Abstract
Although root growth and mortality play critical regulatory roles in terrestrial ecosystems, little is known about the temporal scale of these dynamics. In temperate grasslands, root dynamics may be particularly rapid because of the high proportion of production allocated to very fine root biomass. In this study, we used minirhizotron tubes to estimate root growth and mortality in an upland grassland in Yellowstone National Park that was grazed by migratory herds of ungulates. Monthly rates of root growth and mortality were estimated from May to September 2005, by measuring the elongation (growth) and disappearance (mortality) of roots at 3-day intervals. Average daily growth (millimeters of root length) was approximately 5 times greater in May and June than in July, August, and September. Average daily mortality (millimeters of root length) did not differ among months. A comparison of the June–September rates of root growth and mortality derived from sampling at short (3-day) and long (1-month) time intervals indicated that the long sampling intervals underestimated both growth and mortality by approximately 60% relative to the short intervals. These results suggest that estimates of grassland root dynamics from minirhizotrons are influenced significantly by sampling interval length, and that rapid root turnover may play a more critical role in regulating energy and nutrient fluxes in temperate grasslands than has previously been recognized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Root production and mortality are major regulators of C and nutrient fluxes in terrestrial ecosystems (McClaugherty et al. 1982; Nadelhoffer and Raich 1992; Gill and Jackson 2000; Silver and Miya 2001). In temperate grasslands, these belowground processes play an especially important regulatory role because of the large proportion of biomass allocated belowground and the high rates of root turnover (Stanton 1988; Jackson et al. 1997; Frank 2007). Consequently, the flux of C and nutrients from roots to the soil is the major source of energy and nutrients that sustains the characteristically high levels of biological activity of temperate grassland soils (Stanton 1988; Whittaker 2003).
The timescale on which root growth and mortality occur can be short. Root longevity in forest and shrubland habitat has been found to be less than a few weeks (Eissenstat and Yanai 1997; Ruess et al. 1998; Johnson et al. 2001; Tingey et al 2003). In grasslands, root longevity may be even shorter than in other terrestrial systems, because grasslands support very fine root systems, and studies have shown a positive correlation between root diameter and lifespan (Jackson et al. 1997; Eissenstat et al. 2000; Lauenroth and Gill 2003).
Because root growth and mortality occur simultaneously, the most accurate method to quantify these rates is the repeated measurement of individual roots over time at a frequency that best reflects the potentially fast dynamics of these processes (Johnson et al. 2001). In this study, we re-sampled minirhizotron tubes at 3-day intervals during the growing season in an upland grassland in Yellowstone National Park (YNP). We had four objectives: (1) examine the prevalence of short-term (i.e., ≤3-day) root growth and mortality; (2) compare estimates of root growth and mortality measured at 3-day versus 1-month intervals, the latter a common sampling interval used in field studies of roots; (3) examine the climatic conditions associated with monthly variation in root growth and mortality during the growing season.
Materials and methods
Site description
This study was conducted at Crystal Bench (44°54′N 111°19′W, 1,909 m a. s. l.), a broad bench created by glacial deposits, located on the northern winter range of YNP, Wyoming, USA. The soil at the site was a sandy loam, with a pH of 6.3, bulk density of 1.31 g cm−3, and organic matter and total N content of 5.4 and 0.2%, respectively, as described by Frank and McNaughton (1992). Vegetation at the site included common upland grassland species, such as Festuca idahoensis, Carex stenophylla, Lupinus sericeus, Koeleria cristata, Stipa comata, and Tetradymia canescens. The northern range of YNP is intensely grazed during the winter and early spring by native ungulates, primarily elk (Cervus elaphus), bison (Bison bison), and pronghorn (Antilocapra americana) (Meagher 1973; Houston 1982; Frank and McNaughton 1992). The 1948–2006 weather records at Tower Falls (6 km south-west of the site) indicated that the climate at Crystal Bench is characterized by long, cold winters (October–March monthly mean temperatures ranged −13.0 to −2.8°C) and short, dry summers (April–September total precipitation was 25 cm).
Four minirhizotron tubes (90 cm long × 5 cm inside diameter) were installed at 28° to the soil surface in the spring of 2004, one year before data collection. Prior to installation, 75 numbered frames (9 × 13 mm) were etched along the length of the exterior surface of each tube, and during installation frames were oriented on the top surface of the tubes. The tubes were distributed within an area 24 × 24 m and located more than 5 m apart from one another. Roots were sampled May–September 2005; therefore, winter root dynamics were not examined in this study. Roots growing along the outside of each frame were imaged on the 14th, 17th, 20th, and 23rd day of each month, allowing us to examine the changes in root length over three consecutive 3-day intervals each month. Images were collected using a video camera to a soil depth of approximately 30 cm, a depth that included 80–90% of the total root biomass at the site (D. A. Frank, unpublished data).
Image and data analysis
Video images from minirhizotron tubes were analyzed using the MSU ROOTS software program (Hendrick and Pregitzer 1992, 1993, 1996b). We traced the lengths of all live roots present in even-numbered frames. All observed roots were very fine (diameter ≤0.5 mm). Roots were judged to be alive, rather than decomposing, if they were solid in appearance, as opposed to fragmented. At each sampling date we determined the total standing root length and 3-day rates of root growth and mortality for each minirhizotron tube. Standing length per frame was calculated by summing all root lengths (mm) present in a frame during a sampling date. Total standing root length (mm) per tube was then scaled up to meters of root length per square meter of digitized minirhizotron tube surface area (m m−2). Growth was defined as new root length that appeared as the result of either the growth of a pre-existing root or the growth of a new root that appeared in a frame between sampling dates. Mortality was defined as the disappearance of any pre-existing root length between sampling dates. Three-day rates of growth and mortality per tube were scaled up to monthly rates, and these monthly rates were scaled up to meters of root length that appeared or disappeared per square meter of digitized minirhizotron tube surface area per month (m m−2 month−1). Cumulative May–September 2005 root growth and mortality (m m−2) were calculated by summing these monthly rates.
We compared monthly rates of root growth and mortality derived from 3-day sampling intervals to rates derived from 1-month sampling intervals for each tube. Monthly rates of growth and mortality derived from 1-month sampling intervals were based on observations made on the last sampling day of each month, and these rates were similarly scaled up to meters of root length per square meter of digitized minirhizotron tube surface area per month (m m−2 month−1).
The effect of observer error on estimates of root length was assessed by digitizing 11 previously unanalyzed frames (approximately 90 roots) twice, with 2 weeks between each session to minimize the influence of observer memory on the second set of tracings. Frames were digitized both times without the aid of prior root tracings. Error was calculated by summing the differences in the root lengths between the tracings and dividing this total by the total standing root length of the eleven frames. This procedure allowed us to assess the precision of the methodology (Johnson et al. 2001).
Statistical analysis
Standing root length and rates of growth and mortality derived from 3-day and 1-month sampling intervals were averaged across tubes, and all 3-day estimates were also averaged across the three sampling intervals of each month. Repeated measures ANOVAs were performed on a per tube basis to examine differences in standing root length, growth, and mortality among months. Scheffe’s tests were used to examine pairwise differences between months. Paired t-tests were used to compare the mean monthly rates of growth and mortality at 3-day versus 1-month sampling intervals.
We examined the effects of weather conditions on root dynamics using May–September 2005, local weather data collected at the Tower Falls station (Western Regional Climate Center). Linear regression analysis was used to explore the relationships between the independent weather variables (mean monthly temperature, mean monthly maximum temperature, mean monthly minimum temperature, total monthly precipitation) and the dependent root variables (monthly rates of growth and mortality derived from 3-day sampling intervals). In addition, the ratios of monthly mean, minimum and maximum temperature to monthly precipitation, indices of soil moisture stress, were regressed against the ratio of monthly rates of mortality to growth to determine the influence of variable soil moisture on root processes during the growing season (Gill and Jackson 2000). All statistical analyses were performed using Statistica 5.0 (Statsoft 1995).
Results
Temporal dynamics of roots
Total standing root length varied early in the growing season (P = 0.003). The average (±1 SD) total standing root length increased from May (96.0 ± 14.7 m m−2) to June (120.8 ± 10.6 m m−2), then did not change significantly throughout the rest of the growing season.
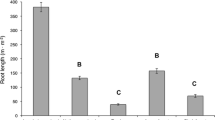
Root growth derived from 3-day sampling intervals varied significantly between the early and late growing season (P = 0.0002; Fig. 1a). Monthly root growth (mean ± 1 SD) was greatest in May (103.4 ± 31.8 m m−2 month−1) and June (83.7 ± 41.5 m m−2 month−1), then decreased in July (22.4 ± 3.8 m m−2 month−1) and remained at consistently low rates in August (23.3 ± 2.5 m m−2 month−1) and September (16.2 ± 4.3 m m−2 month−1). Monthly root mortality (mean ± 1 SD) did not vary among months and averaged 31.3 ± 10.4 m m−2 month−1 (P = 0.12; Fig. 1b). Cumulative May–September 2005 (mean ± 1 SD) root growth derived from 3-day sampling intervals was 239.3 ± 50.2 m m−2, and cumulative root mortality was 162.8 ± 1.4 m m−2.
Mean (±1 SD) monthly (mo) root length a growth (m m−2 month−1) and b mortality (m m−2 month−1) derived from 3-day (black bars) and 1-month sampling intervals (white bars). Different uppercase letters reflect statistical differences (P ≤ 0.05) in 3-day estimates between months, and different lowercase letters reflect statistical differences (P ≤ 0.05) in 1-month estimates. Asterisks indicate no data
Temporal patterns of root growth using images at 1-month intervals were similar to those estimated from 3-day intervals; root growth was greatest early in the growing season, in June, and lower in later months (P ≤ 0.0001; Fig. 1a). However, temporal patterns of root mortality varied between 1-month and 3-day sampling intervals (Fig. 1b). Estimates of mortality from the 3-day interval indicated no temporal variation in root mortality, whereas estimates of mortality from the 1-month sampling interval indicated 2 times greater mortality in July compared to the other months (P = 0.0018).
Effect of sampling interval duration
Rates of growth derived from 3-day sampling intervals were greater than those rates derived from 1-month intervals for all months (P ≤ 0.05, paired t-test; Fig. 1a). The greatest discrepancy between the two estimates occurred in June, when 3-day estimates of growth were 2.5 times greater than estimates from the 1-month sampling interval (Fig. 1a). Mortality estimates derived from 3-day sampling were greater than estimates from 1-month sampling in June (P = 0.032) and August (P = 0.015) and weakly significantly greater in July (P = 0.060) and September (P = 0.067; Fig. 1b). Sampling once per month underestimated cumulative June–September root growth by 60% and mortality by 63% relative to monthly estimates derived from 3-day sampling intervals.
Climatic effects
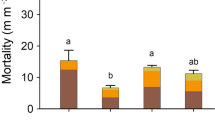
Monthly mean temperature, mean maximum temperature, mean minimum temperature, and total monthly precipitation were not related to either monthly root growth or mortality (P > 0.10). However, the ratio of monthly mean maximum temperature to total monthly precipitation was weakly positively correlated to the ratio of monthly mortality to growth (P = 0.097; Fig. 2). When the weather was cool and wet in June (mean maximum temperature 18.6°C, total precipitation 9.1 cm), fewer roots disappeared relative to the rate of root growth. When the weather was hot and dry in July (mean maximum temperature 27°C, total precipitation 2.5 cm), more roots disappeared relative to the rate of root growth. The increase in the ratio of root mortality to growth from June to July was primarily caused by the decline in root growth.
Methodological error
There was ±5.6% error in tracing root lengths from one digitizing session to the next. This amount of observer error would not influence the conclusions of this study.
Discussion
Rapid root dynamics
We found that a short (i.e., 3-day) sampling interval markedly increased estimates of grassland root growth and mortality, based on the appearance and disappearance of very fine roots (diameter < 0.5 mm), as compared to estimates derived from a longer (i.e., 1-month) sampling interval. In addition, observed temporal patterns of mortality varied between the short (no significant variation) and long sampling intervals (higher in July). The notion that YNP grassland root dynamics were rapid may not have been surprising, considering that: (1) temperate grasses produce very fine roots, and (2) studies have shown that as root diameter decreases, root lifespan decreases (Jackson et al. 1997; Eissenstat et al. 2000; Lauenroth and Gill 2003). However, we were surprised by the relatively large amount of root growth and mortality that occurred at such a short time scale. For example, in May, mean daily root growth and mortality were 0.3 and 0.1%, respectively, of the total standing root length (mm−2).
Our estimates of cumulative May–September 2005 root growth and mortality derived from 3-day sampling are much greater than previous minirhizotron estimates of native grassland annual root growth and mortality (Higgins et al. 2002; Milchunas et al. 2005; Steinaker and Wilson 2005), with the exception of estimates by Arnone et al. (2000). Previously reported rates might be lower than those reported by this study due in part to longer sampling intervals, which ranged from 2 to 12 weeks. Johnson et al. (2001) and Tingey et al. (2003) documented in northern forested systems that reducing sampling intervals down to 2 weeks or less yielded higher estimates of root growth and mortality than longer sampling intervals.
The most common sampling interval used in minirhizotron studies is once per month, although studies have used intervals ranging from 1 to 16 weeks (Johnson et al. 2001). Others have emphasized the need for shorter sampling intervals to better understand patterns of root turnover (Hendrick and Pregitzer 1996a; Johnson et al. 2001; Tingey et al. 2003). Our results support these recommendations and suggest that estimates from longer sampling intervals likely underestimate rates of root production and mortality and may misrepresent temporal variability. Consequently, those who conduct field studies of roots need to sample at a frequency that matches the temporal scale of the root dynamic of interest.
Root mortality
There are two processes that account for root mortality. The first is root death initiated in some way by the plant. Some consider this to be the primary cause of root mortality in terrestrial systems (Aerts et al. 1989; Hendrick and Pregitzer 1993, 1996b; Katterer et al. 1995; Milchunas and Lauenroth 2001). A second cause of root mortality is root herbivory. Wells et al. (2002) demonstrated experimentally that root feeders can greatly reduce root life span. Similarly, Yanai and Eissenstat (2002) proposed a model of optimal root turnover that suggested that under certain circumstances root herbivory could be the major factor determining root lifespan. This model may be particularly applicable to temperate grasslands, which support high densities of root herbivores. For instance, at our study site, Merrill et al (1994) conducted a study of root-feeding nematodes, the major grassland root herbivore, and found phytophagus nematodes at densities of 4.1 × 105 m−2 and 8.5 × 105 m−2 under grazed Festuca idahoensis and Agropyron spicatum, respectively. The presence of such high densities of root herbivores at our YNP site suggests that belowground herbivory may have been significant. However, it is unclear how much of the observed root mortality was a consequence of root herbivory.
Rates of root growth and mortality were estimated at our study site while the grassland was intensely grazed by ungulates. Aboveground herbivory can have a multitude of effects on plant dynamics. Studies have shown that grazing can increase, decrease, or have no effect on shoot growth, root growth, and root mortality, depending on the grazing intensity, climate, and soil conditions (McNaughton 1985; Frank et al. 2002l Frank 2007). However, it is unknown how ungulate grazing may have influenced rates of root growth and mortality during this study.
Implications of climate
Our results demonstrated a positive relation between root turnover and the ratio of mean monthly maximum temperature to total monthly precipitation, an index of the moisture available for plant growth during the growing season. However, Gill and Jackson (2000) reported the inverse relation between inter-annual variation in root turnover in a shortgrass steppe (Colorado, USA). They found that root turnover over 16 years was positively correlated with the ratio of growing season precipitation to maximum monthly temperature. Our results and those of Gill and Jackson (2000) suggest that climate may influence short-term, seasonal root dynamics differently than long-term, annual dynamics.
The increase in the ratio of root mortality to root growth that we observed between cool, wet June and hot, dry July was due primarily to a large decline in root growth. This suggested that a rapid decline in soil moisture influenced seasonal root production more than mortality. Frank (2007) studied the effects of a 2-year drought on grassland primary production in YNP and found that the effect was much more pronounced belowground than aboveground. During the drought, no effect was observed on aboveground net primary productivity, whereas belowground productivity declined dramatically. These results indicated that roots were more sensitive to climatic variation than shoots (Frank 2007). Other studies have also shown that annual temperature is a significant determinant of root lifespan (Hendrick and Pregitzer 1993), due to the effects of temperature on root respiration, nutrient mineralization, and pathogen activity (Lauenroth and Gill 2003; Gill and Jackson 2000). The complex linkages between climate, soil biogeochemical processes, and root turnover suggest that temperate grassland root systems may be particularly responsive to climatic variation.
This study captures very rapid rates of root growth and mortality in a temperate grassland. Our results demonstrate that estimates of grassland root dynamics from minirhizotrons are influenced significantly by length of sampling interval. In order to improve the accuracy of these estimates, we recommend that future studies sample roots at an interval that matches the temporal scale of the root parameter of interest. The findings of this study suggest that rapid root dynamics may play a critical role in grassland functioning, and future research is required to better understand the drivers of these belowground processes.
References
Aerts R, Berendse F, Klerk NM, Bakker C (1989) Root production and root turnover in two dominant species of wet heathlands. Oecologia 81:374–378
Arnone JAIII, Zaller JG, Spehn EM, Niklaus PA, Wells CE, Körner C (2000) Dynamics of root systems in native grasslands: effects of elevated atmospheric CO2. New Phytol 147:73–85
Eissenstat DM, Yanai RB (1997) The ecology of root lifespan. Adv Ecol Res 27:1–60
Eissenstat DM, Wells CE, Yanai RD (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147:33–42
Frank DA, McNaughton SJ (1992) The ecology of plants, large mammalian herbivores, and drought in Yellowstone National Park. Ecology 73:2043–2058
Frank DA, Kuns MM, Guido DR (2002) Consumer control of grassland plant production. Ecology 83:602–606
Frank DA (2007) Drought effects on aboveground and belowground production in a temperate grazed grassland ecosystem. Oecologia 152:131–139
Gill RA, Jackson RB (2000) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147:13–31
Hendrick RL, Pregitzer KS (1992) The demography of fine roots in a northern hardwood forest. Ecology 73:1094–1104
Hendrick RL, Pregitzer KS (1993) Patterns of fine root mortality in two sugar maple forests. Nature 361:59–61
Hendrick RL, Pregitzer KS (1996a) Applications of minirhizotrons to understand root function in forests and other natural ecosystems. Plant Soil 185:293–304
Hendrick RL, Pregitzer KS (1996b) Temporal and depth-related patterns of fine root dynamics in northern hardwood forests. J Ecol 84:167–176
Higgins PA, Jackson RB, Des Rosiers JM, Field CB (2002) Root production and demography in a California annual grassland under elevated atmospheric carbon dioxide. Global Change Biol 8:841–850
Houston DB (1982) The northern Yellowstone elk: ecology and management. Macmillan, New York
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci USA 94:7362–7366
Johnson MG, Tingey DT, Phillips DL, Storm MJ (2001) Advancing fine root research with minirhizotrons. Environ Exp Bot 45:263–289
Katterer T, Fabiao A, Madeira M, Ribeiro C, Steen E (1995) Fine-root dynamics, soil moisture, and soil carbon content in a Eucalyptus globulus plantation under different irrigation and fertilization regimes. For Ecol Manage 74:1–12
Lauenroth WK, Gill (2003) Turnover of root systems. In: de Kroon H, Visser EJW (eds) Root ecology. Springer, New York, pp 61–89
McClaugherty CA, Aber JD, Melillo JM (1982) The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 63:1481–1490
McNaughton SJ (1985) Ecology of a grazing ecosystem: the Serengeti. Ecol Monogr 55:259–294
Meagher MM (1973) The bison of Yellowstone National Park. National Park Service scientific monograph series number 1. United States Department of Interior, Washington
Merrill EH, Stanton NL, Hak JC (1994) Response of bluebunch wheatgrass, Idaho fescue, and nematodes to ungulate grazing in Yellowstone National Park. Oikos 69:231–240
Milchunas DG, Lauenroth WK (2001) Belowground primary production by carbon isotope decay and long-term root biomass dynamics. Ecosystems 4:139–150
Milchunas DG, Morgan JA, Mosier AR, Lecain DR (2005) Root dynamics and demography in shortgrass steppe under elevated CO2, and comments on minirhizotron methodology. Global Change Biol 11:1837–1855
Nadelhoffer KJ, Raich JW (1992) Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecology 73:1139–1147
Ruess RW, Hendrick RL, Bryant JP (1998) Regulation of fine root dynamics by mammalian browsers in early successional Alaskan taiga forests. Ecology 79:2706–2720
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Statsoft (1995) Statistica 5.0. Statsoft, Tulsa
Stanton NL (1988) The underground in grasslands. Annu Rev Ecol Syst 19:573–589
Steinaker DF, Wilson SD (2005) Belowground litter contributions to nitrogen cycling at a northern grassland-forest boundary. Ecology 86:2825–2833
Tingey DT, Phillips DL, Johnson MG (2003) Optimizing minirhizotron sample frequency for an evergreen and deciduous tree species. New Phytol 157:155–161
Wells CE, Glenn DM, Eissenstat DM (2002) Soil insects alter fine root demography in peach (Prunus persica). Plant Cell Environ 25:431–439
Whittaker JB (2003) Root–animal interactions. In: de Kroon H, Visser EJW (eds) Root ecology. Springer, New York, pp 363–385
Yanai RD, Eissenstat DM (2002) Coping with herbivores and pathogens: a model for optimal root turnover. Funct Ecol 16:865–869
Acknowledgements
We would like to thank A. Risch, V. Green, and M. Wysser for assistance in the field. J. Fridley, C. Hellquist, T. Spier, and M. Thorne provided helpful comments on an earlier draft of this paper. This work was supported by the National Science Foundation grant DEB-0318716, the Syracuse University Ruth Meyer grant, and the National Science Foundation GK-12 grant 0638686. All experiments complied with the current laws of the United States.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Tim Seastedt.
Rights and permissions
About this article
Cite this article
Stewart, A.M., Frank, D.A. Short sampling intervals reveal very rapid root turnover in a temperate grassland. Oecologia 157, 453–458 (2008). https://doi.org/10.1007/s00442-008-1088-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1088-9