with fleshy fruits (Keeley 1991; Verdu 2000; Pausas et al. 2004). In contrast, the Australian flora exhibits a very rich array of fire-related traits (Keith et al. 2002), which coexist in different combinations (Myerscough et al. 2000; Pausas et al. 2004). Genera containing serotinous taxa (e.g., Allocasuarina, Banksia, Hakea, Isopogon, Petrophile, Leptospermum, and Melaleuca) include both obligate seeders and resprouters. Moreover, populations of some species may vary in their degree of post-fire resprouting ability (e.g., species of Allocasuarina, Hakea, Keith et al. 2002), and others in their degree of serotiny (e.g., Banksia, Cowling and Lamont 1985; Whelan et al. 1998). Nonserotinous genera with dormant soil seed banks (e.g., Acacia and Dodonaea) also include both obligate seeders and resprouters. This situation enables us to independently examine the influence of the post-fire regeneration mode (resprouter or nonresprouter, including obligate seeders) and seed bank type (serotinous or nonserotinous, including species with soil seed banks) on germination in relation to fire.
Early germination stimulated by heat and smoke, resistance to high ash concentration and its high pH should be advantageous for post-fire seedling recruitment, and thus more crucial for nonresprouters (including obligate seeders) than resprouters. Post-fire recruiting species that regenerate from soil seed banks commonly have dormant seeds that are cued to germinate at the earliest possible time after fire, with heat shock and smoke as the main regulating factors (Bond and van Wilgen 1996). Given their greater dependence on post-fire seedling recruitment for population persistence, we expect nonresprouters to exhibit positive germination responses to fire-related cues (e.g., heat, smoke and ash), while resprouters should exhibit negative responses or no effect. We also expect seeds of nonresprouters to display greater tolerance to ash and high pH, because their dependence on post-fire recruitment exposes them to greater selection pressures from these factors. However, these responses may differ depending on the nature of the seed bank. For instance, retention of seeds within serotinous fruits potentially reduces their exposure to heat, and hence we expect them to experience less selective pressure for development of heat resistance and dormancy. However, after their release into the post-fire environment, serotinous seeds are exposed to chemicals leaching from charred wood, which have similar effects on germination to chemicals in smoke (Keeley and Fotheringham 1997). Thus, we do not expect smoke to negatively affect the germination of serotinous seeds.
Seeds of serotinous species, both resprouters and nonresprouters, are released onto the soil surface in the immediate post-fire environment, where they experience high levels of ash and high pH. Nonserotinous nonresprouters (obligate seeders with soil seed bank) are also dependent on post-fire recruitment. We therefore expect plant species with these combinations of traits to respond more positively to ash and high pH than nonserotinous resprouters (Goubitz et al. 2003). Nonserotinous resprouters, some of which have no soil seed bank (e.g., oaks in the Northern Hemisphere), are not dependent on post-fire recruitment, and some of their seeds may germinate in fire-free intervals. If this means that they have been exposed to less selective pressure associated with heat, smoke, ash and elevated pH, then we expect their germination to be negatively affected when exposed to these conditions.
In this study, we examined the germination of 27 east Australian species to determine whether plant regeneration modes (serotiny and resprouting ability) influenced seed germination response to heat shock or smoke, and to ash and high pH values. Specifically, we sought to test the predictions summarized in Table 1. In addition, we examined two conspecific (Allocasuarina distyla) populations that varied in resprouting ability, two conspecific (Banksia serrata) populations that varied in degree of serotiny, and two conspecific (Hakea dactyloides) populations that did not differ in these traits.
Methods
Study species and seed collection
We selected common and widespread species growing in fire-prone habitats of southeastern Australia, representing post-fire resprouters and nonresprouters (obligate seeders), as well as serotinous and nonserotinous (some with soil seed bank) species, in all possible combinations. To increase phylogenetic independence, we sought closely related species that differ in their post-fire responses. We succeeded in doing so with regard to resprouting ability; however, sampling choices for serotiny were limited because this trait is restricted to a few plant families (e.g., Proteaceae and Myrtaceae). Constraints on seed availability necessitated the use of Acacia and Dodonaea as the only nonserotinous species. We are aware that our sampled species are phylogenetically biased, but believe that by testing 27 species we could discover any pattern of connections between plant functional types (serotinous or nonserotinous and resprouting or nonresprouting) and germination responses to heat, smoke, ash and high pH. In addition, from three of the 27 species we collected seeds from two populations each: Banksia serrata populations differing in their degree of serotiny; Allocasuarina distyla that differed in their resprouting ability; and Hakea dactyloides with similar post-fire regeneration strategies.
We conducted two germination experiments; the first examined the effects of heat shock and smoke, while the second examined the effects of ash and pH. Seeds of most serotinous species were collected by us in NSW Australia in September–November 2004 with a permit from National Parks and Wildlife Service of NSW, while the rest were no more than two years old, harvested in nature by seed collectors, and purchased from AustraHort Pty Ltd. Seed Merchants (P.O. Box 595, Cleveland, QLD 4163, Australia).
The tested species list, their source, their post-fire regeneration traits and the number of seeds used for the germination experiment are listed in S1 of the “Electronic Supplementary Material” (or “ESM”). Nomenclature follows Harden (1990–2003). Post-fire regeneration traits were determined according to the New South Wales Flora Fire Response Database (Department of Environment and Conservation, National Parks and Wildlife Service, NSW, Australia) and personal knowledge (D.K. and R.W.).
Experimental design
Seeds were collected from several individual plants and pooled together for further treatments. Heat treatment was applied to a part of the seed stock of each species before initiating germination. Large seeds were disinfected for 5 min in 10% hypochlorite solution and rinsed thoroughly for 5 min in running tap water. Seeds were set for germination on one layer of Whatman No. 1 filter paper in 9 cm Petri dishes with 5 ml water or treatment solution at room temperature (20–25°C). Petri dishes were set in trays inserted into sealed plastic bags to limit evaporation, and the location of the trays was rotated daily. Seed number per dish varied with species depending on seed size and availability (for details, see S1 of the ESM). Each treatment included ten dishes. Because smoke, ash and pH were applied at the Petri dish level, each dish was regarded as an independent sample (although heat shock was applied at the seed stock level).
In the heat and smoke experiment, seeds were exposed to four treatments: (C) control: untreated seeds; (H) heat: dry seeds, covered with thin aluminum foil, were heated for 5 min in an oven that was preheated to 100°C; (S) smoke: dry seeds were germinated on smoked filter paper; smoked filter paper was exposed for 15 min to cold smoke produced by burning Eucalyptus leaf litter in a custom-made device; (SH) smoke and heat: heated seeds were germinated on smoked filter paper, combining the two previous treatments. All Acacia species were heated, but because of a shortage of seeds and the previous knowledge that their germination is mainly regulated by heat (Bell 1999), they were not tested for the effect of smoke.
For the ash and pH experiment, seeds of Acacia and Dodonaea were individually scarified using a razor blade to ensure that any seed-coat induced dormancy was broken. All seeds were germinated in 5 ml of one of the following treatment solutions: distilled water (W); pH 10, 0.05 M MCAPS [3-(cyclohexamino)-1-propanesulfonic acid] (pH 10); Eucalyptus ash diluted to pH 7 (A7); Eucalyptus ash diluted to pH 8 (A8); Eucalyptus ash diluted to pH 9 (A9); and Eucalyptus ash diluted to pH 10 (A10). MCAPS buffer was purchased from Sigma-Aldrich, Inc (St. Louis, MO, USA). This buffer had no negative side effects on the germination of Rhus coriaria and Pinus halepensis when tested by Ne’eman et al. (1999) and by Henig-Sever et al. (2000).
Only species that germinated more than 5% of the seeds in more than one treatment were included in the analyses; Eucalyptus grandis, E. saligna and Leptospermum laevigatum failed to germinate to this level.
Seeds were considered to have germinated when the root length equaled the seed diameter. Germination was monitored every 4–7 days, until no more seedlings emerged for 14 days, and the final germination proportion was calculated for each Petri dish.
Data analyses
Germination hypotheses were tested by fitting logit-linear models with binomial error distributions to the germination data (Keith 1997). The models used for the heat and smoke experiment were of the form G = β0 + β1 × H + β2 × S + β3 × H*S, where G is the proportion of seeds germinated, H is the effect of heat shock (two levels), S is the effect of smoke (two levels), H*S the interaction between heat and smoke, and β0–β3 are the coefficients. A similar model was applied to the ash and pH experiment, G = β0 + β1 × A + β2 × P + β3 × A*P, where A is the effect of ash (two levels), P is the effect of pH (three levels), A*P is the interaction between ash and pH, and G and β0–β3 are as described above. Due to the exclusion of the bis-tris propane buffer solution (pH 8) treatment, only one degree of freedom was available to test the interaction term. The number of potentially viable seeds in each replicate (varying from 6 to 45, depending on species) was used as the binomial denominator. There were five replicate Petri dishes within each orthogonal combination of heat and smoke treatments and each combination of ash and pH. Only a heat treatment was applied to the six Acacia species due to limited seed availability. The models were checked by examining plots of the residuals. Where the mean residual deviance indicated overdispersion, the models were refitted using the Williams procedure to obtain a scale parameter that was close to unity (Crawley 1993). Main effects and interaction terms were tested by backward stepwise elimination from the full model, and by constructing F ratios from the mean deviance of each model term using the mean residual deviance as the denominator.
We classified the responses of each of the 27 tested species (randomly excluding one of the two conspecific sampled populations) as “supporting” or “rejecting” our specific hypotheses concerning the response to heat, smoke and the combined response to ash and high pH, and grouped the responses by plant functional types (serotiny and resprouting) in a table. Then we counted, for each plant functional type and experimental factor, the number of species that supported or rejected our specific hypotheses, and used the binomial distribution to examine whether the responses of any given species in each plant functional group differed significantly (P < 0.05) from the randomly expected ratio of 1:1 in order to support or reject our hypothesis.
Results
No significant differences were found among the four regeneration modes in their relative germination rates (control = 100%) as a result of heat-shock, smoke, smoke and heat, (Fig. 1a, Kruskal Wallis test χ 2 = 0.858, df = 3, P = 0.836; χ 2 = 0.640, df = 2, P = 0.726; χ 2 = 0.769, df = 2, P = 0.681, respectively). The nonserotinous resprouting species had an extremely high but variable germination after heat shock. Note that Acacia species were not smoke treated. There were also no differences between regeneration modes in relative germination in buffer (pH 10) and ash solution (A10) (Fig. 1b, Kruskal Wallis test χ 2 = 3.544, df = 3, P = 0.315 and χ 2 = 1.798, df = 3, P = 0.615, respectively).
Mean (+SE) relative (%) germination rates (control = 100%) of tested species and populations classified by their post-fire regeneration traits—resprouting serotinous (RSP/SR); resprouting nonserotinous (RSP/NSR); nonresprouting serotinous (NRSP/SR) and nonresprouting nonserotinous (NRSP/NSR)—in response to a heat-shock H, smoke S, heat and smoke HS and control C treatments, and b pH 10 (pH 10), ash solution at pH 10 (A10) and water W. Numbers above bars indicate the number of species
The results for the responses of all tested species to all experiments are presented in S1 and S3 of the ESM and are summarized in Table 2. The results for 36 cases supported our hypotheses and those for 25 cases rejected them, and the ratio between them was not significantly different from 1:1.
Species-specific responses
Heat and smoke
Heat significantly affected the germinations of 16 out of the 21 tested species and populations; 14 species negatively and only two positively (S2 of the ESM). Smoke significantly affected the germinations of eight out of the 16 tested species and populations; all of the significant effects were negative (S2 of the ESM). The interaction term for heat and smoke treatments was significant for seven out of the 16 tested species and populations (S2 of the ESM). In all cases, the effect of one treatment became more negative when combined with the other.
Out of the 20 heat-tested species, 12 supported our predictions (Table 1), but their ratio did not differ significantly from 0.5 (binomial test, P > 0.05) (Table 2). Out of the five serotinous nonresprouting species, four supported our prediction by having significantly reduced germination after the heat treatment, and out of eight serotinous resprouting species, seven supported our prediction by having a positive or no effect of heat on their germination (Table 2, S2 of the ESM). Leptospermum juniperinum and Melaleuca hypericifolia also had a negative response to heat, but only when it was combined with smoke. Seven species also had a significant H*S interaction, and the effect of heat became more negative when combined with smoke (e.g., B. spinulosa, M. squarrosa, Fig. 2, S2 of the ESM). The remaining species (e.g., L. squarrosum) were unaffected by heat, smoke, or their combination. All four nonserotinous nonresprouting species failed to support our predictions by having a negative or no effect of heat, and two of the three nonserotinous resprouters, contrary to our prediction, had positive germination responses to heat (Table 2, S2 of the ESM).
Final germination percentages (mean + SE) of untreated control seeds C, heat shock H, smoke S and combined heat and smoke treatments HS, demonstrating a negative synergistic effect of the combined heat and smoke treatment on a B. spinulosa and b M. squarrosa. Differences among treatments in all species are significant (P < 0.05, S2 of the ESM)
Out of the 13 species tested for smoke response, only four supported our predictions (Table 1), but their ratios did not differ significantly from 0.5 (binomial test, P > 0.05) (Table 2). All five serotinous nonresprouting species rejected our prediction by having no effect of smoke on germination, but only four out of the eight serotinous resprouting species demonstrated a positive or no effect of smoke, supporting our predictions (Table 2, S2 of the ESM).
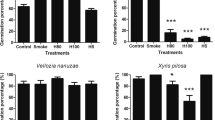
We discovered large differences in germination responses to heat between conspecific populations that varied in their degree of resprouting (A. distyla, Fig. 3a, S2 of the ESM) and degree of serotiny (B. serrata, Fig. 3b, S2 of the ESM). These differences were mainly due to much stronger negative effects of heat in the nonresprouting population of A. distyla and the more serotinous population of B. serrata. However, such a difference also occurred between similar populations of H. dactyloides (Fig. 3c, S2 of the ESM). The differences in response to smoke were small.
Final germination percentages (mean + SE) of untreated control seeds C, heat shock H, smoke S and combined heat and smoke HS treatments, demonstrating intraspecific differences between populations of: a resprouting and b weakly resprouting A. distyla; c fully serotinous and d partially serotinous B. serrata; and e, f two H. laevipes populations with similar post-fire regeneration traits. Differences among treatments in all species are significant (P < 0.05, S2 of the ESM)
Ash and pH
The germinations of only five out of 30 tested species were significantly affected by ash, and another one by pH treatment (S3 of the ESM). Germination in water was lower than in most of the significant ash and pH treatments, demonstrating positive effects of ash and elevated pH (S3 of the ESM). Out of the 30 tested species, 20 supported our predictions (Table 1), but their ratios did not differ significantly from 0.5 (binomial test, P > 0.05) (Table 2). The responses of six serotinous resprouting species rejected our prediction (Tables 1, 2) by showing a negative or no effect of high pH or ash on germination, and only M. hypericifolia (Fig. 4a, S3 of the ESM) had a positive effect that supported our prediction. All 12 serotinous resprouting species supported our prediction by having positive or nonsignificant effects of pH or ash (Table 2, S3 of the ESM). Among these, M. acuminata (Fig. 4b, S3 of the ESM) had a positive effect of ash on germination, while E. elata (Fig. 4c, S3 of the ESM) and B. serrata BG (Fig. 4d, S3 of the ESM) did not show significant effects. The data for all four nonserotinous resprouting species supported our prediction by showing negative or no effects of ash or pH (Table 2, S3), but only three out of five nonserotinous nonresprouting species had positive effects that supported our prediction (Table 2, S3 of the ESM).
Final germination percentages (mean + SE) of untreated control seeds in water (no ash) and ash solutions adjusted to pH 7, pH 8, pH 9 and pH 10 for a M. hypericifolia, b M. acuminata, c E. elata and d B. serrata. Differences among treatments in all species are significant (P < 0.05, S3 of the ESM). Water treatments buffered at pH 8 and pH 9 were not examined (see text)
Discussion
Heat and smoke
The average germination of serotinous seeds after heat shock tended to be lower than that of untreated seeds (Fig. 2, S2 of the ESM); this was expected because nonserotinous seeds are protected from the heat by the insulation provided by the fruit tissue (Gill 1976, 1981) and are thus not exposed to direct selection pressure from heat. In contrast, the average germination after heat shock of nonserotinous seeds tended to be higher than that of untreated seeds (Fig. 2, S2 of the ESM); this result was expected (despite phylogenetic bias) because this group consists mainly of Acacia species, many of which have a soil-stored seed bank. However, we did not find the expected positive or tolerant germination response to heat in nonserotinous nonresprouters (Table 2, S2 of the ESM). Our results (S2 of the ESM) demonstrated differential responses to heat within this group, including species of Acacia, many of which have hard seeds that are known to require heat shock before germination (Brown et al. 2003). The lack of any heat response in Acacia, as found here for A. calamifolia and A. pycnantha, is uncommon, but has been reported previously for a small number of species (Jeffrey et al. 1988; Auld and O’Connell 1991). Some Acacia species can produce a significant fraction of nondormant seeds with soft seed coats (Auld 1995), limiting their response to heat. Thus, high temperatures (110–120°C) and long durations can be lethal for a range of Acacia species (Auld and O’Connell 1991).
Smoke has been reported to be seed germination cue in South African fynbos species (De Lange and Boucher 1990; Brown 1993), California chaparral species (Keeley 1991; Keeley and Fotheringham 1997) and Australian sclerophyllous species (Dixon et al. 1995; Thomas et al. 2003). We found little relationship between smoke response and resprouting ability, a result that concurs with Dixon and Roche (1995), who suggested that the promotive effect of smoke is independent of plant life form and resprouting ability. Smoke has been investigated as a germination cue mainly for species with dormant soil seed banks (Brown and van Staden 1997). The negative smoke effects that we observed in many serotinous species (S2 of the ESM) were unexpected, because these seeds would be exposed to chemicals associated with smoke and charred wood after their post-fire release. However, germination responses are sensitive to concentrations of these chemicals (Dixon et al. 1995), and delayed dispersal may reduce the exposure of serotinous seeds to smoke chemicals relative to seeds stored in the soil.
In this research, we examined the effect of smoke on only one nonserotinous species (D. falcata). However, results of previous studies support our predictions (Table 1). Positive effects of both heat and smoke have been demonstrated separately, and in combination, for a range of species with soil-stored seed banks from the Sydney region (Auld and O’Connell 1991; Kenny 2000; Morris 2000; Thomas et al. 2003) and elsewhere (Keeley 1991; Dixon et al. 1995; Keith 1997).
Ash and pH
Ash inhibited germination of the Mediterranean pine Pinus halepensis (Ne’eman et al. 1993; Henig-Sever et al. 1996, 2000) and Californian cypress Cupressus sargentii (Ne’eman et al. 1999). Inhibition of germination by ash has been demonstrated in a range of fire-prone northern hemisphere plant species (Thomas and Wein 1985; Gonzales-Rabanal et al. 1989; Ne’eman et al. 1993; Gonzalez-Rabanal and Casal 1995; Eshel et al. 2000). Only two published studies report the effects of ash on germination in the Australian flora. In both cases, the addition of ash to soil seed bank samples reduced densities and species richness of the emerged seedlings compared to control (Facelli and Kerrigan 1996; Enright et al. 1997).
We hypothesized that because nonresprouting (serotinous and nonserotinous) as well as serotinous resprouting species germinate in the post-fire environment, they should positively respond to high pH and ash treatments (Table 1). However, almost all serotinous nonresprouting species rejected our hypothesis by showing no positive responses to ash or elevated pH, while nonserotinous nonresprouters did not demonstrate any clear pattern. In contrast, all resprouters (serotinous and nonserotinous) supported our predictions; serotinous resprouters had no negative responses and nonserotinous resprouters had no positive responses (Table 2, S2 of the ESM). This suggests that resprouters and nonresprouters, as well as serotinous and nonserotinous species, of the fire-prone east Australian flora are similarly adapted to germinate in the post-fire environment by having positive or at least no negative effects of ash. Unfortunately, due to limited seed availability, our study included mainly serotinous species, few nonserotinous species (mainly Acacia), and did not examine the functional group of resprouting species with no dormant seed bank (R+S− species of Pausas et al. 2004).
Linkage between germination cues and life history traits
Morphological and life history traits are useful for determining plant functional types, and are used to predict responses of plants to various disturbances (Noble and Slatyer 1980; Noble and Gitay 1996; Lavorel and Cramer 1999). However, the ability of plants to regenerate after disturbances in general, and fire in particular, also depends on traits that determine their germination. If fire regimes act as a selective force on life history traits (Pausas et al. 2004, 2006; Verdu 2000), we expect them to act similarly to regulate post-fire germination.
Despite these expectations, our results did not demonstrate general strong relationships between life history traits and germination response. At the species level, there were some negative relationships between serotiny and germination responses to heat and smoke, but there were no clear relationships between resprouting ability and germination. In general, the responses of species were similar regardless of their membership of any of the four post-fire functional groups. Heat and smoke generally had negative effects (S2 of the ESM), while ash and pH had nonsignificant effects on germination or positive effects of ash in a few species (S3 of the ESM).
There are several possible explanations for the absence of tight relations between life history traits (resprouting and serotiny) and germination responses to environmental variables associated with the post-fire environment. First, even though nonserotiny and resprouting provide opportunities for recruitment in the absence of fire, other factors may prevent this from occurring, maintaining a dependence on post-fire recruitment, and hence an exposure of seeds and seedlings to selective pressures associated with fires. Second, the relatively poor sample of nonserotinous species, represented mainly by Acacia, affected our results, which were phylogenetically biased. Third, the differences in the degree of serotiny among populations within single species may mask differences that exist among species. For example, the degree of serotiny may vary in relation to variation in fire frequency (Muir and Lotan 1985; Daskalakou and Thanos 1996; Whelan et al. 1998; Goubitz et al. 2004; Ne’eman et al. 2004) and along climatic gradients (Cowling and Lamont 1985). Fourth, post-fire resprouting ability of species also varies among populations. For example, resprouting ability has been found to be age- or size-dependent in Australian species (Gill and Bradstock 1992; Keith 1996), as well as in oaks (Pausas 1997) and pines (Stephens and Libby 2006). Fifth, our results demonstrate some differences in the response of seeds to heat and smoke between populations that varied in their degree of resprouting (Allocasuarina distyla) or degree of serotiny (Banksia serrata), as well as between populations that had similar traits (Hakea dactyloides). Thus, neither serotiny nor resprouting ability appear to be strictly categorical traits (Keeley and Zedler 1978). Sixth, the type of serotiny and seed-releasing mechanism may also vary between species. For example, in species with dead cones or fruits (e.g. Banksia and Pinus) seed release is activated only by heat, whereas in other species with living fruits or cones (e.g., Eucalyptus, Hakea, Leptospermum, Melaleuca, Cupressus), seeds are released after branch death, irrespective of the cause (senescence, pest attack or fire). Consequently, such species may have both canopy and soil-stored seed banks that could be under contradictory selection pressures with regard to regulation of their germination. For our study species, however, there was little evidence of different responses between serotinous species with dead or living fruits (i.e., Banksia; cf. other genera).
Conclusions
Only one combination of factors (heat, smoke and ash) and post-fire regeneration functional group significantly supported our prediction. In four groups, almost all of the species supported our predictions, while all of the species in two groups rejected our predictions, but it seems that sample number avoided significance in the binomial test. In three groups, the number of supporting and rejecting species was similar (Table 2). Thus, we conclude that the germination responses of the investigated species to post-fire germination cues are not tightly connected to their post-fire regeneration functional types.
References
Auld TD (1995) Soil seed bank patterns of four trees and shrubs from arid Australia. J Arid Environ 29:33–45
Auld TD, O’Connell MA (1991) Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Aust J Ecol 16:53–70
Bell DT (1999) The process of germination of Australian species. Aust J Bot 47:475–517
Bell DT, Williams DS (1998) Tolerance of thermal shock in seeds. Aust J Bot 46:221–223
Biswell HH (1974) Effects of fire on chaparral. In: Kozlowski TT, Ahlgren CE (eds) Fire and ecosystems. Academic, New York, pp 321–364
Bond WJ, van Wilgen BW (1996) Fire and plants. Chapman and Hall, London
Brown NAC (1993) Promotion of germination of fynbos seeds by plant-driven smoke. New Phytol 123:575–583
Brown NAC, van Staden J (1997) Smoke as germination cue: a review. Plant Growth Regul 22:115–124
Brown J, Enright NJ, Miller BP (2003) Seed production and germination in two rare and three common co-occurring Acacia species from south-east Australia. Aust J Ecol 28:271–280
Cocks MP, Stock WD (1997) Heat stimulated germination in relation to seed characteristics in fynbos legumes of the Western Cape Province, South Africa. S Afr J Bot 63:129–132
Cowling RM, Lamont BB (1985) Variation in the serotiny of three Banksia species along a climatic gradient. Aust J Ecol 10:345–350
Crawley MJ (1993) GLIM for ecologists. Blackwell, Oxford
Daskalakou EN, Thanos CA (1996) Aleppo pine (Pinus halepensis) post-fire regeneration: the role of canopy and soil seed banks. Int J Wildland Fire 6:59–66
De Lange JH, Boucher C (1990) Autecological studies on Audounia capitata (Bruniaceae) 1. Plant derived smoke as a germination cue. S Afr J Bot 56:700–703
Dixon KW, Roche S (1995) The role of combustion products (smoke) in stimulating ex-situ and in-situ germination of West Australian plants. Proc Int Plant Prop Soc 45:53–56
Dixon KW, Roche S, Pate JS (1995) The promotive effect of smoke derived from native vegetation on seed germination of Western Australian plants. Oecologia 101:185–192
Enright NI, Goldblum D, Ata P, Ashton DH (1997) The independent effects of heat, smoke and ash on emergence of seedlings from the soil seed bank of a heathy Eucalyptus woodland in Grampians (Gariwerd) National Park, western Victoria. Aust J Ecol 22:81–88
Eshel A, Henig-Sever N, Ne’eman G (2000) Spatial variation of seedling distribution in an east Mediterranean pine woodland at the beginning of post-fire succession. Plant Ecol 148:175–182
Facelli JM, Kerrigan R (1996) Effects of ash and four types of litter on the establishment of Eucalyptus obliqua. Ecoscience 3:319–324
Gill AM (1975) Fire and the Australian flora: a review. Aust For 38:4–25
Gill AM (1976) Fire and the opening of Banksia ornata F. Muell. follicles. Aust J Bot 24:329–335
Gill AM (1981) Adaptive responses of Australian vascular plant species to fires. In: Gill AM, Groves RH, Nobel IR (eds) Fire and the Australian biota. Australian Academy of Science, Canberra, pp 243–271
Gill AM, Bradstock RA (1992) A national register for the fire responses of plant species. Cunninghamia 2:653–660
Gonzales-Rabanal F, Casal M, Trabaud L (1989) Effects of high temperatures, ash and seed position in the inflorescence on the germination of 3 Spanish grasses. J Veg Sci 5:289–294
Gonzalez-Rabanal F, Casal M (1995) Effect of high temperatures and ash on germination of ten species from gorse shrubland. Plant Ecol 116:123–131
Goubitz S, Werger M, Ne’eman G (2003) Germination response to fire related factors of seeds from non-serotinous and serotinous cones. Plant Ecol 169:195–204
Goubitz S, Nathan R, Roitemberg D, Shmida A, Ne’eman G (2004) Canopy seed bank structure in relation to: fire, tree size and density. Plant Ecol 173:191–201
Harden GJ (1990–2003) Flora of New South Wales, vols 1–4. University of New South Wales Press, Kensington
Henig-Sever N, Eshel A, Ne’eman G (1996) pH and osmotic potential of pine ash as post-fire germination inhibitors. Physiol Plant 96:71–76
Henig-Sever N, Eshel A, Ne’eman G (2000) Regulation of the germination of Aleppo pine (Pinus halepensis) by nitrate, ammonium and gibberellin, and its role in post-fire forest regeneration. Physiol Plant 108:390–397
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Jeffrey DJ, Holmes PM, Rebelo A (1988) Effects of dry heat on seed germination in selected indigenous and alien legume species in South Africa. S Afr J Bot 54:28–34
Keeley JE (1991) Seed germination and life history syndromes in the California chaparral. Bot Rev 57:81–116
Keeley JE, Fotheringham CJ (1997) Trace gas emissions and smoke-induced seed germination. Science 276:1248–1250
Keeley JE, Zedler PH (1978) Reproduction of chaparral shrubs after fire: a comparison of resprouting and seedling strategies. Am Mid Nat 99:142–161
Keith DA (1996) Fire-driven extinction of plant populations: a synthesis of theory and evidence from Australian vegetation. Proc Linn Soc NSW 116:37–78
Keith DA (1997) Combined effect of heatshock, smoke and darkness on germination of Epacris stuartii Stap., an endangered fire-prone Australian shrub. Oecologia 112:340–344
Keith DA, McCaw WL, Whelan RJ (2002) Fire regimes in Australian heathlands and their effects on plants and animals. In: Bradctock RA, Wiliams JE, Gill AM (eds) Flammable Australia: the fire regimes and biodiversity of a continent. Cambridge University Press, Cambridge, pp 199–237
Kenny BJ (2000) Influence of multiple fire-related germination cues on three Sydney Grevillea (Proteaceae) species. Aust Ecol 25:664–666
Kruger LM, Midgley JJ, Cowling RM (1977) Resprouters vs. reseeders in south African forest trees: a model based on forest canopy height. Funct Ecol 11:101–105
Lamont BB, Le Maitre DC, Cowling RM, Enright NJ (1991) Canopy seed storage in woody plants. Bot Rev 57:277–317
Lavorel S, Cramer W (eds) (1999) Special feature: plant functional types and disturbance. J Veg Sci 10:603–730
McMaster GS, Zedler PH (1981) Delayed seed dispersal in Pinus torreyana (Torrey Pine). Oecologia 51:62–66
Moreno JM, Oechel W (1991) Fire intensity effects on germination of shrubs and herbs in southern California chaparral. Ecology 72:1993–2004
Morris EC (2000) Germination response of seven east Australian Grevillea species (Proteaceae) to smoke, heat exposure and scarification. Aust J Bot 48:179–189
Muir PS, Lotan JE (1985) Disturbance history and serotiny of Pinus cornata in western Montana. Ecology 66:1658–1668
Myerscough PJ, Whelan RJ, Bradstock RA (2000) Ecology of Proteaceae with special reference to Sydney region. Cunninghamia 6:951–1016
Naveh Z (1975) The evolutionary significance of fire in the Mediterranean region. Vegetatio 29:199–208
Ne’eman G, Meir I, Ne’eman R (1993) The effect of ash on the germination and early growth of shoots and roots of Pinus, Cistus and annuals. Seed Sci Technol 21:339–349
Ne’eman G, Fotheringham CJ, Keeley JE (1999) Patch to landscape patterns in post-fire recruitment of a serotinous conifer. Plant Ecol 145:235–242
Ne’eman G, Goubitz S, Nathan R (2004) Reproductive traits of Pinus halepensis in the light of fire—a critical review. Plant Ecol 171:69–79
Noble IR, Gitay H (1996) A functional classification for predicting the dynamics of landscapes. J Veg Sci 7:329–336
Noble IR, Slatyer RO (1980) The use of vital attributes to predict successional changes in plant communities subject to recurrent disturbance. Vegetatio 43:5–12
Pausas JG (1997) Resprouting of Quercus suber after fire in NE Spain. J Veg Sci 8:703–706
Pausas JG (1999) The response of plant functional types to changes in the fire regime in Mediterranean ecosystems. A simulation approach. J Veg Sci 10:717–722
Pausas JG, Bradstock RA, Keith DA, Keeley JE, GCTE Fire Network (2004) Plant functional traits in relation to fire in crown fire ecosystems. Ecology 85:1085–1100
Pausas JG, Keeley JE, Verdu M (2006) Inferring differential evolutionary processes of plant persistence traits in Northern Hemisphere Mediterranean fire prone ecosystems. J Ecol 94:31–39
Stephens C, Libby WJ (2006) Anthropogenic fire and bark thickness in coastal and island pine populations from Alta and Baja California. J Biogeogr 33:648–652
Thomas PA, Wein RW (1985) The influence of shelter and the hypothetical effect of fire severity on the post-fire establishment of conifers from seed. Can J For Res 15:148–155
Thomas PB, Morris EC, Auld TD (2003) Interactive effects of heat shock and smoke on germination of nine species forming soil seed banks within the Sydney region. Aust Ecol 28:674–683
Trabaud L (1987) The role of fire in ecological systems. SPB, The Hague
Trabaud L, Oustric J (1989) Heat requirements for seed germination of three Cistus species in the garrigue of southern France. Flora (Jena) 183:321–325
Verdu M (2000) Ecological and evolutionary differences between Mediterranean seeders and resprouters. J Veg Sci 11:265–268
Whelan RJ (1995) The ecology of fire. Cambridge University Press, Cambridge
Whelan RJ, De Jong NH, von der Burg S (1998) Variation in bradyspory and seedling recruitment without fire among populations of Banksia serrata (Proteaceae). Aust J Ecol 23:121–128
Acknowledgments
We thank the Institute for Conservation Biology and Law, University of Wollongong, for the research fellowship to G.N., and the NSW Department of Environment and Conservation for permitting the collection of seeds in National Parks. All experiments comply with the laws of NSW in Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jon Keeley.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ne’eman, G., Ne’eman, R., Keith, D.A. et al. Does post-fire plant regeneration mode affect the germination response to fire-related cues?. Oecologia 159, 483–492 (2009). https://doi.org/10.1007/s00442-008-1237-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1237-1