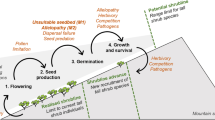
Abstract
Identifying the environmental constraints that affect the distribution of an invasive species is fundamental to its effective control. Triadica sebifera (Chinese tallow tree) has invaded the southeastern United States, but its potential for further range and habitat extension has been unresolved. We explored experimentally environmental factors in macro- and microhabitats that affect its persistence at five widely separated sites along the Atlantic seaboard of the United States and at two sites inland; three sites occur well beyond the tree’s current range. At each site, seeds and young vegetative plants (0.5–0.65 m tall) of T. sebifera were placed in four microhabitats (closed-canopy upland, closed-canopy lowland, open-canopy upland, and open-canopy lowland). Plant growth, leaf CO2 assimilation rates, leaf N concentrations and δ13C ratios, and stem water potential were measured for two growing seasons. Percent seed germination was consistently higher in open-canopy microhabitats and lowest at northern and inland sites. T. sebifera grew in all open-canopy microhabitats, even 300–500 km beyond its current distribution. Plant growth in closed-canopy habitats was lower, attributable to lower carbon gain per unit leaf area in shaded compared with open-canopy environments, especially at northern and inland sites. Neither competition, other than canopy shade, nor grazing was a key constraint on distribution at any scale. Our results demonstrate that T. sebifera is dispersal limited at landscape scales but limited locally by dispersal and overstory shade; it has yet to occupy the full extent of its new range in North America. Quantifying environmental factors both within and well beyond a species’ current range can effectively highlight the limits on its distribution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological invasions involve the spread of a non-native species’ descendants across what often becomes a huge new range (Mack 1981; Humphries et al. 1991). Yet few, if any, invaders occupy all habitats within a landscape, a composite consequence of the localized forces that either foster or constrain their advance. Quantifying these forces’ roles at the habitat level then becomes an important goal not only for understanding the cause(s) of the invasion but also to focus control efforts on the habitats most in jeopardy of invasion (McFadyen and Skarratt 1996; Welk et al. 2002). Despite the usefulness of experiments in controlled growth facilities in meeting these goals (Patterson 1994), their unresolved applicability in nature (Hairston 1989) means that manipulative field experiments remain strongly advisable, if not mandatory.
Triadica sebifera (L.) Small [formerly Sapium sebiferum (L.) Roxb.], the Chinese tallow tree, is native to China, Taiwan, and northern Vietnam. It has invaded the fringes of wetlands, lakes and riparian areas, coastal forests, and prairies (Bruce et al.1997; Conner et al. 2005) and has spread locally into closed-canopy upland forests throughout the southeastern United States (Renne et al. 2002). The tree’s wide range in the United States and recent predictions of its potential distribution using the climate-matching model CLIMEX (Pattison and Mack 2008) caused us to examine experimentally whether T. sebifera will expand its current range northward and inland, and if so, in which habitats.
To answer this general question, we used field experiments within and well beyond the range of T. sebifera to evaluate constraints at macro- and micro-environmental scales on the tree’s distribution in the eastern United States. Three questions shaped our investigation. (1) How do seed germination and the performance of young vegetative plants of T. sebifera vary at a macro-environmental scale across this region? We predicted that its distribution at the macro-environmental scale would be dispersal limited and that seed germination and young plant performance would be constrained at northerly and inland sites (Pattison and Mack 2008). (2) Given the paucity of T. sebifera in closed-canopy stands (Bruce et al. 1997; Renne et al. 2002), we asked how seed germination and the performance of young plants would vary between habitats frequently invaded (open-canopy lowlands) to those less frequently invaded (closed-canopy upland)? And (3), to what degree does competition restrict T. sebifera across macro and micro-environmental scales? Based on Renne et al.'s (2002) observation, we predicted that reduced competition would increase plant performance in closed-canopy habitats.
Materials and methods
Study species
Seeds of T. sebifera were collected from approx. 50 trees near Georgetown, SC, USA, during November to December (1998, 2000). (Georgetown is well within the current range of T. sebifera in the US.) Seed collections were mixed and stored in paper bags in an unheated room until sown in field germination trials or used to produce seedlings for field trials.
Seed germination trials
Samples of 44 T. sebifera seeds each were mixed into 1.5–2.0 L of A-horizon soil collected at each field site in December 2000 and early January 2001. Each seed sample/soil mixture was placed in an aluminum wire (1-mm mesh) container (30 × 30 × 4 cm deep) to prevent seed dispersal and predation; one wire container was buried level with the soil surface adjacent to the two plots in each of eight to ten blocks (see below). Emergent seedlings were censused every 3–4 weeks. Whole seeds and empty endocarps in the containers were counted in November 2001 to account for seedlings that may have gone undetected.
Twenty samples of 44 seeds each were also sown in flats (30 × 60 × 10 cm deep) in January 2001 to determine percent seed germination. Flats were maintained in growth chambers at Washington State University (Pullman, WA, USA) at 18°C/32°C (night/day) and irrigated to maintain field capacity. Results from growth chamber studies demonstrated that endocarp counts closely correlated with germination percentages in the field (Pattison 2003).
Plant field trials
Plantings of T. sebifera were conducted in 1999 and 2000. Plants for field planting were grown initially in greenhouses. Seeds were imbibed overnight and sown into flats with Peters Professional Potting Soil® (United Industries, St Louis, MO, USA) in a temperature-controlled greenhouse in Charleston, SC, USA, in February 1999 and 2000. Once 7–10 cm tall, seedlings were transplanted into Styrofoam potting trays (Super block 60/250 Beaver Plastics, Edmonton, Alberta) filled with Peters Professional Potting Soil. Seedlings were watered daily to soil saturation. Positions of potting trays were randomized every 1–3 weeks to minimize bench effects. Once 20–25 cm tall, seedlings were moved to an outdoor structure with a single layer of neutral density shade cloth overhead (IGC, Georgetown, IL, USA) and watered once (3–5 min) daily. Light levels under the shade cloth averaged 370 μmol m−2 s−1 PAR, as measured with an ACCUPAR linear PAR ceptometer (Decagon, Pullman, WA, USA). The trays were randomly assigned to positions every 1–3 weeks until randomly selected for planting into field sites.
Study sites
Field experiments evaluated the performance of seeds and young plants of T. sebifera at seven widely spaced locations. Six field sites were employed in 1999; a seventh (PP) was added in 2000. The sites are along two orthogonal transects: one transect (five sites) extended 1,200 km along the Atlantic seaboard; the other transect (two sites) extended 320 km inland along the Savannah River. Two sites (PP, BAR) have already been invaded by T. sebifera; two others (HOF, SRS) occur within the transition zone (approx. 200 km wide) in the southeastern United States in which Chinese tallow tree currently occurs locally but has not yet invaded. The remaining sites (CLEM, FTE, SERC) occur 300–500 km beyond its current range. Sites differ in maximum and minimum temperatures in January and July, annual precipitation, as well as community composition and soil characteristics (Table 1).
Environmental measurements
The light environment in each microhabitat (see below) was quantified using an ACCUPAR linear PAR ceptometer. Leaf area index (LAI) was calculated for each experimental block at 1-m heights adjacent to each block between 1000 and 1400 hours (EST) on cloud-free days in July 2000. Long-term (20–40 year averages) and monthly weather during the study were collected from the nearest (1–20 km) meteorological station (http://www.ncdc.noaa.gov/oa/ncdc.html) to each site. Cover of the herb layer adjacent to plots was determined in a 20 × 50 cm plot (Daubenmire 1959) beside each of the eight to ten control plots.
Experimental design
At each site, eight to ten blocks (1.5 × 6 m), containing two plots (1.5 × 1.5 m), were established in each of four microhabitats [open- and closed-canopy upland (>2 m vertical distance from a creek, pond or river), and open- and closed-canopy lowland (<1 m vertical distance from a perennial source of water)]. Blocks were widely separated (8–20 m apart) across each microhabitat, and plots were 3 m apart within each block.
Three to five tagged plants (0.5–0.65 m tall, 2.23 g, SE ± 0.18) were planted approx. 0.75 m apart in each plot in the four microhabitats at each site (2 plots × 8–10 blocks × 4 microhabitats) in spring 1999 and 2000. Each plant received 4.5 L of supplemental water upon planting. Among T. sebifera to be planted at each site, 20 were randomly selected, harvested, and their mean biomass was the basis for plant relative growth rates. Plants were inspected every 3–4 weeks from June to September for general condition and the percentage of defoliation.
Both the 1999 and 2000 plantings were harvested between late October to early November 2001. Excavating all roots proved unreliable, so root biomass was not included in growth analyses. Harvested plants were transported to the laboratory under ice and stored (−20°C) until measured. Total leaf area of each plant was measured with a LI-COR 3100 leaf area meter (LI-COR, Lincoln, NE, USA). Plants were separated into stems and leaves, oven dried (55°C, 72–96 h) and weighed to the nearest 0.1 g. Sites were revisited 1 and 2 years after the harvest to ensure that all tagged seeds and plants had been removed.
Competition
At the time of planting, one plot in each block was randomly assigned to a reduced competition treatment. The treatment was prepared by spading (25-cm deep) around the plot and hand-pulling all aboveground vegetation within the plot. Re-growth of vegetation was removed every 3–6 weeks during the growing season. If a T. sebifera in a plot grew 2 m tall, the remaining experimental plants were removed to eliminate intra-specific competition.
Plant relative growth rates
Plant relative growth rates (g g−1 day−1) (RGR) (Hunt 1982) were calculated as follows:
RGR = [ln (final plant above ground biomass) − ln (initial plant above ground biomass)]/plant duration at field site. The effect of competition, other than from the forest canopy, on plant performance was examined by comparisons of RGR and physiological measurements of plants in control and treatment plots. Differences in plant performance between control and reduced competition were also assessed with a relative competitive index, the “log response ratio” (lnRR). The value of lnRR for plant growth was calculated using the expression lnRR = ln (biomass of plants in control/biomass of plants in the treatment). Final above ground biomass was used, rather than RGR, to calculate lnRR because negative values cannot be used in lnRR calculations (Goldberg and Scheiner 2001).
Photosynthetic and water potential measurements
Photosynthetic CO2 assimilation per unit leaf area (A sat) was compared among treatments, microhabitats, and sites in August 2001 for plants planted in 2000. Measurements were made under ambient conditions with a portable differential photosynthesis system (LCA-3; ADC, Hertfordshire, UK). Photosynthetic capacity was measured in the field at mid to late morning (0800–1100 hours EST). A Sylvan halogen automobile headlight was used to generate photosynthetic active radiation (PAR) levels of 1,000–1,100 μmol m−2 s−1. PAR was measured with a quantum sensor mounted on the ADC photosynthesis system. In each microhabitat, A sat was measured for the youngest fully expanded leaf of a randomly chosen plant in each treatment plot of randomly chosen blocks (n = 3–6). Leaf area was determined with a LI-COR 3100 leaf area meter. Leaf mass per area (LMA) was determined by dividing leaf mass by leaf area.
Predawn (Ψpd) and midday (Ψmd) xylem water potentials were measured to determine instantaneous differences in whole plant water status attributable to treatments, microhabitats or sites. Measurements were made on one fully expanded leaf on the same plants used for A sat measurements, using a pressure chamber (PMS, Corvallis, Oregon, USA) and standard techniques (Turner 1988). Water potential was determined within one day of A sat measurements.
Nitrogen and δ13C analysis
Foliar values of nitrogen and δ13C can gauge soil nutrient availability (Hobbie and Gough 2002) and integrated long-term stomatal conductance (Dawson et al. 2002 and references therein), respectively. We used these measures to assess treatment, microhabitat, and site specific differences between plants. Leaf N and δ13C analyses were conducted for three randomly selected leaves in each treatment and microhabitat at each site. Samples were oven dried (55°C, 72 h) and ground to a fine powder. A 2 to 3-mg sample of each leaf was combusted in an elemental analyzer (NC2500; CE Instruments, Milan, Italy). Combustion products were swept via a helium carrier gas and continuous-flow interface in an isotope ratio mass spectrometer (Delta Plus; Finnegan MAT, Bremen, Germany) in which carbon isotope ratios were determined. All N and δ13C analyses were performed at the University of Idaho Stable Isotopes Laboratory (Moscow, ID, USA).
Data analysis
Plant growth, seed germination, A sat, foliar N and δ13C, and water potential data were analyzed using a split-plot analysis of variance (Proc GLM in SAS, v. 8.2; SAS Institute). Growth data from 1999 and 2000 plantings were analyzed separately. In all analyses, type III sums of squares were employed to determine levels of significance because of missing data (Goldberg and Scheiner 2001). The plot (site × habitat) variable was treated as a random effect throughout. Least squares means were used to test for significant differences. The least squares means for the three-way component (site × habitat × treatment) were not estimable as a result we examined least squares means for habitat × treatment at each site to determine three-way means. We tested the residuals from all analyses for normality and homogeneity of variance. Levels of significance were determined at P < 0.05. We used Fisher’s LSD test for post-hoc comparisons of least squares means with Bonferroni adjustments to α levels. Germination rates were arcsine-transformed to provide normality. Results from non-transformed data and arcsine-transformed data do not differ, consequently, non-transformed data are presented here. Individual testing of lnRR for each site by habitat combination to determine if values were significantly different (P < 0.05) from zero was performed using Fisher’s LSD test for post-hoc comparisons of least squares means (Proc GLM in SAS). Simple regression analyses were used to examine the relationships between seed germination, growth (RGR), seedling physiological measurements, lnRR, and environmental parameters. Minimum temperature can be a key constraint on plant distribution (Woodward 1990), consequently, we used mean minimum daily temperature in January (consistently the month of lowest regional winter temperature, http://www.ncdc.noaa.gov/oa/ncdc.htm) as an index of site suitability. We used LAI as an indicator of light availability. Leaf N and δ13C values were only measured for seedlings planted in 2000. A few plants were accidentally destroyed: e.g., seeds in low-lying microhabitats at SRS were frequently submerged in 2000 (R.R. Pattison, personal observation), and their loss resulted in data gaps in the statistical analyses.
Results
Environmental measurements
Meteorological stations near five of the seven sites received less precipitation (from 96 to 457 mm) during 2000 than the averages of long-term (20–40 years) records (Table 1). Much of this decrease in precipitation occurred during the growing season, suggesting that seeds and seedlings may have experienced unusually low water availability in 2000. Mean minimum daily temperature during the coldest month (January) and maximum temperature during the warmest month (July) throughout the study were similar to long-term averages (Table 1; http://www.ncdc.noaa.gov/oa/ncdc.htm).
Seed germination
The highest percentage of germination consistently occurred in open-canopy microhabitats within the current range of T. sebifera. The lowest percentage occurred in closed-canopy lowland microhabitats beyond the tree’s current range (SRS, SERC, CLEM) (Fig. 1). There was a significant site × habitat interaction for seed germination (Table 2). Percent germination within microhabitats across sites was positively correlated to mean minimum temperature in January, 2001, except for germination in the closed-canopy low-lying microhabitats (Fig. 2).
Regression of means (n = 7–9) and SE of percent germination of T. sebifera and the mean minimum daily temperature (January 2001) at the closed-canopy upland (r 2 = 0.646, P = 0.029, % germ. = 55.2 + 5.66 × min. temp.), the open-canopy upland (r 2 = 0.918, P < 0.001, % germ. = −71.8 + 7.67 × min. temp.), and the open-canopy lowland (r 2 = 0.94, P < 0.001, % germ. = 69.1 + 5.14 × min. temp.) microhabitats. The regression among the closed-canopy lowland microhabitats was not significant (P > 0.05) and is not shown
Plant growth
Relative growth rates varied from −0.0059 to 0.0059 (g g−1 day−1) across microhabitats, sites, and years (Fig. 3, Table 2). In most cases, relative growth rates were lowest (−0.0059 to 0.0026) for plants in closed-canopy microhabitats and higher (−0.0015 to 0.0059) among plants in open-canopy microhabitats. Highest values for RGR (0.0003–0.0059) were in open-canopy microhabitats beyond the current distribution of T. sebifera (FTE in 2000 and CLEM in 1999) (Fig. 3). Lowest values of RGR (−0.0059 to −0.0026) were among plants in closed-canopy microhabitats at a site farthest from the tree’s current range (SERC) (Fig. 3). However, negative values were detected among plants even within the current distribution (BAR in 2000 and HOF in 1999). A significant interaction occurred between site and habitat for RGR for both sets (1999 and 2000) of outplantings (Table 2). RGR and LAI were negatively correlated at the four sites most distant from the current distribution (Fig. 4) but not at other sites. When pooled across years, sites, habitats, and treatments, LAI and RGR (RGR = 0.00263 − 0.000523 × LAI, P < 0.0001) also displayed a negative relationship.
Least squares means (n = 3–8) and SE of relative growth rates (RGR) of T. sebifera (control and reduced competition) in four microhabitats at six sites in 1999 (a), and seven sites in 2000 (b), which were all harvested in 2001. Different letters indicate significant (P < 0.05) differences among microhabitats and treatments within the same site. * Denotes plants that were inadvertently destroyed; † too few surviving plants for statistical analysis
Relationship between plot RGR values and plot leaf area index (LAI) for T. sebifera planted in 1999 and 2000 in four microhabitats at seven sites (CLEM r 2 = 0.02, P = 0.003; SERC r 2 = 0.34, P < 0.001; HOF r 2 = 0.34, P < 0.001; FTE r 2 = 0.47, P < 0.001). Data for T. sebifera in control and reduced competition plots are shown by open and closed symbols, respectively. No significant (P < 0.05) correlations were found for SRS, BAR and PP, and are therefore not shown
T. sebifera responded to competition. Plants under low competition had higher RGR in 6 of the 44 microhabitat × site × year combinations (P < 0.05) (Fig. 3). This response occurred, however, in different microhabitats among 4 of the 7 sites. Values of lnRR were significantly negative (biomass under reduced competition > biomass in control) in 7 of 18 and 7 of 24 microhabitat × site combinations for seedlings planted in 1999 and 2000, respectively. Values of lnRR did not correlate with the percent cover of neighboring vegetation (data not shown). For plants in closed-canopy upland microhabitats, only values of lnRR negatively correlated with average minimum daily temperature in January 2000 among 1999 (r2 = 0.72, P = 0.02) and 2000 (r2 = 0.55, P = 0.034) plantings.
Plant above ground biomass allocation and physiology
The ratio of plant leaf area to above ground biomass correlated with the LAI measured above each block for plants planted in 1999 (r 2 = 0.32, P < 0.001) and 2000 (r 2 = 0.52, P < 0.001). Control and treatment Ψpd or Ψmd did not differ for plants in any microhabitat (Table 3). Pooled (control and treatment) Ψpd and Ψmd ranged from −0.25 to −1.37 Mpa and −0.3 to −1.89 Mpa, respectively. The most negative Ψpd occurred among plants at the CLEM closed-canopy (mean, ±1 SE: −1.37 MPa, ±0.40) and open-canopy (−1.33 MPa, ±0.03) upland microhabitats. The most negative Ψmd occurred in the open-canopy upland microhabitat (−1.89 MPa, ±0.19) at PP (data not shown).
Values of A sat were negatively correlated with LAI, when compared across all microhabitats at all sites (Fig. 5), i.e., the capacity for carbon gain per unit leaf area was lower under greater canopy cover. Competition did not reduce photosynthetic rates at any microhabitat or site (Table 3).
Relationship between CO2 assimilation on a leaf area basis (A sat) and LAI for T. sebifera planted in 2000 in four microhabitats at seven sites (r 2 = 0.51, P < 0.001, A sat = 9.25 − 1.168 × LAI). Data points are means of n = 3–7. Too few plants survived for measurements at the closed-canopy upland microhabitats at HOF, CLEM and SERC and the closed-canopy lowland at HOF. Flooding prevented measurement at the open-canopy lowland at SRS
Competition also did not alter foliar δ13C in any microhabitat or site (Table 3). Pooled values of δ13C values for control and treatments were more negative (indicating greater internal leaf CO2 levels, more open stomata and potentially greater water availability) in closed- compared with open-canopy communities, in both upland and lowland habitats. Differences in δ13C among microhabitats are partially attributable to higher carboxylation capacity of leaves in open-canopy microhabitats as there is a weak relationship between δ13C and A sat (r 2 = 0.194, P = 0.004) (Evans et al. 1986). The most negative δ13C values were detected in closed-canopy microhabitats at sites beyond the tree’s current range. The least negative values occurred in open-canopy microhabitats at the SERC and PP sites. There was a negative correlation (r 2 = 0.82, P = 0.034) across sites between lnRR biomass and lnRRδ13C for plants in open-canopy upland microhabitats but not in other microhabitats.
Leaf N values did not differ between control and treatment plants (Table 3). The log response ratio of plant biomass (lnRR) was positively correlated (r 2 = 0.78, P = 0.02) with lnRRN across sites for plants in closed-canopy lowland microhabitats but not with lnRRN for plants in other microhabitats.
Plant herbivory in both years was negligible. Among the approx. 2,500 seedlings planted at the sites throughout the study, 2 were probably destroyed by beaver (Castor canadensis), 1 by an unidentified rodent, and 1 damaged by unidentified scale insects.
Discussion
Our results support earlier predictions based on the climate-matching model CLIMEX (Pattison and Mack 2008): T. sebifera has yet to occupy its full potential range within the eastern US. Given the performance of the invader at all sites, dispersal limitation appears to be a key constraint on its distribution across the region. Low temperatures are, however, likely to ultimately define the tree’s geographic limit—a commonly observed environmental response among temperate and subtropical trees (Sakai 1982). Low light levels in closed-canopy microhabitats negatively affect plant performance but do not prevent establishment. While competition did not prevent establishment in any microhabitat, the results suggest that competition for water and N may limit, respectively, establishment in open-canopy upland and closed-canopy lowland microhabitats.
Seed germination
Low temperature and shade can each negatively affect T. sebifera germination. Unlike vegetative growth, seed germination for T. sebifera strongly correlates with daily minimum temperatures—results that highlight the variation in constraints acting on different life history stages (Rousett and Lepart 2007). Percent germination was lower in more northern and inland sites, as predicted by climate-matching (Pattison and Mack 2008). Predicted increases in daily minimum temperatures with global climate change of 2°C (IPPC 2001) and regressions of germination with temperature (Fig. 2) suggest T. sebifera germination could increase regionally by 10–15% in three of the microhabitats. The negative correlation between germination and LAI (r 2 = 0.45, P < 0.001) indicates that low light in closed-canopy microhabitats negatively affects tree recruitment. Consequently, a reduction in canopy cover is likely to facilitate T. sebifera establishment. For example, removal of the canopy by Hurricane Hugo led to prolific recruitment of T. sebifera seedlings in a coastal South Carolina forest (Conner et al. 2005).
Risk of plant escape outside the study area precluded our surface sowing seeds in our trials. Consequently, our germination results with buried seeds are likely greater than had seeds been dispersed across the soil surface by birds, gravity, or water; in greenhouse experiments, buried T. sebifera seeds had twice the germination percentage of surface sown seeds (Renne et al. 2001). Samuels (2004) found that a thick litter layer reduced surface sown seed germination at the PP site. Although seed viability was not tested here, seed viability in earlier tests from the same source population was 96% (Renne et al. 2001).
The role of seed predation for T. sebifera is equivocal. Earlier research at BAR site found that predation of T. sebifera seeds was inconsequential (Renne et al. 2000, 2001), consistent with results in coastal Texas (Siemann and Rogers 2003a). Samuels (2004) working at the PP site found, however, that seed predation by the invasive fire ant (Solenopsis invicta) was varied (21–82%) but nonetheless substantial. A persistent seed bank could, however, offset predation. For example, the viability of T. sebifera seeds remaining in the field at the BAR site for 1 and 2 years was 29–68 and 16–69%, respectively (Renne et al. 2001).
Plant performance
Plant RGR values indicate that T. sebifera could grow well beyond (500 km) its current distribution in the southern United States; the open-canopy microhabitats in some inland and northern sites may be even more favorable for its growth than sites it currently occupies. These results substantially strengthen geographic range predictions based on climatic constraints alone (Pattison and Mack 2008). In contrast to CLIMEX predictions, RGR was higher at some sites beyond the current range. This result is unlikely an artifact of unusually favorable weather: mean monthly precipitation was consistently lower, i.e., presumably less favorable for T. sebifera, than long-term averages at FTE and CLEM (Table 1; Pattison 2003).
The highest values for RGR often occurred in open-canopy lowland microhabitats (Fig. 3), results consistent with the common habitat of T. sebifera (Bruce et al. 1997, Renne et al. 2002). Correlation of lower A sat values with larger LAI (Fig. 5) suggests that low carbon gain under shade is a primary constraint on plant RGR at local scales. The higher ratio of leaf area to stem biomass under closed canopies compared with the results on open-canopy sites indicates, however, that T. sebifera acclimates to low light, supporting general conclusions for greenhouse grown seedlings (Jones and McLeod 1990) and field studies (Siemann and Rogers 2003a) that Chinese tallow tree is shade tolerant but grows more rapidly in full sun. Nevertheless, closed-canopy microhabitats appear less susceptible to the tree’s invasion, particularly at more inland and northerly sites, based on the negative correlation between RGR and LAI at our inland and northernmost sites (Fig. 4). As a result, the occurrence of T. sebifera is likely to be restricted to a set of microhabitats (e.g., open-canopy lowland) at the perimeter of its potential distribution (Guo et al. 2005); the diminishing occurrence of such habitats inland and northward could slow the invader’s spread.
Biotic interactions
Competition reduced the growth of our transplants (Fig. 3), but its influence was neither universal nor specific to particular microhabitats or sites. Consequently, it does not appear to be a key constraint. Estimates of competition may have been higher had we sown seeds in the field rather than planted juvenile plants (cf. Siemann and Rogers 2003b). The negative relationship between lnRR and mean minimum temperature in January for plants in closed-canopy upland microhabitats suggests that the relative reduction in growth due to competition increased in warmer climates. As a result, competition could become an increasingly important constraint on T. sebifera distribution in this microhabitat in warmer climates, a response documented for other temperate woody species (Vetaas 2002). Root biomass accumulation of T. sebifera seedlings was greater, however, than accumulation for native woody competitors under an elevated soil temperature regimen in a greenhouse with high light and ample irrigation (Jones 1993). The negative correlation between lnRR biomass and lnRRδ13C for plants in open-canopy upland microhabitats indicates that in this microhabitat the largest differences in aboveground biomass between treatment and control (treatment > control) also had the largest differences in δ13C (treatment had greater water availability than control). Consequently, competition for water may limit distribution in open-canopy upland microhabitats. The positive correlation (r 2 = 0.78, P = 0.02) between lnRR biomass with lnRRN for plants in closed-canopy lowland microhabitats indicates that a greater difference in treatment and control aboveground biomass was associated with greater differences in leaf level measures of N availability (greater foliar N in treatment than control). Competition for N may consequently limit distribution in closed-canopy lowland microhabitats.
Facilitation can be an important determinant of plant community structure (Brooker et al. 2007 and references therein). Overstory shade may facilitate T. sebifera establishment when plants experience high evaporative demand and low soil moisture. For example, at the PP site, plants in the closed-canopy upland habitats had greater RGR values than plants in the open-canopy upland microhabitat (Fig. 3). Midday water potentials (−1.89 MPa, SE = ±0.19) and the δ13C values (−28.58, SE = ±0.647) of leaves in the open-canopy upland microhabitat at this site were among the most and least negative, respectively, in this study. Overstory shade may also indirectly benefit plant growth by reducing the growth of understory competitors (Siemann and Rogers 2003a).
We found only nominal evidence of vertebrate grazing on T. sebifera, although Siemann and Rogers (2003b) caution that reductions in seedling leaf area of as little as 2.1% by invertebrates can decrease survival. Such low levels of defoliation are likely to have gone undetected in our study.
Implications
These field trials provide evidence for the likely spread of Chinese tallow tree to 38°N latitude and inland along the Savannah River. Our trials were, however, limited to 2 years, while the amplitude of variation in regional weather plays out over much longer time frames (http://www.ncdc.noaa.gov/oa/ncdc.htm). Infrequent extremes events, such as severe freezes, can be important determinants of plant distribution (Gaston 2003). Although we investigated seed germination and the performance of young plants, we did not follow these plants until sexual maturity. But if these first stages in the life cycle do not survive, the fate of later stages becomes irrelevant.
We had earlier found evidence for potential northward and inland spread for this invader, based on projections from the CLIMEX model (Pattison and Mack 2008). Our field results largely reinforce those predictions as well as provide insight into the tree’s response to different microhabitats. Spread of T. sebifera, based on both lines of evidence, appears far from complete in the United States. But the extent to which on-going global atmospheric change will influence this range occupation complicates any predictions (Pattison and Mack 2008).
Our approach, with manipulative field trials within and beyond the current range of T. sebifera, draws on Harper’s (1982) admonition: in seeking to explain species’ environmental limits many investigations document the environment where a species occurs but fail to address the equally powerful question “what are the limits and constraints that prevent it from living elsewhere?” Field application of this two-pronged approach seems particularly germane for thwarting a still spreading invasion. An invader is most effectively checked when its still isolated, nascent foci are destroyed (Moody and Mack 1988). But the search for new foci, the ability to safely by-pass invulnerable sites, and the timely marshalling of control measures are dependent on accurately knowing an invader’s potential habitats (Simberloff 2003 and references therein). Our field studies can assist in combating T. sebifera in eastern North America; a conceptually similar approach could be productively applied to other on-going invasions.
Simultaneous field trials within and beyond a species’ current range can also weigh two conflicting hypotheses for any species’ range (native or introduced): (1) has evolutionary specialization narrowed the range of habitats that allow survival and reproduction or (2) has geographic isolation prevented colonists ever reaching these sites (Harper et al. 1997)? As Harper (1982) succinctly stated, studies that explain both where a species does (and does not) occur “…would appear to be the ideal way to demonstrate the real extent and proximal cause of the narrow specialization of most plant forms.”
References
Brooker RW, Maestre FT, Callaway RM et al (2007) Facilitation in plant communities: the past, the present, and the future. J Ecol 96:18–34
Bruce KA, Cameron GN, Harcombe PA, Jubinsky G (1997) Introduction, impact on native habitats, and management of a woody invader, the Chinese tallow tree, Sapium sebiferum. Nat Areas J 17:255–260
Conner WH, Mixon WD, Wood GW (2005) Maritime forest habitat dynamics on Bulls Island, Cape Romain National Wildlife Refuge, SC following Hurricane Hugo. For Ecol Manage 212:127–134
Daubenmire R (1959) A canopy-coverage method. Northwest Sci 33:43–64
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–509
Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas ex-change to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13:281–292
Gaston KJ (2003) The structure and dynamics of geographic ranges. Oxford University Press, Oxford
Goldberg DE, Scheiner SM (2001) ANOVA and ANCOVA field competition Experiments. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments, 2nd edn. Oxford University Press, Oxford, pp 77–98
Guo Q, Taper M, Schoenberger M, Brandle J (2005) Spatial–temporal population dynamics across species range: from centre to margin. Oikos 108:47–57
Hairston NG (1989) Ecological experiments: purpose, design, and execution. Cambridge University Press, New York
Harper JL (1982) After description. In: Newman EI (ed) The plant community as a working mechanism. British Ecological Society Special Publications, vol 1. Blackwell, Oxford, pp 11–25
Harper JL, Silvertown J, Franco M (1997) Preface. In: Silvertown J, Franco M, Harper JL (eds) Plant life histories: ecology, phylogeny, and evolution. Cambridge University Press, New York, pp 11–18
Hobbie SE, Gough L (2002) Foliar and soil nutrients in tundra on glacial landscapes of contrasting ages in northern Alaska. Oecologia 131:453–462
Humphries SE, Groves RH, Mitchell DS (1991) Plant invasions: the incidence of environmental weeds in Australia. Kowari 2. Australian National Parks and Wildlife Service, Canberra
Hunt R (1982) Plant growth curves: the functional approach to plant growth analysis. University Park Press, Baltimore
Intergovernmental Panel on Climate Change [IPPC] (2001) Climate change 2001: synthesis report summary for policy makers. http://www.ipcc.ch/
Jones RH (1993) Influence of soil temperature on competition in seedlings of Acer rubrum, Liquidambar styraciflua and Sapium sebiferum. Am Midl Nat 130:116–126
Jones RH, McLeod KW (1990) Growth and photosynthetic responses to a range of light environments in Chinese tallow tree and Carolina ash seedlings. For Sci 36:851–862
Mack RN (1981) Invasion of Bromus tectorum L. into western North America: an ecological chronicle. Agro-Ecosyst 7:145–165
McFadyen RC, Skarratt B (1996) Potential distribution of Chromolaena odorata (siam weed) in Australia, Africa and Oceania. Agric Ecosyst Environ 59:89–96
Moody ME, Mack RN (1988) Controlling the spread of plant invasions: the importance of nascent foci. J Appl Ecol 25:1009–1021
Patterson DT (1994) Temperature responses and potential range of the grass weed, serrated tussock (Nasella trichotoma), in the United States. Weed Technol 8:703–712
Pattison RR (2003) Ecological constraints on the distribution of the Chinese tallow tree (Sapium sebiferum) in the southeastern USA. PhD dissertation. Washington State University, Pullman
Pattison RR, Mack RN (2008) Potential distribution of the invasive tree Triadica sebifera (Euphorbiaceae) in the United States: evaluating CLIMEX predictions with field trials. Glob Change Biol 14:813–826
Radford AE, Ahles HE, Bell CR (1968) Manual of the vascular flora of the Carolinas. University of North Carolina Press, Chapel Hill
Renne IJ, Gauthreaux SA, Gresham CA (2000) Seed dispersal of the Chinese tallow tree (Sapium sebiferum (L.) Roxb.) by birds in coastal South Carolina. Am Midl Nat 144:202–215
Renne IJ, Spira TP, Bridges WC (2001) Effects of habitat, burial, age and passage through birds on germination and establishment of Chinese tallow tree in coastal South Carolina. J Torrey Bot Soc 128:109–119
Renne IJ, Barrow WC, Johnson-Randall LA, Bridges WC (2002) Generalized avian dispersal syndrome contributes to Chinese tallow tree (Sapium sebiferum, Euphorbiaceae) invasiveness. Divers Distrib 8:285–295
Rousett O, Lepart J (2007) Positive and negative interactions at different life stages of a colonizing species (Quercus humilis). J Ecol 88:401–412
Sakai A (1982) Freezing resistance of ornamental trees and shrubs. J Am Soc Hortic Sci 107:572–581
Samuels I (2004) Invasion of Chinese tallow (Sapium sebiferum): a test of dispersal and recruitment limitation in multiple habitats. MS thesis. University of Florida, Gainesville. http://etd.fcla.edu/UF/UFE0004706/samuels_i.pdf
Siemann E, Rogers WE (2003a) Changes in light and nitrogen availability under pioneer trees may indirectly facilitate tree invasions of grasslands. J Ecol 91:923–931
Siemann E, Rogers WE (2003b) Herbivory, disease, recruitment limitation, and the success of alien and native tree species. Ecology 84:1489–1505
Simberloff D (2003) Why not eradication? In: Rapport DJ, Lasley BL, Rolston DE et al (eds) Managing for healthy ecosystems. Lewiss, Boca Raton, pp 541–548
Turner NC (1988) Measurement of plant water status by the pressure chamber technique. Irrigation Sci 9:289–308
Vetaas OR (2002) Realized and potential climate niches: a comparison of four Rhododendron tree species. J Biogeogr 29:545–554
Welk E, Schubert K, Hoffmann MH (2002) Present and potential distribution of invasive garlic mustard (Alliaria petiolata) in North America. Divers Distrib 8:219–233
Woodward FI (1990) The impact of low temperatures in controlling the geographical distribution of plants. Proc R Soc Lond B 326:585–593
Acknowledgments
We thank M. Burke (USFS), J. Wiemer (Paynes Prairie State Preserve), K. McLeod (Savannah River Ecology Laboratory), W. Conner and the staff of the Belle W. Baruch Institute for Coastal and Marine Sciences, K. Cox (Clemson University Forest), the Hofmann Forest staff, T. Sanders (Ft. Eustis Army Base), D.F. Whigham and J.P. O’Neill and the staff at the Smithsonian Environmental Research Center (SERC). We also thank K.D. Rode and J.J. Brunette for valuable assistance in all stages of research and M. Evans (Washington State University) and M.S. Minton (SERC) for statistical consulting. We thank D.G. Williams and J.P. Sparks, R.K. Monson, M. Lerdau and two anonymous reviewers for constructive criticism. This research was supported by grants to R.R.P. from the Betty Higinbotham Trust, the Florida Exotic Pest Plant Council and a Natural Resources Conservation Grant at WSU. Experiments reported in this work comply with the current laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Manuel Lerdau.
Rights and permissions
About this article
Cite this article
Pattison, R.R., Mack, R.N. Environmental constraints on the invasion of Triadica sebifera in the eastern United States: an experimental field assessment. Oecologia 158, 591–602 (2009). https://doi.org/10.1007/s00442-008-1187-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1187-7