Abstract
Repercussions of species loss on ecosystem processes depend on the effects of the lost species as well as the compensatory responses of the remaining species in the community. We experimentally removed two co-dominant plant species and added a 15N tracer in alpine tundra to compare how species’ functional differences influence community structure and N cycling. For both of the species, production compensated for the biomass removed by the second year. However, the responses of the remaining species depended on which species was removed. These differences in compensation influenced how species loss impacted ecosystem processes. After the removal of one of the co-dominant species, Acomastylis rossii, there were few changes in the relative abundance of the remaining species, and differences in functioning could be predicted based on effects associated with the removed species. In contrast, the removal of the other co-dominant, Deschampsia caespitosa, was associated with subsequent changes in community structure (species relative abundances and diversity) and impacts on ecosystem properties (microbial biomass N, dissolved organic N, and N uptake of subordinate species). Variation in compensation may contribute to the resulting effects on ecosystem functioning, with the potential to buffer or accelerate the effects of species loss.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accelerating rates of species loss may have severe consequences for the functioning of ecosystems. Most studies to date have focused on the relationship between species richness and ecosystem function (Hooper et al. 2005; Naeem and Wright 2003). However, other impacts associated with biodiversity decline, such as non-random patterns of species loss (Schlapfer et al. 2005; Smith and Knapp 2003) and functional aspects of compositional change independent of richness (Chapin 2003; Diaz and Cabido 2001; Symstad et al. 2003) have been shown to strongly influence the repercussions of species loss. Compensation, how the remaining species respond, has been less studied but may be an important mechanism determining the severity of species loss (Larsen et al. 2005; Ruesink and Srivastava 2001).
There are several ways in which loss and compensation may influence ecosystem processes. First, consequences of species loss could be manifested by how those lost species contribute to ecosystem functioning (Chapin 2003). It is well documented that individuals of different species can differ in litter quality, resource impacts, and disturbance feedbacks (e.g., Craine et al. 2002; Ehrenfeld 2003; Hobbie 1992), characteristics that determine the per capita functional contribution of a species (Lavorel and Garnier 2002). If this were the primary effect of biodiversity loss, then knowledge of the functional effects of the lost species would be sufficient to predict the consequences of such a loss. For instance, the removal of Lolium perenne, a species with high root allocation, resulted in decreased total root mass due to its strong contribution to this ecosystem characteristic (Wardle et al. 1999).
However, compensation of the remaining species in the community may influence ecosystem processes as much or more than the direct effects of the species lost. The ability for the remaining species to compensate for species loss can depend on recruitment, reproductive output, and density-related factors regulating population size. Some of the remaining species may respond strongly to the loss of species, while other species may show little response, resulting in functional changes in the community (Diaz et al. 1999; Lavorel and Garnier 2002). Thus, an alternative prediction would be that the response of the remaining species, rather than the identity of the species lost, should primarily influence the consequences of species loss at the ecosystem level. A removal study by Symstad and Tilman (2001) supports this pattern: dynamics largely driven by recruitment limitations strongly influenced community response, regardless of what functional group was removed. Bret-Harte et al. (2004) and Buonopane et al. (2005) also found that limitations to compensation, possibly due to rigid niche complementarity, superseded direct effects of lost species.
Lastly, an interaction between loss and compensation could exist, where the identity of the species lost will influence what species in the community will compensate for the loss. This interaction could either buffer or accelerate changes in ecosystems processes. Buffering interactions would occur if the characteristics of lost and compensating species were similar. For instance, Wardle et al. (1999) found that exclusion of one functional group was largely compensated for other groups with similar effects on ecosystem function, resulting in few unidirectional effects of species removal on ecosystem-level processes. Changes would be accelerated rather than buffered if characteristics of lost and compensating species differ in a consistent and directional manner.
Here we test a critical assumption in biodiversity research: that compensation contributes strongly to the resultant consequences on ecosystem functioning, with the potential to buffer or accelerate the effects of species loss. We test this idea with removal experiments combined with 15N tracer additions, focusing on two co-dominants of alpine moist meadows. Divergent functional effects on N cycling of the two co-dominants have been shown previously (Bowman et al. 2004; Steltzer and Bowman 1998): one is associated with slow net rates of N mineralization and high N retention, whereas the other is associated with faster rates of net N mineralization. We ask two main questions
-
1.
Can we predict the ecosystem-level consequences of the loss of these species based on our knowledge of their respective species effects, or is compensation of the remaining species important?
-
2.
If compensation through the remaining species is important, does it depend on the attributes of the species lost?
Materials and methods
Experimental system
The field experiments were conducted in a moist meadow alpine site, 3,450 m in elevation, located on Niwot Ridge (40°03′N, 105°35′W) in the Front Range of the Rocky Mountains, Colorado. Plant productivity in moist meadow communities is generally N limited (Bowman et al. 1995). We focus on Acomastylis rossii (R. Br) Greene, a rosaceous forb, and Deschampsia caespitosa (L.) P. Beauv., a tillering bunchgrass, the two most abundant species in the study site (May and Webber 1982). These species respond to nutrient supply, reabsorb nutrients, and influence nutrient cycling very differently. Deschampsia takes up N 4 times faster, grows 3 times faster, and produces litter with a lower C–N ratio than Acomastylis (Bowman et al. 2004; Miller and Bowman 2002; Steltzer and Bowman 1998). Acomastylis, possibly due to high labile C concentrations in its litter, is associated with higher microbial activity and increased N immobilization (Bowman et al. 2004; Steltzer and Bowman 1998). Growing season net N mineralization rates are over 10 times greater in Deschampsia-dominated compared to Acomastylis-dominated neighborhoods (Steltzer and Bowman 1998). Increasing N availability associated with increased atmospheric N deposition or climatic effects is predicted to disrupt the stable co-dominance in this system and shift dominance toward Deschampsia (Bowman and Steltzer 1998; Suding et al. 2004).
Other abundant species at this site include Artemisia scopulorum Gray (Compositeae), Bistorta bistortoides Small (Polygonaceae), and Caltha leptosepala DC (Ranunculaceae) (Weber 1976). Hereafter, we will refer to species by genus.
Experimental species removals
In June 2000, we selected fifteen 0.5×0.5-m plots that had equal proportions of Acomastylis and Deschampsia cover. Cover was determined non-destructively with point-quadrant frame sampling. Plots were separated by at least 2 m. Of the 15 plots, we randomly selected five in which we removed Acomastylis and five in which we removed Deschampsia. Five were left undisturbed as controls. Removal treatments consisted of repeatedly clipping the selected species to ground level. The biomass was collected, dried to a constant weight, and weighed. All 15 plots were trenched to a depth of 15 cm twice a year. These treatments were initiated in 2000 and continued through the 2001, 2002, and 2003 growing seasons.
In years 2 and 4 following the removals (July 2001 and 2003), we measured soil inorganic N, microbial biomass N, inorganic N loss (resin-extractable), and species composition. To measure inorganic N and microbial biomass N, soil samples were taken from the top 10 cm using a 2-cm-diameter soil corer and kept on snow until extraction. Inorganic N was extracted within 24 h of collection with K2SO4 (50 ml of 0.5 N K2SO4 to 10 g dry soil) and analyzed on a Lachat autoanalyzer in the Kiowa Analytical Lab. Microbial biomass N was determined by the chloroform fumigation procedure (Brookes et al. 1985) followed by Kjeldahl digestion. To estimate N loss from the rooting zone, ion-exchange resin bags (2-cm lengths of 2-cm-diameter cylinders wrapped in nylon mesh) were placed at a depth of 10 cm for 25 days, collected, and extracted with 2 N KCl. Resin bags were collected prior to the 15N label addition (see below). Species frequency was measured as the presence of species within each of twenty-five 10×10-cm quadrats within a plot. Relative abundance was estimated as the proportion of quadrats containing the species and diversity indices were calculated following Magurran (1988).
N tracer experiment
In year 4, we applied low levels of enriched 15N-labeled NH4NO3 to the plots and followed the fate of N in the plant–soil–microbe system. A solution of 15N-labeled NH4NO3 (15NH4 15NO3 98 atom% 15N; Aldrich Chemical, St. Louis, Mo.) was sprayed on the plots in an aqueous solution on 20 July 2003. The application of the tracer was timed to coincide with the period of active growth and peak biomass at this site. In order to minimize the foliar uptake of the 15N, the tracer solution was applied in the evening when stomata were closed and was followed by a water rinse to wash the solution off plants and into the soil. The tracer was added at a low level (0.011 g N/m2) to minimize any fertilization effects. We followed the tracer in five pools: (1) exchangeable inorganic N (NH4 + and NO3 −), (2) exchangeable dissolved organic N (DON), (3) CHCl3-labile microbial N, (4) aboveground shoot N, and (5) fine root N.
We collected soil samples 3 and 16 days following the addition of the 15N tracer. On each sampling date in each plot, we collected plant aboveground biomass in three 5-cm-diameter areas on a predetermined grid and then took 5-cm-diameter×10-cm-deep soil cores at these same locations. We removed roots from the cores and extracted soils in 0.5 N K2SO4 for inorganic N and total N (TN). We measured inorganic N by colorimetry on a continuous flow autoanalyzer and extractable TN and CHCl3-N as inorganic N following high-temperature persulfate digestion. We calculated concentrations and 15N enrichments of extractable DON as the extractable TN minus extractable (NH4 ++NO3 −) inorganic N. Chloroform-labile N was determined using the chloroform fumigation extraction technique, as above. We calculated microbial N as CHCl3-labile N minus extractable TN, using a factor of 0.54 to correct for extraction efficiency (Brookes et al. 1985). Soil extracts were diffused for 7 days for inorganic N, TN, and CHCl3–N (Khan et al. 1998; Stark and Hart 1996) and analyzed at the 15N Analysis Service at the University of Illinois.
Lastly, from these same cores, we harvested total above- and belowground biomass. We separated the shoots and roots in each sample, rinsing the roots in deionized water and 0.5 mM CaCl to remove 15N label adhering to the surface of the roots. We also took aboveground leaf samples from five abundant species (Acomastylis, Deschampsia, Artemisia, Bistorta, and Caltha) growing within each plot to examine the effects of removal treatments on species N uptake dynamics. These tissue samples were dried at 60°C, weighed, ground with a mortar and pestle, and analyzed for 15N enrichment at the Stable Isotope Facility at the University of California Davis. We also used the biomass samples from the cores to estimate belowground biomass allocation and root–shoot ratios.
Calculations and statistical analyses
We estimated TN pools on a per unit area basis by multiplying the sample N concentration by either the soil bulk density to the sampling depth or, for vegetation pools, plant biomass. We subtracted natural abundance 15N values for each pool from enriched values to determine atom percent excess 15N. We then determined the 15N recoveries according to a mass balance equation (Nadelhoffer and Fry 1994), as the mass of 15N in a pool above natural abundance divided by the 15N added. N uptake rates were determined as μg 15N/g dry weight per day where μg 15N equals the aboveground plant N pool multiplied by the proportion of N as 15N. Uptake rates were natural log-transformed prior to analysis.
We conducted three types of analyses. First, we analyzed for the effect of removal treatments on species abundance, diversity, microbial N, and resin N in year 2 and year 4 with repeated measures ANOVA models. We first used planned comparisons (removal versus non-removal) to confirm that we were able to control for numerical effects stemming from biomass removal. Second, we analyzed recoveries of 15N from all five N pools and the five selected species 3 and 16 days following the application of the 15N tracer in year 4. We also used repeated measures ANOVA to analyze recoveries of 15N, although the temporal comparison in this model was the 2-week interval between samplings in 2003 and not the 2-year interval in the former analysis. Third, to simultaneously analyze all the 15N recoveries, we used a multiple ANOVA mixed effects model for each sampling date.
We used these analyses to test the influences of direct loss effects and compensation on ecosystem processes. If direct effects of species lost were primarily important, we would expect that removal treatments effects on N-cycling measures would be consistent with previous work on species effects in naturally occurring high-density patches (Bowman et al. 2004; Steltzer and Bowman 1998). Thus, this mechanism would be supported if the removal of Acomastylis resulted in increased rates of N loss and decreased microbial biomass, and the removal of Deschampsia resulted in decreased rates of N loss. Alternatively, if compensation were the predominant mechanism, then the same set of species should respond to the removals of both Acomastylis and Deschampsia, influencing ecosystem function in these treatments similarly. An interaction between loss and compensation could be a third outcome, exerting a buffering or accelerating effect of ecosystem processes. If compensation buffered species loss, then we expect species that increase due to Acomastylis removal should be similar functionally to Acomastylis and species that increase due to Deschampsia removal should be those similar functionally to Deschampsia. Thus, even in spite of the loss of these species, Acomastylis removal treatments will maintain relatively low rates of N loss and vice versa. If compensation accelerated the effects of species loss, we would expect species that increase due to Acomastylis removal should be those inhibited by slow N cycling associated with Acomastylis (such as Deschampsia) and species that increase due to Deschampsia removal should be those inhibited by fast-growing species (such as Acomastylis). This divergent species response would contribute to differential functional compensation, where the Acomastylis removal treatments should have lower rates of N retention and microbial biomass than the Deschampsia removal treatments.
Results
Aboveground biomass
As intended with the species removal treatments, we removed equal amounts of aboveground biomass and both species removal treatments recovered to control biomass levels. Over the four growing seasons, 56–87 g/m2 of aboveground biomass was removed from plots, with the majority of the biomass (80–95%) removed in the first growing season. Species removal treatments did not differ in the amount of biomass removed (F 1,7=0.10, P>0.05). In year 4, aboveground production did not vary among treatments (F 2,12=0.35, P>0.05) and averaged 195 g/m2 (range 125–250 g/m2). Biomass allocation (root–shoot ratio) also did not vary among treatments (F 2,12=0.04, P>0.05).
Species composition and diversity
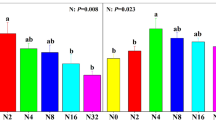
The remaining community responded differently to the removal of each co-dominant species. Acomastylis significantly increased in relative abundance following the removal of Deschampsia (Fig. 1); Acomastylis abundance was 30% greater in the Deschampsia removal than in the non-removal control. In addition, other graminoids (including Festuca brachyphylla, Trisetum spicatum, and Poa alpina) that are absent or in low abundance in the presence of Deschampsia significantly increased when Deschampsia was removed. These responses occurred by year 2 and were maintained for the four growing seasons (Fig. 1).
While total biomass compensated for the removal of Acomastylis similarly to removal of Deschampsia, few species-specific cover responses occurred due to the removal of Acomastylis. The relative cover of Deschampsia only marginally (P<0.10) increased in the absence of Acomastylis. Otherwise, no one species changed in relative abundance (Table 1), as indicated by small and/or variable species composition shifts in response to Acomastylis removal.
Consistent with the changes in species composition, both richness and evenness significantly increased due to the removal of Deschampsia but not due to the removal of Acomastylis (Table 1). The effect of Deschampsia removal on these measures of diversity grew stronger with time (Fig. 2).
Changes in species richness and evenness of the non-dominants (excluding Acomastylis and Deschampsia) in year 2 following removals (2001) and in year 4 following removals (2003) (means±1 SE). *P<0.05, **P<0.01. Abbreviations as in Fig. 1
N cycling and loss
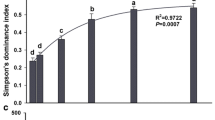
We measured two variables associated with N cycling: microbial biomass N and inorganic N loss. Microbial biomass N was significantly greater in the plots where Deschampsia was removed (and where the abundance of Acomastylis subsequently increased) than in both the control (no removals) and Acomastylis removal treatment (F 2,10=5.6, P<0.05; Fig. 3). This effect occurred by year 2 and was maintained over the course of the experiment. Inorganic N loss, as measured by resin-extractable N, significantly increased in the Acomastylis removals (F 2,10=11.3, P<0.01) relative to the controls and Deschampsia removal treatment (Table 2; Fig. 3). This effect also occurred soon after the removal treatments were initiated and was maintained over time.
Changes in microbial biomass N (upper) and inorganic N loss (lower) due to species removals in year 2 (2001) and 4 (2003) (means±1 SE). The yearly variability is predominantly due to weather differences; soil moisture was a significant covariate for both variables. *P<0.05, **P<0.01. Abbreviations as in Fig. 1
Species-specific N uptake
While the 15N uptake rates of the two co-dominant species were not affected by removals, the three less-abundant focal species did change their uptake rates (Table 3, Fig. 4). Artemisia, Bistorta, and Caltha all increased their 15N uptake rate in the plots where Deschampsia had been removed (for each species, respectively, F 2,10=7.9, P<0.01; F 2,10=5.6, P<0.05; F 2,11=6.09, P<0.05). In contrast, uptake rates of these species were similar between control and Acomastylis removal treatments. Uptake rates after the 3- and 16-day incubation periods were generally similar, although 15N recoveries were lower at the later sampling date. The exception to this pattern was Acomastylis, where there was a weak treatment by time interaction (F 1,8=5.98, P < 0.05): N uptake by Acomastylis in the Deschampsia removal treatment was less than in the control treatment on Day 3, but was higher than the control on Day 16.
Focal species 15N uptake rates in year four, 3, and 16 days after the addition of a 15N tracer (means±1 SE). Acomastylis and Deschampsia were not present in their respective removal plots and thus no uptake values are shown in these cases. Artemisia, Bistorta, and Caltha increased N uptake rates in plots where Deschampsia was removed. *P<0.05, **P<0.01, ***P<0.001. Abbreviations as in Fig. 1
15N tracer recovery in plant and soil pools
Total 15N recoveries averaged approximately 65% and did not differ among treatments (Table 4, Fig. 5). We followed 15N in five pools of N: aboveground plant biomass, root biomass, microbial biomass, and DON and inorganic N pools. Inorganic and microbial fractions decreased and DON and plant fractions increased between days 3 and 16, regardless of species removal (Table 3). However, DON recoveries on day 3 were significantly lower in Deschampsia removal treatments than in the Acomastylis removal or non-removal control plots. This difference was not detectable after 16 days (significant interaction between removal treatment and time, F 2,12=9.3, P<0.01). 15N recovery in root biomass was marginally less in the Deschampsia removal treatment at both sampling dates (F 2,12=2.8, P<0.10). Removal treatments did not affect 15N recoveries in aboveground plant biomass. Microbial biomass and inorganic N pools also did not change due to removal treatment (Table 4, Fig. 5).
Percent of 15N recovery in five N pools (inorganic N, dissolved organic N, microbial biomass N, shoot N, root N) 3 days (a) and 16 days (b) after the addition of the tracer in 2003. Error bars are shown for each pool and represent 1 SE. Multiple ANOVA analyses indicate significant treatment differences at 3 days (Wilk’s λ=0.04 F 10,16=6.74, P<0.005) but not 16 days (Wilk’s λ=0.31, F 10,16=1.29, P=0.31). The differences at Day 3 are predominately due to reductions in dissolved organic N pools in the Deschampsia removal treatment (F 2,12=57.00, P<0.005)
Discussion
In this study we experimentally removed two dominant species with different functional effects while controlling for numerical effects due to the reduction of biomass with the removals. From previous studies of species functional effects in this system, we know that Acomastylis is associated with high microbial N and N retention, thought to be mediated by litter C effects. Thus, with the removal of Acomastylis, we expected lower N immobilization and greater inorganic N losses. In contrast, we expected that the loss of Deschampsia would result in slower rates of N cycling and a decrease in N losses. As with many studies of species effects, these predictions stem from studies of species effects in high-density monospecific patches and assume two things: (1) that effects are not frequency dependent (i.e., that effects at high density are linearly related to effects at lower density), and (2) compensatory effects would be neutral.
When a species is lost from a community, it is generally expected that the remaining species of the community respond strongly, either through release from competition or reduction due to loss of a facilitative relationship (Allen and Forman 1976; Pinder 1975; Silander and Antonovics 1982; Wardle et al. 1999). For both species that we removed, other species compensated for the species removals, resulting in little overall biomass or production change. This occurred by year 2 following the initiation of the treatments even though we eliminated approximately a third of the biomass in these treatments. This pattern indicates general competitive release due to the loss of either species.
While biomass compensation was similar for both of the species, patterns of species response to the removals differed. There were only slight changes in community structure in one case (Acomastylis removal) but more substantial directional changes in the other (Deschampsia removal). By year 2, the remaining community offset the loss of Acomastylis through an increase in subdominants in about equal proportions, without strong compositional changes. In contrast, the loss of Deschampsia allowed Acomastylis and several graminoid species to increase in relative abundance. The loss of Dischampsia was also associated with an increase in both evenness and species richness of the community.
These differences in species response to removals translated to differences in ecosystem processes. The plots where we removed Acomastylis did not functionally differ from the control plots in the majority of soil characteristics we measured. The exception to this pattern was that Acomastylis removal increased inorganic N loss, an effect that could be predicted from the effects associated with the simple absence of this species. It is unclear why we were not able to identify an increase in microbial biomass N, either in soil samples or 15N tracer addition, another response that would be predicted from the absence of Acomastylis. On the whole, however, it appears that the loss of Acomastylis resulted in changes in ecosystem function (increased N losses) that were relatively predictable based on the knowledge of the effects of Acomastlyis in intact communities.
Substantial change in community structure due to the removal of Deschampsia was associated with more changes in ecosystem-level processes: microbial biomass N increased, DON decreased, and uptake of inorganic N increased in the focal subdominant species (Artemisia, Bistorta, Caltha). Again there was a discrepancy between the soil pool and 15N tracer measurements for microbial biomass N, indicating that the effect on microbial N may be slight and/or take a long time to accumulate. Overall, however, these results suggest that many of the effects associated with Deschampsia loss were a consequence of the subsequent increase in the abundance of Acomastylis rather than the absence of Deschampsia. Following Deschampsia removal, Acomastylis biomass in Deschampsia removal plots increased by approximately 30% over control levels. Thus, a decrease in Deschampsia translated into an increase in Acomastylis, with an increase in Acomastylis’s associated effects. For instance, because Acomastylis promotes N immobilization, including DON immobilization, through microbially mediated interactions (e.g., C inputs) (Bowman et al. 2004), the increase in Acomastylis in Deschampsia removal plots could explain the increase in microbial N and the short-term decrease in 15N-DON enrichment that we observed. Thus, it appears that the loss of Deschampsia resulted in changes in ecosystem function that depended on both the identity of the species lost as well as the compensation of the remaining community.
The contrast between the effects associated with the removal of these two species suggests that approaches to predict the ramifications of loss may vary among species. In the case of Acomstalyis, predictions based on knowledge of species effects would be fairly robust. In the case of Deschampsia, however, understanding how the remaining species compensate for the loss would be important. These differences suggest that predicting the ramifications of species loss may depend on the traits of the species and remaining members in the community, a focus that deserves further investigation.
While we predicted different functional consequences due to the loss of these species, we did not anticipate that they would occur through different mechanisms. Particularly, we did not foresee that community structure would change much more strongly due to Deschampsia removal compared to Acomastylis removal, even when we were able to keep the amount of biomass removed consistent among both species. We speculate that Deschampsia exerts most of its effects through the rapid uptake of N, which may have individual-level inhibitory effects on many species particularly with the pulsed N dynamics in the study system (Lipson et al. 1999). In contrast, the effects of Acomastylis are likely through litter–microbial feedbacks, which may exert strong effects at high-density levels but have less impact at lower densities (Suding et al. 2004). The cover of species at the start of this experiment (approximately 30% cover) may have been below the point at which strong effects of Acomastylis on N cycling are realized. There may be a threshold density before there is a functional consequence of its presence. It remains to be tested whether the functional effects of a species with a strong litter feedback is more directly related to density than that of a species with strong uptake effects.
Other community-level species removal experiments suggest that species loss may have different ramifications in different systems. For instance, Bret-Harte et al. (2004) found that biomass compensation was incomplete after 2 years of removals in arctic tundra. In arctic and alpine tundra, some studies have found evidence of facilitation, where the removal of a species caused the decline of other species, while others have found predominately competitive effects, as we did in this study (Aksenova et al. 1998; Bret-Harte et al. 2004; Callaway et al. 2002; Choler et al. 2001; Gerdol et al. 2002). Effects are likely to be often more subtle, however, in more realistic cases of species loss where rarer species have a higher probability of loss (Suding et al. 2005).
N deposition from anthropogenic sources is increasing on Niwot Ridge (currently approximately 6 kg N/ha per year) (Baron et al. 2000; Williams et al. 1996; Williams and Tonnessen 2000). N fertilization experiments suggest that Deschampsia may increase more than Acomastylis with increased N deposition (Bowman and Steltzer 1998). Long-term records indicate that species composition has been relatively stable in unmanipulated areas, although Deschampsia is starting to increase in abundance (K. N. Suding, unpublished data). Although we did not test how enhanced N availability would influence these dynamics, our results suggest that measures in naturally occurring monospecific patches of Acomastylis may be able to estimate the effects of the loss of this species. The remaining species in the community showed strong buffering effects and biomass compensation was fairly rapid, which could reduce the negative consequences of a shift in structure in this community.
Species loss can cause wide-ranging dynamics, making it challenging to predict consequences. Species differences in functional contribution, differences in abundance/biomass, the compensatory response of a system following species loss, short-term legacy effects and longer-term feedbacks resulting from altered community structure and function can all influence how a system responds to species loss. This study indicates that compensation of the remaining species following species loss can depend on the attributes of the lost species, and may be an important but understudied mechanism determining the severity of species loss. The attributes of the species lost may help predict both the functional consequences of the loss as well as the compensation dynamics of the remaining species.
References
Aksenova AA, Onipchenko VG, Blinnikov MS (1998) Plant interactions in alpine tundra: 13 years of experimental removal of dominant species. Ecoscience 5:258–270
Allen EB, Forman RTT (1976) Plant species removals and old-field community structure and stability. Ecology 57:1233–1243
Baron JS, et al. (2000) Ecosystem responses to nitrogen deposition in the Colorado Front Range. Ecosystems 3:352–368
Bowman WD, Steltzer H (1998) Positive feedbacks to anthropogenic nitrogen deposition in Rocky Mountain alpine tundra. Ambio 27:514–517
Bowman WD, Theodose TA, Fisk MC (1995) Physiological and production responses of plant-growth forms to increases in limiting resources in alpine tundra—implications for differential community response to environmental-change. Oecologia 101:217–227
Bowman WD, Steltzer H, Rosenstiel TN, Cleveland CC, Meier CL (2004) Litter effects of two co-occurring alpine species on plant growth, microbial activity and immobilization of nitrogen. Oikos 104:336–344
Bret-Harte MS, et al. (2004) Plant and soil responses to neighbour removal and fertilization in Alaskan tussock tundra. J Ecol 92:635–647
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil-nitrogen—a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Buonopane M, Huenneke LF, Remmenga M (2005) Community response to removals of plant functional groups and species from a Chihuahuan desert shrubland. Oikos 110:67–80
Callaway RM, et al. (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Chapin FS (2003) Effects of plant traits on ecosystem and regional processes: a conceptual framework for predicting the consequences of global change. Ann Bot 91:455–463
Choler P, Michalet R, Callaway RM (2001) Facilitation and competition on gradients in alpine plant communities. Ecology 82:3295–3308
Craine JM, Tilman D, Wedin D, Reich P, Tjoelker M, Knops J (2002) Functional traits, productivity and effects on nitrogen cycling of 33 grassland species. Funct Ecol 16:563–574
Diaz S, Cabido M (2001) Vive la difference: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Diaz S, Cabido M, Casanoves F (1999) Functional implications of trait-environment linkages in plant communities. In: Weiher E, Keddy P (eds) Ecological assembly rules. Cambridge University Press, Cambridge, pp 338–362
Ehrenfeld JG (2003) Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6:503–523
Gerdol R, Brancaleoni L, Marchesini R, Bragazza L (2002) Nutrient and carbon relations in subalpine dwarf shrubs after neighbour removal or fertilization in northern Italy. Oecologia 130:476–483
Hobbie SE (1992) Effects of plant–species on nutrient cycling. Trends Ecol Evol 7:336–339
Hooper DU, et al. (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge and needs for future research. Ecol Monogr 75(1):3–35
Khan SA, Mulvaney RL, Brooks PD (1998) Diffusion methods for automated nitrogen-15 analysis using acidified disks. Soil Sci Soc Am J 62:406–412
Larsen TH, Williams NM, Kremin C (2005) Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecol Lett 8:538–547
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16:545–556
Lipson DA, Schmidt SK, Monson RK (1999) Links between microbial population dynamics and nitrogen availability in an alpine ecosystem. Ecology 80(5):1623–1631
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton, N.J.
May DE, Webber PJ (1982) Spatial and temporal variation of the vegetation and its productivity, Niwot Ridge, Colorado. In: Halfpenny J (ed) Ecological studies in the Colorado Alpine: a festschrift for John W. Marr. Institute of Arctic and Alpine Research University of Colorado, Boulder, Colo., pp 35–62
Miller AE, Bowman WD (2002) Variation in nitrogen-15 natural abundance and nitrogen uptake traits among co-occurring alpine species: do species partition by nitrogen form? Oecologia 131:635–635
Nadelhoffer KJ, Fry B (1994) Nitrogen isotope studies in forest ecosystems. In: Lajtha K, Michener R (eds) Stable isotopes in ecology and environmental science. Blackwell, Oxford, pp 22–44
Naeem S, Wright JP (2003) Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecol Lett 6:567–579
Pinder JE (1975) Effects of species removal on an old-field plant community. Ecology 56:747–751
Ruesink JL, Srivastava DS (2001) Numerical and per capita responses to species loss: mechanisms maintaining ecosystem function in a community of stream insect detritivores. Oikos 93:221–234
Schlapfer F, Pfisterer AB, Schmid B (2005) Non-random species extinction and plant production: implications for ecosystem functioning. J Appl Ecol 42:13–24
Silander JA, Antonovics J (1982) Analysis of interspecific interactions in a coastal plant community—a perturbation approach. Nature 298:557–560
Smith MD, Knapp AK (2003) Dominant species maintain ecosystem function with non-random species loss. Ecol Lett 6:509–517
Stark JM, Hart SC (1996) Diffusion technique for preparing salt solutions, Kjeldahl digests, and persulfate digests for nitrogen-15 analysis. Soil Sci Soc Am J 60:1846–1855
Steltzer H, Bowman WD (1998) Differential influence of plant species on soil nitrogen transformations within moist meadow Alpine tundra. Ecosystems 1:464–474
Suding KN, Larson JR, Thorsos E, Steltzer H, Bowman WD (2004) Species effects on resource supply rates: do they influence competitive interactions? Plant Ecol 175:45–58
Suding KN, et al. (2005) Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc Natl Acad Sci USA 102:4387–4392
Symstad AJ, Tilman D (2001) Diversity loss, recruitment limitation, and ecosystem functioning: lessons learned from a removal experiment. Oikos 92:424–435
Symstad AJ, et al. (2003) Long-term and large-scale perspectives on the relationship between biodiversity and ecosystem functioning. Bioscience 53:89–98
Wardle DA, et al. (1999) Plant removals in perennial grassland: vegetation dynamics, decomposers, soil biodiversity, and ecosystem properties. Ecol Monogr 69:535–568
Weber W (1976) Rocky Mountain flora. University of Colorado Press, Niwot, Colo.
Williams MW, Tonnessen KA (2000) Critical loads for inorganic nitrogen deposition in the Colorado Front Range, USA. Ecol Appl 10:1648–1665
Williams MW, Baron JS, Caine N, Sommerfeld R, Sanford R (1996) Nitrogen saturation in the Rocky Mountains. Environ Sci Technol 30:640–646
Acknowledgements
This work was funded by the Andrew W. Mellon Foundation, with support from the Niwot Ridge Long-Term Ecological Research Program (NSF 0423662). We thank I. Ashton, R. Inouye, A. Kahmen, B. Schmid, and an anonymous reviewer for critical comments on this manuscript, E. Hayes, J. Larson, K. Lohnas, M. Talluto, and E. Thorsos for help in the field and laboratory, and C. Seibold for analytical support. The experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bernhard Schmid
Rights and permissions
About this article
Cite this article
Suding, K.N., Miller, A.E., Bechtold, H. et al. The consequence of species loss on ecosystem nitrogen cycling depends on community compensation. Oecologia 149, 141–149 (2006). https://doi.org/10.1007/s00442-006-0421-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0421-4