Abstract
The 2019 coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has brought an enormous public health burden to the global society. The duration of the epidemic, the number of infected people, and the widespread of the epidemic are extremely rare in modern society. In the initial stage of infection, people generally show fever, cough, and dyspnea, which can lead to pneumonia, acute respiratory syndrome, kidney failure, and even death in severe cases. The strong infectivity and pathogenicity of SARS-CoV-2 make it more urgent to find an effective treatment. Mesenchymal stem cells (MSCs) are a kind of pluripotent stem cells with the potential for self-renewal and multi-directional differentiation. They are widely used in clinical experiments because of their low immunogenicity and immunomodulatory function. Mesenchymal stem cell–derived exosomes (MSC-Exo) can play a physiological role similar to that of stem cells. Since the COVID-19 pandemic, a series of clinical trials based on MSC therapy have been carried out. The results show that MSCs are safe and can significantly improve patients’ respiratory function and prognosis of COVID-19. Here, the effects of MSCs and MSC-Exo in the treatment of COVID-19 are reviewed, and the clinical challenges that may be faced in the future are clarified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since COVID-19 was discovered in December 2019 (Hu et al. 2021a; Huang et al. 2020; Hui et al. 2020), up to March 10, 2023, the data released on the official website of the World Health Organization show that the COVID-19 epidemic has involved more than 200 countries around the world, and the cumulative confirmed cases are more than 690 million, the cumulative deaths are more than 7.6 million (Organization 2023), and the case fatality rate is about 0.9%. The epidemic of COVID-19 has lasted for a long time and spread widely, and the number of people diagnosed is unprecedented. The ongoing outbreak of COVID-19 has caused widespread concern.
COVID-19 can cause pathological changes in the lung parenchyma and interstitium (Xu et al. 2020b), and chest CT shows patchy ground glass–like shadows, solid shadows, traction bronchiectasis, and bronchial vascular bundle thickening (Shi et al. 2021a; Yang et al. 2020). In patients with severe COVID-19, SARS-CoV-2 is mainly distributed in the respiratory tract, including the lungs, and increased infiltration of immune cells (neutrophils, monocytes/macrophages, natural killer (NK), CD4 + T, CD8 + T, Th17, B cells) and followed by cytokine storms (including interferon-α (IFN-α), IL-1, IL-6, and tumor necrosis factor-α (TNF-α)). Hyaline membrane formation, the release of cellular fibromyxoid exudates, and pneumocyte desquamation are also observed (Shi et al. 2021b).
There is no specific treatment for COVID-19, and most treatment options use symptomatic supportive therapy, including antiviral therapy (Holshue et al. 2020; Wang et al. 2020b; Zhu et al. 2020), glucocorticoid therapy (Stern et al. 2017), convalescent plasma therapy (Dhawan et al. 2022; Zimmerli et al. 2021), IL-6 receptor inhibitor therapy (Xu et al. 2020a), oxygen therapy (Teng et al. 2021), anticoagulation (Shi et al. 2020), and traditional Chinese medicine treatment (Ni et al. 2021), but the prognosis is poor, and some COVID-19 patients still have sequelae after cure (Fonseca et al. 2021; Huang et al. 2021a, b), for example, interstitial lung disease, decreased tolerance to cardiopulmonary exercise, easy to feel fatigued, loss of smell and taste, sleep disorders, anxiety, or depression. To address the urgent need for effective and safe treatments to alleviate the physical damage caused by COVID-19 to patients, drug development for COVID-19 is in full swing, multiple vaccines have been marketed, and many clinical drugs have shown some efficacy, MSCs are one of them.
MSCs are pluripotent stem cells found in a variety of tissues, including bone marrow, umbilical cord, fat, and dental pulp (Bernardo et al. 2009). It is safely available without major ethical issues. MSCs also have powerful tissue repair, anti-inflammatory, and immunomodulatory functions (Jiang and Xu 2020), so MSCs are considered an effective and safe source of stem cell therapy and are clinically mainly used for autoimmune diseases (Heidari et al. 2018), repair of damaged tissues (Liu et al. 2021), and drug therapy vectors (Litvinova et al. 2022). MSC-Exo is an exosome secreted by MSCs, typically between 40 and 150 nm in diameter, and contains substances such as proteins, mRNA, miRNA, and lncRNA (Jayaramayya et al. 2020). MSC-Exo also has a certain regulatory function, and as a non-cellular biologically active substance, it has gradually attracted widespread attention. In the early stage of the SARS-CoV-2 pandemic, it was found in phase I clinical trial using MSCs for the treatment of COVID-19 that C-reactive protein (CRP) and IL-6 levels in the serum of patients with severe COVID-19 in the umbilical cord–derived MSC (UC-MSC) treatment group were significantly reduced on the third day after UC-MSC infusion compared with the control group. In addition, the time for the lymphocyte count to return to normal was significantly accelerated, and the absorption time of inflammatory lesions in the lungs was significantly shortened. No serious adverse events associated with UC-MSC transfusion were observed (ChiCTR2000031494) (Shu et al. 2020). In most patients with severe COVID-19, arterial partial pressure of oxygen-to-percentage inhaled oxygen (PaO2/FiO2) ratio improved after treatment with UC-MSCs, and chest CT scan images show that lung lesions are well controlled after infusion of UC-MSCs (NCT04252118) (Meng et al. 2020). This demonstrates the safety and efficacy of intravenous infusion of UC-MSCs in the treatment of severe COVID-19, so MSCs can be considered one of the treatment options for patients with severe COVID-19.
The objective of this review is to summarize clinical studies of MSCs in the treatment of COVID-19 as of 2023, elucidate the therapeutic effects of MSCs on COVID-19, and discuss the prospects and challenges of MSC-based treatment of COVID-19.
SARS-CoV-2 and its route of infection
Features of SARS-CoV-2
SARS-CoV-2 is an RNA virus with high variability that belongs to the coronavirus family, beta coronavirus genus, and severe acute respiratory syndrome–related coronavirus species (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses 2020). CoV-2 has an envelope (DiMaio et al. 2020); is round or oval, granular, with a diameter of about 60–140 nm; has strong infectious power; can spread from person to person (Chan et al. 2020); and can reach the respiratory tract through droplets and air and survive (Sungnak et al. 2020). According to studies, virus particles can survive for a long time on air and on surfaces, and viruses can remain infectious for up to 2 h to 9 days on surfaces composed of different materials (Kampf et al. 2020). SARS-CoV-2 is sensitive to medical alcohol, chlorine-containing disinfectants, ultraviolet light, and high heat. At 56 °C, the virus can be killed in 30 min, and in an environment of 92 °C, the virus can reach a completely inactivated state in 15 min (Pastorino et al. 2020).
SARS-CoV-2 infection pathway
The main sources of infection for SARS-CoV-2 are asymptomatic virus carriers and people with COVID-19. The main modes of transmission are droplet transmission and contact transmission (Cheng et al. 2020; Lai et al. 2020). There is also the possibility of airborne transmission through aerosol forms, especially in tightly confined spaces where the risk of aerosol transmission is greater.
SARS-CoV-2 surface glycosylated spike protein binds to host angiotensin-converting enzyme 2 (ACE2) to mediate virus entry into cells (Yan et al. 2020). When the spike protein of SARS-CoV-2 binds to the ACE2 receptor, type 2 transmembrane serine protease (TMPRSS2) located on the host cell membrane promotes virus entry into the cell by activating the spike protein (Bestle et al. 2020; Hoffmann et al. 2020). Due to the high expression of ACE2 and TMPRSS2 in alveolar type II cells, the lungs are severely affected by viruses (Devaux et al. 2020).
Clinical manifestations of SARS-CoV-2
COVID-19 is a serious disease caused by SARS-CoV-2, and the virus can lurk in infected people for about 14 days, but most patients develop symptoms within 3 to 7 days (Li et al. 2020)—initially presents with fatigue, fever, myalgia, and dry cough (Chen et al. 2020b; Wang et al. 2020a) and, less commonly, diarrhea, nausea, headache, vomiting, loss of smell, loss of taste, and sore throat (Giacomelli et al. 2020; Guan et al. 2020). Breathing difficulties occur as the condition worsens, and severe patients present with respiratory distress syndrome (ARDS), sepsis shock, difficult-to-correct metabolic acidosis, coagulation dysfunction, lung, and multi-organ damage, and even death (Chen et al. 2020c).
Imaging examination found that patients had multiple localized ground glass shadows under the pleura of both lungs in the early stages, rapid progression of advanced lesions, and multiple ground glass shadows in both lungs with consolidation (Yang et al. 2020), and some patients could have fibrous strip shadows, and in severe lesions, “white lung” manifestations were present.
COVID-19 sequelae
Studies have found that the sequelae of COVID-19 are mostly manifested as fatigue, dyspnea, difficulty sleeping, chest pain, diabetes, brain atrophy, male dysfunction, dry eye, cardiovascular disease, loss of smell, taste, and mental illness (Carfi et al. 2020; de Melo et al. 2021; Douaud et al. 2022; Kresch et al. 2021; Li et al. 2022; Wan et al. 2022; Xie and Al-Aly 2022; Xie et al. 2022).
An article published in The Lancet said that 1276 COVID-19 survivors were followed for 12 months and found that they had sequelae after being cured and discharged from the hospital, such as pulmonary diffusion dysfunction and radiological abnormalities that persisted for 12 months. Particularly in patients who were critically ill during hospitalization, health status remained lower than in the control population after 12 months (Huang et al. 2021b). Xie et al. analyzed data from 181,280 COVID-19 patients and found that SARS-CoV-2 infection increased the risk of developing diabetes by 40% compared to healthy controls and that Black people over the age of 65, with cardiovascular disease, and those with prediabetes with a BMI of > 25 were at greater risk of developing diabetes and taking hypoglycemic drugs (Xie and Al-Aly 2022). Douaud et al. selected 785 subjects from the UK Biobank and compared the brain images of these participants. They found that SARS-CoV-2 causes brain volume to shrink, and the shrunken brain tissue is related to cognitive function, olfactory function, etc., which can lead to the executive ability and cognitive decline of infected people. In addition, they also found that the older the infected person, the more severe the disease, and the more obvious the brain tissue damage (Douaud et al. 2022). Kresch et al. found that after infection with SARS-CoV-2, the virus is present in the penis of male patients and that extensive endothelial cell disorders caused by viral infections can lead to erectile dysfunction (Kresch et al. 2021). Li et al. found in hamster models of SARS-CoV-2 infection that the virus may cause acute testicular injury, chronic asymmetric testicular atrophy, and associated hormonal changes and proposed that vaccination could prevent this complication (Li et al. 2022). Wan et al. found that one in five people who have recovered from COVID-19 experience at least one symptom associated with dry eyes, such as blurred vision, itchy eyes, pain, or burning sensations. In addition, studies have found that the more severe a patient’s condition, the higher the risk of developing dry eye (Wan et al. 2022). In a large study involving 153,760 patients with COVID-19, researchers analyzed the risk of cardiovascular disease in patients 12 months after SARS-CoV-2 infection and found that infection with the virus increased the long-term risk of a variety of cardiovascular diseases, including cerebrovascular disease, arrhythmias, ischemic and non-ischemic heart disease, pericarditis, myocarditis, heart failure, and thromboembolic disease (Xie et al. 2022). In a study that analyzed the effects of SARS-CoV-2 on the human olfactory system, researchers detected persistent SARS-CoV-2 virus particles in the olfactory mucosa of COVID-19 patients with persistent olfactory loss, indicating that long-term recurrent olfactory loss in COVID-19 patients may be caused by persistent viral infection (de Melo et al. 2021).
Molecular mechanisms by which SARS-CoV-2 triggers lung injury
Histological examination by Xu et al. has determined that people who die of COVID-19 have a bilateral diffuse alveolar injury, signs of respiratory inflammation, microvascular complications, and pulmonary fibrosis (Carsana et al. 2020; Xu et al. 2020b). For effective targeted treatment, comprehensive cellular and molecular levels of lung pathology in patients with COVID-19 are required.
Wang et al. synthesized transcriptomics, proteomics, and histopathology techniques to construct a complete multi-omics and mononuclear transcriptomics atlas of lung tissue in patients with COVID-19 and found that ACE2 and TMPRSS2 were mainly expressed in epithelial cells, such as type I and type II alveolar epithelial cells (AT1 and AT2, respectively), which are also the main target cell types of SARS-CoV-2 (Wang et al. 2021). It was found that the binding of SARS-CoV-2 to ACE2 can mediate the down-regulation of ACE2 expression in the lungs, resulting in the accumulation of angiotensin II (Ang II) (Kai and Kai 2020; Kuba et al. 2005). Excessive Ang II activates the Fas/FasL system to induce apoptosis of lung cells, prompting impaired lung epithelial barrier function (Wang et al. 1999). An increase in M1 macrophages in the alveoli can worsen diffuse alveolar damage by releasing a large number of pro-inflammatory cytokines. The study also found that the activation of HIF-1A and the expression silencing of FOXO3 may promote the transition from fibroblasts to myofibroblasts in the lungs, thereby mediating the occurrence of pulmonary fibrosis (Wang et al. 2021).
Existing strategies for treating COVID-19
The existing treatments and drugs for COVID-19, depending on the object of action, can be divided into two categories: one acting on the human immune system or cells and the other acting on the coronavirus.
At present, the treatment methods for COVID-19 are mainly treatment based on MSCs, general treatment, antiviral therapy, immunotherapy, anticoagulation therapy, traditional Chinese medicine treatment, etc., but there is no effective antiviral treatment for COVID-19. Clinical treatment is mostly based on the experience of fighting SARS, and the use of integrated traditional Chinese and Western medicine for symptomatic supportive treatment and comprehensive intervention is still the main treatment for severe COVID-19 patients.
Existing drugs in antiviral therapy do not kill the virus directly but inhibit the replication and spread of the virus in the human body. For example, lopinavir interferes with viral synthesis, and remdesivir prevents viral RNA replication (Choy et al. 2020; Uzunova et al. 2020). Glucocorticoids are used in immunomodulatory therapy to reduce the over-stressed immune response and regulate the unbalanced immune system, thus slowing down the progression of the disease and saving the lives of patients.
Treatment based on MSCs
MSCs play a supportive role in the treatment of critically ill patients and are nonspecific therapies. It does not directly destroy the virus but helps relieve inflammation, improve lung function, and improve survival rates in severely ill patients. Compared with other treatment options, MSCs, in addition to exerting the body’s immunomodulatory effect to suppress inflammation and viral infections, also have tissue repair functions, helping the recovery of damaged tissues and organs. So the mechanism by which MSCs treat patients with COVID-19 may be due to immunomodulatory and supportive repair effects (Harrell et al. 2019; Sadeghi et al. 2020; Zhu et al. 2021).
General treatment
Bed rest is usually given to the patient, supportive care is intensified, adequate energy and water intake are ensured, and effective oxygen therapy is given promptly, including mask oxygen and nasal high-flow oxygen therapy. Antimicrobial therapy may be used if necessary, but a combination of broad-spectrum antimicrobials should be avoided.
Antiviral therapy
At present, there are no highly targeted antiviral drugs, clinically trying “old drugs and new uses.” We reviewed the “Diagnosis and Treatment Plan for Novel Coronavirus Pneumonia” (1–8 editions) published on the official website of the National Health Commission of China. Lopinavir/ritonavir (LPV/r) was found to be consistently recommended as an anti-SARS-CoV-2 drug in versions 3–8. They also recommend LPV/r in combination with ribavirin. One study showed that after treatment with LPV/r, patients had a reduced viral load and reduced clinical symptoms (Lim et al. 2020). Antiviral therapy rebuilds the immune function of the host and is recommended early in the patient’s course to prevent a transition to a high-risk condition of severe illness.
Immunomodulatory therapy
Immunomodulatory therapy strategies may potentially halt disease progression and save patients with COVID-19, especially in critical cases. There are many immunotherapies for COVID-19, including glucocorticoid therapy, convalescent plasma therapy, and IL-6 receptor inhibitor therapy.
Glucocorticoid therapy
The use of glucocorticoids in the treatment of COVID-19 is controversial, with some researchers not recommending the use of glucocorticoids in the treatment of patients with COVID-19 (Russell et al. 2020), while others recommend the use of glucocorticoids as appropriate (Shang et al. 2020). The WHO guidelines for COVID-19 recommend that systemic corticosteroids should not be routinely given outside clinical trials for viral pneumonia or acute respiratory distress syndrome except for specific reasons, as high-dose glucocorticoids can delay coronavirus clearance due to immunosuppressive effects. Studies have shown that the early use of small doses of short-course glucocorticoids in some patients with “ordinary COVID-19” (with fever, respiratory symptoms, etc., imaging can show pneumonia) has the potential to rapidly inhibit the inflammatory response and block its transition to severe/critical illness (Li et al. 2021a).
Convalescent plasma therapy
Clinical trials have used high-titer convalescent plasma from COVID-19 recovery patients to be transfused into elderly patients with a mild infection, then observed the relevant clinical indicators and efficacy, and found that the administration of high-titer convalescent plasma early in the course of the disease can effectively prevent disease progression (Libster et al. 2021). However, the disadvantage is that this treatment is expensive and complex to operate.
Treatment with IL-6 receptor inhibitors
Tocilizumab is an IL-6 receptor antagonist that binds to IL-6 receptors on the cell surface, preventing the occurrence of cytokine storms and weakening the inflammatory response. In hospitalized patients with severe COVID-19 with hypoxia and systemic inflammation, the use of tocilizumab improves survival and reduces the probability of invasive mechanical ventilation (Group et al. 2021).
Traditional Chinese medicine treatment
Chinese medicine pays attention to the right medicine according to the condition and personal constitution. During the period of medical observation, it is recommended to take proprietary Chinese medicines: Jinhua Qinggan granules, wind and detoxification capsules (granules); in the clinical treatment period of confirmed cases, lung detoxification soup is suitable for mild, ordinary, and severe patients, and in critically ill patients, it should be used rationally according to the actual situation of patients.
Clinical trials of MSCs and their derivatives
MSCs have been used clinically as a supportive treatment for patients with severe COVID-19, and as of May 20, 2023, 16 trials exploring the potential of MSCs in treating patients with COVID-19 have been registered at the Chinese Clinical Trials Registry (http://www.chictr.org.cn).
We found 9012 trials about COVID-19 registered on the International Clinical Trials Registry Platform (http://ClinicalTrials.gov). These include more than 70 trials exploring the potential of MSCs and their derivatives to treat COVID-19 patients. In addition to China, countries such as the United States, Brazil, Spain, Indonesia, France, Mexico, Pakistan, Russia, Turkey, Canada, and Belgium are also actively conducting clinical trial studies on the subject. Table 1 Clinical trials of MSCs for COVID-19-related diseases.
Therapeutic effects of MSCs
The analysis of Table 1 found that most clinical trials used UC-MSCs as a source of MSCs, at doses of 1 × 106 cells/kg, and administered intravenously, to explore safety and efficacy in patients with COVID-19. This may be because intravenous administration is the most common, least invasive, and most reproducible method. The doses fall into the range of 0.5–3 × 106 cells/kg, which overlaps with a group of MSC trials that reported efficacy for MSC for various indications. The article shows that the median dose for IV delivery is 100 million MSCs/patient/dose. They found only four trials that reported dose–response data showing differences in efficacy for improving outcomes, suggesting a narrower MED range of 100–150 million MSCs/patient with lower and higher IV doses being less effective (Kabat et al. 2020).
Multiple clinical trials have shown that MSCs have good therapeutic potential. In February 2020, in a clinical study conducted by Leng et al. (ChiCTR2000029990), it was explored whether MSC transplantation improved the prognosis of 7 patients with COVID-19. The trial found that seven patients had significant improvements in lung function and symptoms within 2 days after transplantation of MSCs. TNF-α levels were significantly reduced in the MSC-treated group compared to the control group, while IL-10 levels were significantly elevated. The therapeutic potential of transplanted MSCs in improving lung function in patients with COVID-19 was confirmed (Leng et al. 2020). An exploratory clinical trial of Li et al. (ChiCTR2000029606) showed that patients with severe and critical COVID-19 had significant improvements in dyspnea and oxygen saturation compared with the control group after receiving intravenous transfusions of transvenous MSCs of menstrual blood sources (Xu et al. 2021). In the phase I clinical trial conducted by Shu et al. (ChiCTR2000031494), it was found that the clinical improvement time of the UC-MSC treatment group was significantly shorter than that of the control group, the incidence of critical progression in the treatment group was 0, the mortality rate at 28 days was 0 in the treatment group, and the 28-day mortality rate in the control group was 10.34% (Shu et al. 2020). From the phase I clinical trial conducted by Meng et al. (NCT04252118), it was found that patients with severe COVID-19 decreased IL-6 levels within 3 days after UC-MSC infusion, and lung lesions were well controlled within 6 days and completely resolved within 2 weeks after infusion. In contrast, patients with severe illness with COVID-19 in the control group remained with lung lesions at the time of discharge (Meng et al. 2020); on this basis, the team conducted a phase II clinical trial (NCT04288102) and found that compared with the control group, the 6-min walk test (6-MWT) distance of patients treated with UC-MSCs increased, and the volume of total lung lesions improved significantly (Shi et al. 2021a). In the phase II clinical trial conducted by Feng et al. (NCT04269525), there were no infusion-related or allergic reactions. The Oxygenation index was improved, and the CT scan improved. However, it is not indicated whether these two results are statistically significant (Feng et al. 2020). In a multicenter randomized double-blind trial conducted by Monsel et al. (NCT04333368), PaO2/FiO2 changes between D0 and D7 did not differ significantly between the UC-MSC and placebo groups. Repeated UC-MSC infusions were not associated with serious adverse events during treatment or after that (until D28). Despite the lack of statistically significant differences, UC-MSC-treated patients’ more tremendous PaO2/FiO2 ratio increase between D0 and D7, compared to placebo-infused controls might represent a signal warranting further investigation on a larger patient population (Monsel et al. 2022). In a double-blind, phase 1/2a, randomized controlled trial conducted by Lanzoni et al. (NCT04355728), UC-MSC infusions in COVID-19 ARDS were found to be safe. Inflammatory cytokines were significantly decreased in UC-MSC-treated subjects at day 6. Treatment was associated with significantly improved patient survival (91% vs. 42%, P = 0.015), SAE-free survival (P = 0.008), and time to recovery (P = 0.03) (Lanzoni et al. 2021). In a prospective double-controlled trial conducted by Adas et al. (NCT04392778), all the indicators of anti-inflammation, anti-fibrosis signs in the lungs, and levels of immunoregulatory markers were dramatically improved (Adas et al. 2021). In a randomized controlled trial conducted by Dilogo et al. (NCT04457609), the length of stay in the intensive care unit and ventilator usage were not statistically significant, and no adverse events were reported. The application of infusion UC-MSCs significantly decreased interleukin 6 in the recovered patients (P = 0.023) (Dilogo et al. 2021). In the phase II/III clinical trial conducted by Soetjahjo et al. (NCT05132972), UC-MSCs led to a better recuperation in oxygenation index and oxygen saturation. Additionally, compared to the placebo group, the treatment group had a significantly smaller increase in PCT level at D + 22 (1.43 vs. 12.76, P = 0.011). No adverse effects, including serious ones, were recorded until D + 91 (Soetjahjo et al. 2023). In the phase I/II clinical trial conducted by Grégoire et al. (NCT04445454), MSC infusions were well tolerated, and no adverse effects related to MSC infusions were reported. Survival was significantly higher in the MSC group, both at 28 and 60 days (100% vs. 79.2%, P = 0.025 and 100% vs. 70.8%, P = 0.0082, respectively) (Grégoire et al. 2022). In a randomized, double-blind, placebo-controlled trial conducted by Karyana et al. (NCT04535856), there were no apparent differences in clinical characteristics between study groups (TL, TH, and C) at baseline. All patients did not show the progression of severity during the study period (Karyana et al. 2022). In a randomized, double-blind, single-center, efficacy, and safety study of allogeneic HB-adMSCs against COVID-19 (NCT04348435), results were first posted on November 17, 2022, and there were no serious adverse events (SAEs) reported during the study period. Primary outcome measures are the number of participants who were hospitalized due to COVID-19 symptoms. However, the number of subjects hospitalized due to COVID-19 symptoms during the study was 0 in both the experimental and placebo groups. Sadeghi et al. used placenta-derived decidua stromal cells (DSCs) to treat COVID-19 patients. DSCs were administered 1–2 times at a dose of 1 × 106/kg. The trial found that after DSC infusion, pulmonary infiltrates disappeared in all patients, and IL-6 levels were significantly reduced (Sadeghi et al. 2021). We believe that further research is needed on the effects of MSC in people with COVID-19, including the need for controlled randomized trials. These clinical trials have demonstrated that transplantation of MSCs by intravenous infusion is an effective approach that can be considered as a potential treatment option for treating patients with COVID-19.
Limitations of using current adult tissue–derived MSCs
The treatment effect using MSCs from bone marrow, adipose tissue, umbilical cord tissue, etc., has been inconsistent. MSC derived from different tissue donors makes it hard to keep quality consistency.
A rigorous quality control system reduces batch-to-batch variability, including secretome, allowing MSCs to maintain consistent quality. MSCs derived from induced pluripotent stem cells (iPSC-MSCs) have recently been established to induce targeted differentiation of pluripotent stem cells to MSCs by employing clinically compliant protocols, chemically defined media, feeder-free conditions, and CD105-positive and CD24-negative selection and proposed that it can be used as an alternative to reduce batch-to-batch variation of MSC products (Lian et al. 2016).
Aging and aging-related disorders impair the survival and differentiation potential of BM-MSCs and limit their therapeutic efficacy (Roobrouck et al. 2008; Xin et al. 2010). iPSCs have the ability to differentiate and proliferate indefinitely in multiple lineages. Compared with adult BM-MSCs, iPSC-MSCs have a higher proliferative capacity, are highly expandable up to 40 passages (120 population doublings) without obvious loss of self-renewal capacity, and constitutively express surface antigens of multipotent MSCs (Lian et al. 2010). iPSC-MSCs have many applications, including large-scale cell population expansion for tissue engineering and cell therapy development. At present, iPSC-MSCs have been used to treat severe hindlimb ischemia and heart failure in mice and have achieved good therapeutic results (Lian et al. 2010; Liao et al. 2019). Engineered iPSC-MSCs transfected with glutathione peroxidase 3 (GPx3) have also significantly inhibited liver aging and reduced liver transplantation injury in mice (Qi et al. 2018). In addition, GMP-grade iPSC-MSCs (CYP-001) have been used in clinical trials for steroid-resistant acute graft-versus-host disease (SR-aGvHD), the first full human clinical trial report using iPSC-derived cells to treat disease to evaluate the safety, tolerability, and efficacy of CYP-001. The study found no serious adverse events associated with CYP-001, indicating that it was safe and well tolerated, and the 100-day survival rate was 87.5% (low dose) and 85.7% (high dose), demonstrating that CYP-001 has some therapeutic effect on steroid-resistant acute graft-versus-host disease (Bloor et al. 2020).
Therapeutic effect of MSC-Exo
MSC-Exo has similar biological functions to MSCs, and in addition, MSC-Exo also has the advantages of non-toxicity, low immunogenicity, high stability, easy storage, etc., making them widely used as an alternative to MSCs in the clinic (Jafari et al. 2019; Joo et al. 2020).
It is well recognized to measure the concentration of MSC-exosomes using nanoparticle tracking analysis (NTA). Some experimental articles have mentioned that they use NTA to measure the concentration of MSC-exosomes (Hu et al. 2021b; Shi et al. 2021c; Yu et al. 2020). Of the seven trials using MSC-exosomes listed in Table 2, four had an infusion/inhalation dose of 109, two at an infusion/inhalation dose of 108, and one at an inhalation dose of 1010. The doses varied widely from 108 to 2 × 1010, perhaps because of different sources of MSC-exosomes, different ways of use, and different stages of disease that may lead to different doses. In addition, since there are few clinical trials on MSC-exosomes for the treatment of COVID-19, we may need to determine what the optimal dose is from a larger dose range.
In clinical trials for the treatment of COVID-19 patients, intravenous infusion and aerosol inhalation are often used to transplant MSC-Exo. A pilot clinical study (NCT04276987) in the inhalation of allogeneic adipose-derived MSC-Exo (AD-MSC-Exo) in the treatment of patients with severe COVID-19 found that MSC-Exo has the potential to cure patients with severe COVID-19. A clinical trial using MSC-Exo nebulized inhalation for the treatment of COVID-19 (ChiCTR2000030261) found that MSC-Exo has the potential to inhibit inflammatory factors, enhance the body’s immunity, and promote the early recovery of COVID-19 patients and reduce complications by atomizing into the lungs and directly contacting the lesion. A clinical trial evaluating the safety and efficacy of stem cell exosome inhalation in SARS-CoV-2-associated pneumonia (NCT04491240) found a significant decrease in serum CRP levels in patients after inhalation of exosomes.
Security assessment
An exploratory clinical trial conducted by Li et al. (ChiCTR2000029606) found that adverse reactions associated with clinical indicators after receiving intravenous infusions of MSCs of menstrual blood sources, including blood test results, liver function indicators, renal function, blood lipid level, and myocardial enzyme level were not significantly different from the control group (P > 0.05) (Xu et al. 2021).
In a previous phase I clinical trial (NCT04252118), researchers found that patients with severe COVID-19 had no serious adverse events after intravenous infusion of UC-MSCs, demonstrating that UC-MSCs by intravenous infusion were safe and well tolerated (Meng et al. 2020). A randomized, double-blind, placebo-controlled phase II clinical trial (NCT04288102) based on this basis also evaluated the safety and efficacy of intravenous infusion of UC-MSCs in patients with COVID-19 lung injury and found that the use of UC-MSCs was safe and had a tendency to improve overall lung damage in patients (Shi et al. 2021a).
From a clinical trial of the safety and efficacy of BM-MSC-derived exosomes (ExoFlo™) in the treatment of patients with severe COVID-19 conducted by the Grossman School of Medicine of New York University, no adverse events were observed within 72 h of receiving ExoFlo intravenous infusion, demonstrating the safety of stem cell exosomes in humans (Sengupta et al. 2020).
Potential mechanisms of MSCs in the treatment of COVID-19
The homing capability of MSCs
MSCs can be homebound to the site of injury after intravenous infusion, and the homing of MSCs is regulated by platelet-derived growth factor (PDGF) or insulin-like growth factor-1 (IGF-1) and chemokines such as CCR2, CCR3, CCR4, or CCL5 (Yagi et al. 2010). In addition, IL-8 can also induce the migration of MSCs to the injured area (Bi et al. 2014).
Some researchers have found that MSCs can differentiate into type II alveolar epithelial cells through typical Wnt pathways and atypical Wnt pathways (Liu et al. 2013, 2014). Differentiation of MSCs into epithelial type II cells may partially restore the stem cell pool, leading to increased genesis of alveolar cells for the resolution of disrupted alveolar surfaces, thereby augmenting the repair process (Ortiz et al. 2003). Type I alveolar epithelial cells have no ability to divide and are supplemented by type II alveolar epithelial cell proliferation and differentiation after injury (Chen et al. 2020a). Therefore, after infection with COVID-19, transplanted MSCs may first differentiate into type II alveolar epithelial cells and then differentiate into type I alveolar epithelial cells by type II alveolar epithelial cells, thereby reducing lung damage.
So one of the mechanisms by which MSCs are transplanted to treat COVID-19 patients may be due to inflammation of the patient’s lungs triggering the homing of transplanted MSCs in the lungs. Homing MSCs can differentiate into alveolar cells, alleviating lung damage caused by apoptosis of alveolar cells and thereby improving lung function. In addition, researchers have found that the Wnt/β-catenin signaling pathway plays an important role in the differentiation of MSCs into alveolar epithelial cells, and blocking the Wnt/β-catenin signaling pathway may promote the differentiation of MSCs into alveolar epithelial cells (Shi et al. 2015).
Paracrine function of MSCs
Due to the small number of exogenous MSCs migrating to the lesion and the smaller number of differentiated into damaged tissue, the paracrine function of MSCs may play a more important role in promoting damaged tissue repair. Stimulated by inflammatory factors (e.g., TNF-α), MSCs can balance pro-inflammatory and anti-inflammatory responses by secreting immunomodulatory cytokines such as indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), IL-6, and IL-10 (Shi et al. 2018; Ullah et al. 2015).
When a patient with COVID-19 exhibits symptoms of pulmonary fibrosis, the pulmonary capillaries are invaded and destroyed by fibrous tissue, which can affect breathing. After transplantation, MSCs can express and produce vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), monocyte chemotactic-1 (MCP-1), stromal cell–derived factor 1 (SDF-1), etc., to rebuild the vascular network (Kinnaird et al. 2004a, b; Liang et al. 2014a; Reiter et al. 2017).
However, MSCs are vulnerable under stressful environments. Studies have found that hypoxia can affect the paracrine function of MSCs. Compared with normoxia, the concentrations of pro-inflammatory cytokines IL-6, TNF-α, and MCP-1 in BM-MSCs were increased under hypoxic conditions, which was not conducive to cardiac repair treatment after myocardial infarction (Zhang et al. 2015). Hypoxic conditions can also cause apoptosis of BM-MSCs, and this type of apoptosis is more prevalent in aged BM-MSCs (Yang et al. 2018; Zhang et al. 2014). Compared with BM-MSCs, the ratio of phosphorylated NF-κB to NF-κB-p65 of Rap1−/−-BM-MSCs obtained by knocking out Rap1 (a telomeric repeat-binding factor 2-interacting protein 1, Terf2IP) with shRap1 was significantly reduced, the ratio of Bax to Bcl-2 was significantly reduced, and the terminal deoxynucleotidyl transferase–mediated dUTP notched end labeling (TUNEL) experiment showed that Rap1−/−-BM-MSCs had enhanced resistance to hypoxia-induced apoptosis (Zhang et al. 2015). Nonetheless, in the context of allogeneic heart transplantation which arouses a violent immune response, Rap1 expression is irreplaceable for MSCs to execute immune-suppressive functions. In vitro, deletion of Rap1 in MSCs leads to paracrine insufficiency: both pro- and anti-inflammatory cytokines are downregulated. In vivo, transplantation of encapsulated WT-MSCs more effectively prolongs the survival of the allogeneic heart than Rap1−/−-MSCs (Ding et al. 2018). The paradoxical effect of Rap1 may be due to MSCs being exposed to different environments in myocardial infarction and heart transplantation.
The functional and regenerative capacities of MSCs decline with senescence. Compared with MSCs isolated from young donors, those derived from aged donors showed a lower level of fibroblast growth factor 21 (FGF21) and a higher level of senescent activity. The study found that FGF21 can mediate MSC aging by regulating mitochondrial dynamics and ROS production. Overexpression of FGF21 in aged MSCs can delay cellular aging, and targeting FGF21 may be a new strategy to improve the quality and quantity of MSCs (Li et al. 2019). In addition, Erb-B2 receptor tyrosine kinase 4 (ERBB4) in aged MSCs was significantly reduced compared to young MSCs, suggesting that ERBB4 may be involved in regulating cellular senescence. ERBB4 overexpression significantly protects aged MSCs from H2O2-induced apoptosis, better preservation of telomere length, and increased telomerase activity. In mouse myocardial infarction models, transplantation of aged MSCs overexpressing ERBB4 contributed to the recovery of myocardial function (Liang et al. 2019). It can be seen that rejuvenating aging MSCs by overexpressing FGF21 and ERBB4 may be a new therapeutic strategy.
Immunomodulatory capacity of MSCs
The immunomodulatory function of MSCs is mainly exerted through cell-to-cell contact (e.g., interactions with T cells, B cells, NK cells, macrophages, monocytes, dendritic cells (DCs), and neutrophils) and paracrine effects (Lu et al. 2022; Song et al. 2020; Weiss and Dahlke 2019; Zhou et al. 2019). MSCs can downregulate the secretion of TNF-α and interferon-γ (IFN-γ) by T cells, upregulate IL-4 secretion, and promote the transformation of cells from a pro-inflammatory state to an anti-inflammatory state. It can also inhibit abnormally activated Th1 and Th17 differentiation, induce regulatory T-cell (Tregs) expansion and differentiation, and restore the Th1/Th2 ratio balance, thereby improving immune status (Uccelli and de Rosbo 2015). MSCs can inhibit the excessive proliferation of B cells, downregulate transcription factor expression, prevent their differentiation to plasma cells, and reduce the level of immunoglobulin secretion, while inhibiting B-cell chemotaxis to lymph nodes (Che et al. 2012). MSCs can also inhibit the proliferation of inflammatory cytokine–stimulated NK cells by secreting human leukocyte antigen-G5 (HLA-G5) and transforming growth factor-β (TGF-β) (Najar et al. 2018).
Studies have found that in environments with high levels of pro-inflammatory factors, MSCs exhibit immunosuppressive effects, while in settings with low levels of pro-inflammatory factors, MSCs can exacerbate the inflammatory response (Li et al. 2012). SARS-CoV-2 may trigger a cytokine storm after invading the human body. After intravenous infusion of MSCs in patients with COVID-19, MSCs can regulate innate immune activity by secreting IDO, interleukin-1 receptor antagonist (IL-1RA), IL-6, HGF, and PGE2 to induce monocyte differentiation into M2-like macrophages, thereby reducing inflammation (Lu et al. 2022; Shi et al. 2018).
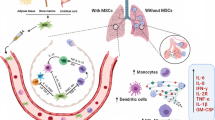
NK cells are the primary effector cells of innate immunity and play an important role in the antiviral response (Biron et al. 1999). When NK cells are co-cultured with MSCs, MSCs have a significant inhibitory effect on the proliferation of NK cells, while blocking the expression of IDO and PGE2 can almost completely restore the proliferation of NK cells (Spaggiari et al. 2008). After transplantation of MSCs in patients with severe COVID-19, MSCs may inhibit NK cell proliferation by expressing IDOs and PGE2 in the lungs, exerting immunosuppressive effects and alleviating excessive immune responses caused by the emergence of the body’s own cytokine storm Fig. 1.
Therapeutic role after intravenous infusion of MSCs in patients with COVID-19. The mechanism of MSCs in the treatment of lung injury caused by COVID-19 may be due to (1) MSCs being able to return to the lungs after transplantation and differentiate into alveolar epithelial cells to alleviate lung damage caused by apoptosis of alveolar epithelial cells. (2) MSCs can rebuild the damaged vascular network of the lungs by secreting VEGF and HGF. (3) MSCs can also induce the differentiation of monocytes into M2-like macrophages by secreting immune regulatory factors such as IDO, IL-1RA, IL-6, HGF, and PGE2 to reduce inflammation, inhibit NK cell proliferation by expressing IDO and PGE2, exert immunosuppressive effects, and reduce excessive immune responses caused by the body’s own cytokine storm. Created with Figdraw.
Mechanisms of MSC-Exo in the treatment of COVID-19
MSC-Exo has different mechanisms for treating patients with COVID-19 than MSCs. MSC-Exo prevents cytokine storms caused by excessive immune responses and promotes endogenous repair of damaged lungs (Hosseini et al. 2022).
MSC-Exo inhibits the high activity of complement and neutrophils
The body’s innate immune system is the first line of defense against viral invasion, and complements and neutrophils are important components of the innate immune system. Complement can directly or indirectly clear the virus by forming a membrane attack complex (MAC) C5b-9 and recruiting other white blood cells (Dunkelberger and Song 2010; Reis et al. 2019).
The nucleocapsid (nucleocapsid, N) protein of SARS-CoV-2 activates the complement system through MBL-associated serine protease 2 (MASP-2); however, overactivation of the complement can lead to endothelial damage and hypercoagulable states, worsening lung damage (Gao et al. 2020). Autopsies of patients with severe COVID-19 found that a large number of complement component (C3, C4, C5, MASP-2) deposits were seen in the patient’s lungs (Diao et al. 2021; Magro et al. 2020). Activated complements are potent chemotactic agents for neutrophils, and the activation complex C5b-9 through the complement terminal drives neutrophils to release Net and IL-17, which can lead to a highly inflammatory immune response in patients with severe COVID-19 (Loh et al. 2022; Price et al. 2015; Vandendriessche et al. 2021). MSC-Exo inhibits the formation of C5b-9 by expressing CD59 (Lai et al. 2013), thereby inhibiting complement-mediated neutrophil activation, inhibiting the amplification and persistence of inflammation during SARS-CoV-2 infection, reducing inflammation levels, and enabling patients with COVID-19 to obtain a good prognosis (Loh et al. 2022).
Antiviral activity of MSC-Exo
MSC-Exo has natural antiviral activity and is determined by endogenous substances mRNA and miRNA (Popowski et al. 2021; Qian et al. 2016). Harrell et al. found that several miRNAs (let-7f, miR-145, miR-199a, and miR-221) contained in UC-MSC-Exo can bind to hepatitis C virus RNA, preventing viral replication. In addition, UC-MSC-Exo has a synergistic effect when used in combination with interferon-α, significantly inhibiting hepatitis C virus replication, so it is considered a novel adjunctive treatment drug for the treatment of hepatitis C patients (Qian et al. 2016).
Khatri et al. found that intratracheal injection of MSC-Exo 12 h after influenza virus induction of acute lung injury in pigs can inhibit the replication of influenza virus and virus-induced apoptosis of lung epithelial cells and alleviate virus-induced pig lung lesions. It was revealed that this antiviral ability is manifested by the transport of mRNA and miRNAs in MSC-Exo to lung epithelial cells (Khatri et al. 2018) Fig. 2.
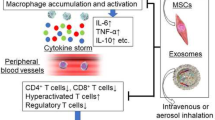
Therapeutic effects of MSC-Exo on COVID-19. The nucleocapsid protein of SARS-CoV-2 is able to activate the complement system through MASP-2; however, overactivation of complement can lead to endothelial cell damage. The mechanism by which MSC-Exo treats lung injury due to COVID-19. (1) MSC-Exo can inhibit complement-mediated neutrophil activation and reduce inflammation levels by expressing CD59. (2) MSC-Exo’s miRNAs can bind to the RNA of the SARS-CoV-2 virus, preventing the virus from replicating. Created with Figdraw.
Long-term efficacy of MSCs on COVID-19
Shi et al. found that in a 1-year follow-up study of patients with severe COVID-19 treated with UC-MSCs after discharge, none of the COVID-19 patients in the placebo group showed that the patient’s lungs were completely normal, and there were still some lesions in their lungs 1 year after recovery and discharge. In the UC-MSC treatment group, 17.9% of patients had normal lung CT images. From a 6-min walking distance (6-MWD) trial that examined the recovery of lung function and comprehensive reserve capacity in both groups, patients in the UC-MSC group had an increase in the value of the 6-MWD at each follow-up node compared with the placebo group (Shi et al. 2022). This may be related to the improvement of lung damage and the restoration of lung reserve capacity after the administration of UC-MSCs. This is the world’s first and most participatory long-term follow-up study of a clinical trial of MSCs for the treatment of patients with severe COVID-19, suggesting that treating patients with COVID-19 with MSCs may weaken and cure the sequelae after discharge compared with other conventional treatments.
Preprocessing of MSCs
In recent years, some researchers have tried to pretreat MSCs in vitro before transplantation, including genetic modification, hypoxia, and drug treatment (Hu et al. 2020; Li et al. 2021b; Zhao et al. 2019). This article mainly introduces the method of genetic modification of MSCs. Among them, the selection of the gene of interest is crucial to improving the therapeutic effect of MSCs.
Bone morphogenesis protein 2 (BMP-2) is one of the important factors in determining the directional differentiation of MSCs into osteoclasts, and the modification of MSCs by genetic modification methods to overexpress BMP-2 can promote bone regeneration, which can be used to treat diseases such as fractures and necrosis of the femoral head (He et al. 2013; Salazar et al. 2016; Tai et al. 2008). In addition, the CXCL12/CXCR4 axis plays an important role in the homing of MSCs to damaged bones (Lapidot and Kollet 2002), and Lien et al. found that modified CXCR4-MSCs have higher retention and homing capacity in the bone marrow than unmodified MSCs, suggesting that it is feasible to enhance the homing capacity of MSCs through genetic modification (Lien et al. 2009).
Antonitsis et al. found that MSCs can differentiate into cardiac lineage cells after treatment with 5-azacitidine, with the potential to treat cardiovascular disease (Antonitsis et al. 2008). Although pilot clinical trials using MSCs for the treatment of myocardial infarction have achieved good results (Gao et al. 2015), MSCs still have the disadvantages of poor retention and easy apoptosis. Caspase8 (Cas8) is closely related to apoptosis, and Liang et al. found that down-regulating the Cas8 gene through RNA interference can significantly improve the survival rate of MSCs and enhance the effect of MSCs in the treatment of myocardial infarction in rats (Liang et al. 2014b).
The exact mechanism by which genetically modified MSCs function in patients is unclear, including the number of modified MSCs that can differentiate into lung epithelial cells, biological distribution, and the survival time of differentiated cells. Until mechanism-related questions are answered, clinical trials of these genetically modified MSCs cannot be conducted. Therefore, there are no trials to improve the effectiveness of MSCs in the treatment of new crown pneumonia patients by genetically modifying MSCs, but the induction of MSCs into the lungs after injury by genetic modification may enhance the effect of MSCs in the treatment of lung injury in patients with COVID-19 to some extent, but this requires a series of clinical trials to prove.
Conclusion
Serious adverse reactions associated with MSC and MSC-Exo transplantation have not occurred in clinical trials of MSCs or MSC-Exo in the treatment of patients with COVID-19. Some of the clinical trials listed in Tables 1 and 2 have been completed, and the results have found that MSCs and MSC-Exo have a better effect on the treatment of COVID-19, which can reduce mortality in patients with severe COVID-19, improve lung function in patients, and reduce symptoms of dyspnea (ChiCTR2000031494, ChiCTR2000029990, NCT04252118, etc.). In terms of clinical safety and efficacy, MSCs and MSC-Exo meet the basic requirements of large-scale promotion and are feasible.
The first major challenge for MSCs and MSC-Exo to treat COVID-19 is the selection of standardized product production and clinical application protocols for MSCs and MSC-Exo. A large number of clinical-grade MSCs and MSC-Exo are required by patients, but their preparation is difficult and cumbersome, making them expensive. The standardization of MSCs or MSC-Exo clinical application regimens may also directly affect treatment effectiveness. The therapeutic properties of MSCs derived from different tissues, such as immunomodulatory and differentiation capacity, may differ. Therefore, it is necessary to screen the optimal plan from a large number of clinical trials, including the source, type, dosing regimen (including dose, interval, and number of cycles), and route of administration of transplanted MSCs. The second challenge is the purification of MSC and MSC-Exo products. This can be achieved using affinity purification of antibodies against MSC or MSC-Exo surface markers to obtain a purer, uniform product. The third challenge is the need for randomized controlled trials with long-term follow-ups to confirm the effectiveness of treatments with MSCs or MSC-Exo. It is believed that with the maturity of the technology of relevant biological product companies and the development of a large number of clinical trials, these challenges will be overcome one by one.
References
Adas G, Cukurova Z, Yasar KK, Yilmaz R, Isiksacan N, Kasapoglu P, Yesilbag Z, Koyuncu ID, Karaoz E (2021) The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transplant 30:9636897211024942
Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, Charokopos N, Kalogeridis A, Kouzi-Koliakou K, Kyriakopoulou I, Klonizakis I, Papakonstantinou C (2008) Cardiomyogenic potential of human adult bone marrow mesenchymal stem cells in vitro. Thorac Cardiovasc Surg 56:77–82
Bernardo ME, Locatelli F, Fibbe WE (2009) Mesenchymal stromal cells. Ann N Y Acad Sci 1176:101–117
Bestle D, Heindl MR, Limburg H, Van Lam van T, Pilgram O, Moulton H, Stein DA, Hardes K, Eickmann M, Dolnik O, Rohde C, Klenk HD, Garten W, Steinmetzer T, Bottcher-Friebertshauser E (2020) TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance 3
Bi L-k, Zhou N, Liu C, Lu F-D, Lin T-X, Xuan X-J, Jiang C, Han J-L, Huang H, Zhang C-X, Dong W, Liu H, Huang J, Xu K-W (2014) Kidney cancer cells secrete IL-8 to activate Akt and promote migration of mesenchymal stem cells. Urol Oncol 32:607–612
Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP (1999) Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol 17:189–220
Bloor AJC, Patel A, Griffin JE, Gilleece MH, Radia R, Yeung DT, Drier D, Larson LS, Uenishi GI, Hei D, Kelly K, Slukvin I, Rasko JEJ (2020) Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: a phase I, multicenter, open-label, dose-escalation study. Nat Med 26:1720–1725
Carfi A, Bernabei R, Landi F, Gemelli Against C-P-ACSG (2020) Persistent symptoms in patients after acute COVID-19. JAMA 324:603–605
Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M (2020) Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 20:1135–1140
Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, Xing F, Liu J, Yip CC-Y, Poon RW-S, Tsoi H-W, Lo SK-F, Chan K-H, Poon VK-M, Chan W-M, Ip JD, Cai J-P, Cheng VC-C, Chen H, Hui CK-M, Yuen K-Y (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet 395:514–523
Che N, Li X, Zhou S, Liu R, Shi D, Lu L, Sun L (2012) Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell Immunol 274:46–53
Chen J, Wu H, Yu Y, Tang N (2020a) Pulmonary alveolar regeneration in adult COVID-19 patients. Cell Res 30:708–710
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia Ja YuT, Zhang X, Zhang L (2020b) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 395:507–513
Chen ZM, Fu JF, Shu Q, Chen YH, Hua CZ, Li FB, Lin R, Tang LF, Wang TL, Wang W, Wang YS, Xu WZ, Yang ZH, Ye S, Yuan TM, Zhang CM, Zhang YY (2020c) Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr 16:240–246
Cheng VCC, Wong SC, Chen JHK, Yip CCY, Chuang VWM, Tsang OTY, Sridhar S, Chan JFW, Ho PL, Yuen KY (2020) Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol 41:493–498
Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP, Huang X, Peiris M, Yen HL (2020) Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res 178:104786
Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (2020) The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5:536–544
de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, Verillaud B, Aparicio C, Wagner S, Gheusi G, Kergoat L, Kornobis E, Donati F, Cokelaer T, Hervochon R, Madec Y, Roze E, Salmon D, Bourhy H, Lecuit M, Lledo P-M (2021) COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med 13
Devaux CA, Rolain JM, Raoult D (2020) ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect 53:425–435
Dhawan M, Priyanka PM, Angural S, Choudhary OP (2022) Convalescent plasma therapy against the emerging SARS-CoV-2 variants: delineation of the potentialities and risks. Int J Surg 97:106204
Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, Tan Y, Wang H, Wang C, Liu L, Liu Y, Liu Y, Wang G, Yuan Z, Hou X, Ren L, Wu Y, Chen Y (2021) Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun 12:2506
Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA, Mariana N, Antarianto RD, Liem IK, Kispa T, Mujadid F, Novialdi N, Luviah E, Kurniawati T, Lubis AMT, Rahmatika D (2021) Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med 10:1279–1287
DiMaio D, Enquist LW, Dermody TS (2020) A new coronavirus emerges, this time causing a pandemic. Annu Rev Virol 7:iii–v
Ding Y, Liang X, Zhang Y, Yi L, Shum HC, Chen Q, Chan BP, Fan H, Liu Z, Tergaonkar V, Qi Z, Tse HF, Lian Q (2018) Rap1 deficiency-provoked paracrine dysfunction impairs immunosuppressive potency of mesenchymal stem cells in allograft rejection of heart transplantation. Cell Death Dis 9:386
Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, Lange F, Andersson JLR, Griffanti L, Duff E, Jbabdi S, Taschler B, Keating P, Winkler AM, Collins R, Matthews PM, Allen N, Miller KL, Nichols TE, Smith SM (2022) SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature
Dunkelberger JR, Song W-C (2010) Complement and its role in innate and adaptive immune responses. Cell Res 20:34–50
Feng Y, Huang J, Wu J, Xu Y, Chen B, Jiang L, Xiang H, Peng Z, Wang X (2020) Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: a pilot study. Cell Prolif 53:e12947
Fonseca M, Summer R, Roman J (2021) Acute exacerbation of interstitial lung disease as a sequela of COVID-19 pneumonia. Am J Med Sci 361:126–129
Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL, Wang ZG, Yan XY, Wang Y, Zhu ZM, Li TC, Wang LH, Chen HY, Chen YD, Huang CL, Qu P, Yao C, Wang B, Chen GH, Wang ZM, Xu ZY, Bai J, Lu D, Shen YH, Guo F, Liu MY, Yang Y, Ding YC, Yang Y, Tian HT, Ding QA, Li LN, Yang XC, Hu X (2015) Intracoronary infusion of Wharton′s jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med 13:162
Gao T, Hu MC, Zhang X, Li H, Zhu L, Liu H, Dong Q, Zhang Z, Wang Z, Hu Y, Fu Y, Jin Y, Li K-d, Zhao S, Xiao Y, Luo S-p, Li L, Zhao L, Liu J, Zhao H, Liu Y, Yang W, Peng J, Chen X, Li P, Liu Y, Xie Y, Song J, Zhang L, Ma Q-j, Bian X-w, Chen W, Liu X, Mao Q, Cao C (2020) Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv
Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S, Galli M (2020) Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis 71:889–890
Grégoire C, Layios N, Lambermont B, Lechanteur C, Briquet A, Bettonville V, Baudoux E, Thys M, Dardenne N, Misset B, Beguin Y (2022) Bone marrow-derived mesenchymal stromal cell therapy in severe COVID-19: preliminary results of a phase I/II clinical trial. Front Immunol 13:932360
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for C (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720
Harrell CR, Sadikot R, Pascual J, Fellabaum C, Jankovic MG, Jovicic N, Djonov V, Arsenijevic N, Volarevic V (2019) Mesenchymal stem cell-based therapy of inflammatory lung diseases: current understanding and future perspectives. Stem Cells Int 2019:4236973
He X, Dziak R, Mao K, Genco R, Swihart M, Swithart M, Li C, Yang S (2013) Integration of a novel injectable nano calcium sulfate/alginate scaffold and BMP2 gene-modified mesenchymal stem cells for bone regeneration. Tissue Eng Part A 19:508–518
Heidari M, Pouya S, Baghaei K, Aghdaei HA, Namaki S, Zali MR, Hashemi SM (2018) The immunomodulatory effects of adipose-derived mesenchymal stem cells and mesenchymal stem cells-conditioned medium in chronic colitis. J Cell Physiol 233:8754–8766
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(271–280):e278
Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK, State W, -nCo VCIT, (2020) First case of 2019 novel coronavirus in the United States. N Engl J Med 382:929–936
Group RC, Horby P, Pessoa-Amorim G, Peto L, Brightling C, Sarkar R, Thomas K, Jeebun V, Ashish A, Tully R, Chadwick D, Sharafat M, Stewart R, Rudran B, Baillie K, Buch M, Chappell L, Day J, Furst S, Jaki T, Jeffery K, Juszczak E, Lim WS, Montgomery A, Mumford A, Rowan K, Thwaites G, Mafham M, Haynes R, Landray M (2021) Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv
Hosseini NF, Dalirfardouei R, Aliramaei MR, Najafi R (2022) Stem cells or their exosomes: which is preferred in COVID-19 treatment? Biotechnol Lett 44:159–177
Hu C, Wu Z, Li L (2020) Pre-treatments enhance the therapeutic effects of mesenchymal stem cells in liver diseases. J Cell Mol Med 24:40–49
Hu B, Guo H, Zhou P, Shi Z-L (2021a) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19:141–154
Hu Z, Yuan Y, Zhang X, Lu Y, Dong N, Jiang X, Xu J, Zheng D (2021b) Human umbilical cord mesenchymal stem cell-derived exosomes attenuate oxygen-glucose deprivation/reperfusion-induced microglial pyroptosis by promoting FOXO3a-dependent mitophagy. Oxid Med Cell Longev 2021:6219715
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B (2021a) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397:220–232
Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, Hu P, Guo L, Liu M, Xu J, Zhang X, Qu Y, Fan Y, Li X, Li C, Yu T, Xia J, Wei M, Chen L, Li Y, Xiao F, Liu D, Wang J, Wang X, Cao B (2021b) 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 398:747–758
Hui DS, Azhar I, E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, McHugh TD, Memish ZA, Drosten C, Zumla A, Petersen E, (2020) The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 91:264–266
Jafari D, Malih S, Eslami SS, Jafari R, Darzi L, Tarighi P, Samadikuchaksaraei A (2019) The relationship between molecular content of mesenchymal stem cells derived exosomes and their potentials: opening the way for exosomes based therapeutics. Biochimie 165:76–89
Jayaramayya K, Mahalaxmi I, Subramaniam MD, Raj N, Dayem AA, Lim KM, Kim SJ, An JY, Lee Y, Choi Y, Kirubhakaran A, Cho S-G, Vellingiri B (2020) Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem-cell-derived exosomes for COVID-19 treatment. BMB Rep 53:400–412
Jiang W, Xu J (2020) Immune modulation by mesenchymal stem cells. Cell Prolif 53:e12712
Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM (2020) Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci 21
Kabat M, Bobkov I, Kumar S, Grumet M (2020) Trends in mesenchymal stem cell clinical trials 2004–2018: is efficacy optimal in a narrow dose range? Stem Cells Transl Med 9:17–27
Kai H, Kai M (2020) Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens Res 43:648–654
Kampf G, Todt D, Pfaender S, Steinmann E (2020) Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 104:246–251
Karyana M, Djaharuddin I, Rif’ati L, Arif M, Choi MK, Angginy N, Yoon A, Han J, Josh F, Arlinda D, Narulita A, Muchtar F, Bakri RA, Irmansyah S (2022) Safety of DW-MSC infusion in patients with low clinical risk COVID-19 infection: a randomized, double-blind, placebo-controlled trial. Stem Cell Res Ther 13:134
Khatri M, Richardson LA, Meulia T (2018) Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther 9:17
Kinnaird T, Stabile E, Burnett MS, Epstein SE (2004a) Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ Res 95:354–363
Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE (2004b) Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109:1543–1549
Kresch E, Achua J, Saltzman R, Khodamoradi K, Arora H, Ibrahim E, Kryvenko ON, Almeida VW, Firdaus F, Hare JM, Ramasamy R (2021) COVID-19 endothelial dysfunction can cause erectile dysfunction: histopathological, immunohistochemical, and ultrastructural study of the human penis. World J Mens Health 39:466–469
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11:875–879
Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 55:105924
Lai RC, Yeo RWY, Tan SS, Zhang B, Yin Y, Sze NSK, Choo A, Lim SK (2013) Mesenchymal stem cell exosomes: the future MSC-based therapy? Mesenchymal Stem Cell Therapy, pp 39–61
Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, Alvarez Gil A, Poggioli R, Ruiz P, Marttos AC, Hirani K, Bell CA, Kusack H, Rafkin L, Baidal D, Pastewski A, Gawri K, Leñero C, Mantero AMA, Metalonis SW, Wang X, Roque L, Masters B, Kenyon NS, Ginzburg E, Xu X, Tan J, Caplan AI, Glassberg MK, Alejandro R, Ricordi C (2021) Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med 10:660–673
Lapidot T, Kollet O (2002) The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia 16:1992–2003
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC (2020) Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis 11:216–228
Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, Zhang L, Yuan ZR, Roberts AI, Shi S, Le AD, Shi Y (2012) Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ 19:1505–1513
Li X, Hong Y, He H, Jiang G, You W, Liang X, Fu Q, Han S, Lian Q, Zhang Y (2019) FGF21 mediates mesenchymal stem cell senescence via regulation of mitochondrial dynamics. Oxid Med Cell Longev 2019:4915149
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207
Li J, Liao X, Zhou Y, Wang L, Yang H, Zhang W, Zhang Z, Kang Y (2021a) Comparison of associations between glucocorticoids treatment and mortality in COVID-19 patients and SARS patients: a systematic review and meta-analysis. Shock 56:215–228
Li N, Guo X-Y, Zhou J, Yan X-L, Yu F-F (2021b) Atorvastatin pretreatment ameliorates mesenchymal stem cell migration through miR-146a/CXCR4 signaling. Tissue Eng Regen Med 18:863–873
Li C, Ye Z, Zhang AJ-X, Chan JF-W, Song W, Liu F, Chen Y, Kwan MY-W, Lee AC-Y, Zhao Y, Wong BH-Y, Yip CC-Y, Cai J-P, Lung DC, Sridhar S, Jin D, Chu H, To KK-W, Yuen K-Y (2022) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections by intranasal or testicular inoculation induces testicular damage preventable by vaccination in golden Syrian hamsters. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America
Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF (2010) Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 121:1113–1123
Lian Q, Zhang Y, Liang X, Gao F, Tse HF (2016) Directed differentiation of human-induced pluripotent stem cells to mesenchymal stem cells. Methods Mol Biol 1416:289–298
Liang X, Ding Y, Zhang Y, Tse H-F, Lian Q (2014a) Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant 23:1045–1059
Liang X, Ding Y, Lin F, Zhang Y, Zhou X, Meng Q, Lu X, Jiang G, Zhu H, Chen Y, Lian Q, Fan H, Liu Z (2019) Overexpression of ERBB4 rejuvenates aged mesenchymal stem cells and enhances angiogenesis via PI3K/AKT and MAPK/ERK pathways. Faseb j 33:4559–4570
Liang Y, Lin Q, Zhu J, Li X, Fu Y, Zou X, Liu X, Tan H, Deng C, Yu X, Shan Z, Yuan W (2014b) The caspase-8 shRNA-modified mesenchymal stem cells improve the function of infarcted heart. Mol Cell Biochem 397
Liao S, Zhang Y, Ting S, Zhen Z, Luo F, Zhu Z, Jiang Y, Sun S, Lai WH, Lian Q, Tse HF (2019) Potent immunomodulation and angiogenic effects of mesenchymal stem cells versus cardiomyocytes derived from pluripotent stem cells for treatment of heart failure. Stem Cell Res Ther 10:78
Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, Esteban I, Caballero MT, Wood C, Berrueta M, Rondan A, Lescano G, Cruz P, Ritou Y, Fernández Viña V, Álvarez Paggi D, Esperante S, Ferreti A, Ofman G, Ciganda Á, Rodriguez R, Lantos J, Valentini R, Itcovici N, Hintze A, Oyarvide ML, Etchegaray C, Neira A, Name I, Alfonso J, López Castelo R, Caruso G, Rapelius S, Alvez F, Etchenique F, Dimase F, Alvarez D, Aranda SS, Sánchez Yanotti C, De Luca J, Jares Baglivo S, Laudanno S, Nowogrodzki F, Larrea R, Silveyra M, Leberzstein G, Debonis A, Molinos J, González M, Perez E, Kreplak N, Pastor Argüello S, Gibbons L, Althabe F, Bergel E, Polack FP (2021) Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med 384:610–618
Lien C-Y, Chih-Yuan Ho K, Lee OK, Blunn GW, Su Y (2009) Restoration of bone mass and strength in glucocorticoid-treated mice by systemic transplantation of CXCR4 and cbfa-1 co-expressing mesenchymal stem cells. J Bone Miner Res 24:837–848
Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, Choe KW, Kang YM, Lee B, Park SJ (2020) Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci 35:e79
Litvinova LS, Shupletsova VV, Khaziakhmatova OG, Daminova AG, Kudryavtseva VL, Yurova KA, Malashchenko VV, Todosenko NM, Popova V, Litvinov RI, Korotkova EI, Sukhorukov GB, Gow AJ, Weissman D, Atochina-Vasserman EN, Khlusov IA (2022) Human mesenchymal stem cells as a carrier for a cell-mediated drug delivery. Front Bioeng Biotechnol 10:796111
Liu AR, Liu L, Chen S, Yang Y, Zhao HJ, Liu L, Guo FM, Lu XM, Qiu HB (2013) Activation of canonical wnt pathway promotes differentiation of mouse bone marrow-derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J Cell Physiol 228:1270–1283
Liu A, Chen S, Cai S, Dong L, Liu L, Yang Y, Guo F, Lu X, He H, Chen Q, Hu S, Qiu H (2014) Wnt5a through noncanonical Wnt/JNK or Wnt/PKC signaling contributes to the differentiation of mesenchymal stem cells into type II alveolar epithelial cells in vitro. PLoS ONE 9:e90229
Liu SC, Bamodu OA, Kuo KT, Fong IH, Lin CC, Yeh CT, Chen SG (2021) Adipose-derived stem cell induced-tissue repair or wound healing is mediated by the concomitant upregulation of miR-21 and miR-29b expression and activation of the AKT signaling pathway. Arch Biochem Biophys 705:108895
Loh JT, Zhang B, Teo JKH, Lai RC, Choo ABH, Lam K-P, Lim SK (2022) Mechanism for the attenuation of neutrophil and complement hyperactivity by MSC exosomes. Cytotherapy
Lu K, Geng S-T, Tang S, Yang H, Xiong W, Xu F, Yuan Q, Xiao X, Huang R, Liang H, Chen Z, Qian C, Li Y, Wang S (2022) Clinical efficacy and mechanism of mesenchymal stromal cells in treatment of COVID-19. Stem Cell Res Ther 13:61
Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J (2020) Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 220
Meng F, Xu R, Wang S, Xu Z, Zhang C, Li Y, Yang T, Shi L, Fu J, Jiang T, Huang L, Zhao P, Yuan X, Fan X, Zhang J-Y, Song J, Zhang D, Jiao Y, Liu L, Zhou C, Maeurer M, Zumla A, Shi M, Wang F-S (2020) Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther 5:172
Monsel A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, Nguekap Tchoumba O, Diehl JL, Demoule A, Annane D, Marois C, Demeret S, Weiss E, Voiriot G, Fartoukh M, Constantin JM, Mégarbane B, Plantefève G, Malard-Castagnet S, Burrel S, Rosenzwajg M, Tchitchek N, Boucher-Pillet H, Churlaud G, Cras A, Maheux C, Pezzana C, Diallo MH, Ropers J, Menasché P, Larghero J (2022) Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care 26:48
Najar M, Fayyad-Kazan M, Meuleman N, Bron D, Fayyad-Kazan H, Lagneaux L (2018) Immunological impact of Wharton’s Jelly mesenchymal stromal cells and natural killer cell co-culture. Mol Cell Biochem 447:111–124
Ni L, Wen Z, Hu X, Tang W, Wang H, Zhou L, Wu L, Wang H, Xu C, Xu X, Xiao Z, Li Z, Li C, Liu Y, Duan J, Chen C, Li D, Zhang R, Li J, Yi Y, Huang W, Chen Y, Zhao J, Zuo J, Weng J, Jiang H, Wang DW (2021) Effects of Shuanghuanglian oral liquids on patients with COVID-19: a randomized, open-label, parallel-controlled, multicenter clinical trial. Front Med 15:704–717
Organization WH (2023) WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/
Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG (2003) Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 100:8407–8411
Pastorino B, Touret F, Gilles M, de Lamballerie X, Charrel R (2020) Evaluation of heating and chemical protocols for inactivating SARS-CoV-2. bioRxiv
Popowski KD, Dinh P-UC, George A, Lutz H, Cheng K (2021) Exosome therapeutics for COVID-19 and respiratory viruses. View (Beijing) 20200186
Price PJR, Bánki Z, Scheideler A, Stoiber H, Verschoor A, Sutter G, Lehmann MH (2015) Complement component C5 recruits neutrophils in the absence of C3 during respiratory infection with modified vaccinia virus Ankara. J Immunol 194:1164–1168
Qi X, Ng KT, Lian Q, Li CX, Geng W, Ling CC, Yeung WH, Ma YY, Liu XB, Liu H, Liu J, Yang XX, Lo CM, Man K (2018) Glutathione peroxidase 3 delivered by hiPSC-MSCs ameliorated hepatic IR injury via Inhibition of hepatic senescence. Theranostics 8:212–222
Qian X, Xu C, Fang S, Zhao P, Wang Y, Liu H, Yuan W, Qi Z (2016) Exosomal microRNAs derived from umbilical mesenchymal stem cells inhibit hepatitis C virus infection. Stem Cells Transl Med 5:1190–1203
Reis ES, Mastellos DC, Hajishengallis G, Lambris JD (2019) New insights into the immune functions of complement. Nat Rev Immunol 19:503–516
Reiter J, Drummond S, Sammour I, Huang J, Florea V, Dornas P, Hare JM, Rodrigues CO, Young KC (2017) Stromal derived factor-1 mediates the lung regenerative effects of mesenchymal stem cells in a rodent model of bronchopulmonary dysplasia. Respir Res 18:137
Roobrouck VD, Ulloa-Montoya F, Verfaillie CM (2008) Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res 314:1937–1944
Russell CD, Millar JE, Baillie JK (2020) Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395:473–475
Sadeghi S, Soudi S, Shafiee A, Hashemi SM (2020) Mesenchymal stem cell therapies for COVID-19: Current status and mechanism of action. Life Sci 262:118493
Sadeghi B, Roshandel E, Pirsalehi A, Kazemi S, Sankanian G, Majidi M, Salimi M, Aghdami N, Sadrosadat H, Samadi Kochaksaraei S, Alaeddini F, Ringden O, Hajifathali A (2021) Conquering the cytokine storm in COVID-19-induced ARDS using placenta-derived decidua stromal cells. J Cell Mol Med 25:10554–10564
Salazar VS, Gamer LW, Rosen V (2016) BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol 12:203–221
Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N (2020) Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev 29:747–754
Shang L, Zhao J, Hu Y, Du R, Cao B (2020) On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 395:683–684
Shi C, Lv T, Xiang Z, Sun Z, Qian W, Han X (2015) Role of Wnt/β-catenin signaling in epithelial differentiation of lung resident mesenchymal stem cells. J Cell Biochem 116:1532–1539
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y (2018) Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 14:493–507
Shi J, Zhang W, Sang L, Qu Z, Zhong M, Jiang L, Song B, Kang L, Zhang Y, Wang X, Zhang D, Zheng X (2020) Coagulation dysfunction in ICU patients with coronavirus disease 2019 in Wuhan, China: a retrospective observational study of 75 fatal cases. Aging (albany NY) 13:1591–1607
Shi L, Huang H, Lu X, Yan X, Jiang X, Xu R, Wang S, Zhang C, Yuan X, Xu Z, Huang L, Fu JL, Li Y, Zhang Y, Yao WQ, Liu T, Song J, Sun L, Yang F, Zhang X, Zhang B, Shi M, Meng F, Song Y, Yu Y, Wen J, Li Q, Mao Q, Maeurer M, Zumla A, Yao C, Xie WF, Wang FS (2021a) Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther 6:58
Shi L, Wang L, Xu R, Zhang C, Xie Y, Liu K, Li T, Hu W, Zhen C, Wang F-S (2021b) Mesenchymal stem cell therapy for severe COVID-19. Signal Transduct Target Ther 6:339
Shi MM, Yang QY, Monsel A, Yan JY, Dai CX, Zhao JY, Shi GC, Zhou M, Zhu XM, Li SK, Li P, Wang J, Li M, Lei JG, Xu D, Zhu YG, Qu JM (2021c) Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J Extracell Vesicles 10:e12134
Shi L, Yuan X, Yao W, Wang S, Zhang C, Zhang B, Song J, Huang L, Xu Z, Fu J-L, Li Y, Xu R, Li T-T, Dong J, Cai J, Li G, Xie Y, Shi M, Li Y, Zhang Y, Xie W-F, Wang F-S (2022) Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine 75:103789
Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, Ji N, Zheng Y, Chen X, Shi L, Wu M, Deng K, Wei J, Wang X, Cao Y, Yan J, Feng G (2020) Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther 11:361
Soetjahjo B, Malueka RG, Nurudhin A, Purwoko S, Wisaksana R, Adhiputri A, Sudadi SAY, Sidharta BRA, Thobari JA, Murni TW, Soewondo W, Herningtyas EH, Sudjud RW, Trisnawati I, Ananda NR, Faried A (2023) Effectiveness and safety of normoxic allogenic umbilical cord mesenchymal stem cells administered as adjunctive treatment in patients with severe COVID-19. Sci Rep 13:12520
Song N, Scholtemeijer M, Shah K (2020) Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci 41:653–664
Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L (2008) Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111:1327–1333
Stern A, Skalsky K, Avni T, Carrara E, Leibovici L, Paul M (2017) Corticosteroids for pneumonia. Cochrane Database Syst Rev 12:CD007720
Sungnak W, Huang N, Becavin C, Berg M, Queen R, Litvinukova M, Talavera-Lopez C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL, Network HCALB (2020) SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26:681–687
Tai K, Pelled G, Sheyn D, Bershteyn A, Han L, Kallai I, Zilberman Y, Ortiz C, Gazit D (2008) Nanobiomechanics of repair bone regenerated by genetically modified mesenchymal stem cells. Tissue Eng Part A 14:1709–1720
Teng XB, Shen Y, Han MF, Yang G, Zha L, Shi JF (2021) The value of high-flow nasal cannula oxygen therapy in treating novel coronavirus pneumonia. Eur J Clin Invest 51:e13435
Uccelli A, de Rosbo NK (2015) The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann N Y Acad Sci 1351:114–126
Ullah I, Subbarao RB, Rho GJ (2015) Human mesenchymal stem cells - current trends and future prospective. Biosci Rep 35
Uzunova K, Filipova E, Pavlova V, Vekov T (2020) Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed Pharmacother 131:110668
Vandendriessche S, Cambier S, Proost P, Marques PE (2021) Complement receptors and their role in leukocyte recruitment and phagocytosis. Front Cell Dev Biol 9:624025
Wan KH, Lui GCY, Poon KCF, Ng SSS, Young AL, Hui DSC, Tham CCY, Chan PKS, Pang CP, Chong KKL (2022) Ocular surface disturbance in patients after acute COVID-19. Clin Exp Ophthalmol
Wang R, Zagariya A, Ang E, Ibarra-Sunga O, Uhal BD (1999) Fas-induced apoptosis of alveolar epithelial cells requires ANG II generation and receptor interaction. Am J Physiol 277:L1245–L1250
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z (2020a) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323:1061–1069
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020b) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271
Wang S, Yao X, Ma S, Ping Y, Fan Y, Sun S, He Z, Shi Y, Sun L, Xiao S, Song M, Cai J, Li J, Tang R, Zhao L, Wang C, Wang Q, Zhao L, Hu H, Liu X, Sun G, Chen L, Pan G, Chen H, Li Q, Zhang P, Xu Y, Feng H, Zhao GG, Wen T, Yang Y, Huang X, Li W, Liu Z, Wang H, Wu H, Hu B, Ren Y, Zhou Q, Qu J, Zhang W, Liu GH, Bian XW (2021) A single-cell transcriptomic landscape of the lungs of patients with COVID-19. Nat Cell Biol 23:1314–1328
Weiss ARR, Dahlke MH (2019) Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol 10:1191
Xie Y, Xu E, Bowe B, Al-Aly Z (2022) Long-term cardiovascular outcomes of COVID-19. Nat Med 28:583–590
Xie Y, Al-Aly Z (2022) Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol
Xin Y, Wang YM, Zhang H, Li J, Wang W, Wei YJ, Hu SS (2010) Aging adversely impacts biological properties of human bone marrow-derived mesenchymal stem cells: implications for tissue engineering heart valve construction. Artif Organs 34:215–222
Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H (2020a) Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 117:10970–10975
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang F-S (2020b) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8:420–422
Xu X, Jiang W, Chen L, Xu Z, Zhang Q, Zhu M, Ye P, Li H, Yu L, Zhou X, Zhou C, Chen X, Zheng X, Xu K, Cai H, Zheng S, Jiang W, Wu X, Li D, Chen L, Luo Q, Wang Y, Qu J, Li Y, Zheng W, Jiang Y, Tang L, Xiang C, Li L (2021) Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clin Transl Med 11:e297
Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML (2010) Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant 19:667–679
Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367:1444–1448
Yang M, Wen T, Chen H, Deng J, Yang C, Zhang Z (2018) Knockdown of insulin-like growth factor 1 exerts a protective effect on hypoxic injury of aged BM-MSCs: role of autophagy. Stem Cell Res Ther 9:284
Yang Q, Liu Q, Xu H, Lu H, Liu S, Li H (2020) Imaging of coronavirus disease 2019: a Chinese expert consensus statement. Eur J Radiol 127:109008
Yu M, Liu W, Li J, Lu J, Lu H, Jia W, Liu F (2020) Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther 11:350
Zhang Z, Zhao C, Liu B, Liang D, Qin X, Li X, Zhang R, Li C, Wang H, Sun D, Cao F (2014) Inositol pyrophosphates mediate the effects of aging on bone marrow mesenchymal stem cells by inhibiting Akt signaling. Stem Cell Res Ther 5:33
Zhang Y, Chiu S, Liang X, Gao F, Zhang Z, Liao S, Liang Y, Chai YH, Low DJ, Tse HF, Tergaonkar V, Lian Q (2015) Rap1-mediated nuclear factor-kappaB (NF-κB) activity regulates the paracrine capacity of mesenchymal stem cells in heart repair following infarction. Cell Death Discov 1:15007
Zhao F, Liu W, Yue S, Yang L, Hua Q, Zhou Y, Cheng H, Luo Z, Tang S (2019) Pretreatment with G-CSF could enhance the antifibrotic effect of BM-MSCs on pulmonary fibrosis. Stem Cells Int 2019:1726743
Zhou Y, Yamamoto Y, Xiao Z, Ochiya T (2019) The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J Clin Med 8
Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, Lu J, Xue Y (2020) Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect 81:e21–e23
Zhu R, Yan T, Feng Y, Liu Y, Cao H, Peng G, Yang Y, Xu Z, Liu J, Hou W, Wang X, Li Z, Deng L, Wang S, Li J, Han Q, Li H, Shan G, Cao Y, An X, Yan J, Zhang Z, Li H, Qu X, Zhu J, Zhou S, Wang J, Zhang F, Gao J, Jin R, Xu D, Ma YQ, Huang T, Peng S, Zheng Z, Stambler I, Gilson E, Lim LW, Moskalev A, Cano A, Chakrabarti S, Ulfhake B, Su H, Xu H, Xu S, Wei F, Brown-Borg HM, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC (2021) Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res 31:1244–1262
Zimmerli A, Monti M, Fenwick C, Eckerle I, Beigelman-Aubry C, Pellaton C, Jaton K, Dumas D, Stamm GM, Infanti L, Andreu-Ullrich H, Germann D, Mean M, Vollenweider P, Stadelmann R, Prella M, Comte D, Guery B, Gachoud D, Rufer N (2021) Case report: stepwise anti-inflammatory and anti-SARS-CoV-2 effects following convalescent plasma therapy with full clinical recovery. Front Immunol 12:613502
Funding
This work was financially supported by the Fundamental Research Funds for the Central Universities (FRF-TP-22-007A1).
Author information
Authors and Affiliations
Contributions
LPL: conceptualization, methodology, software, investigation, data curation, writing (original draft preparation), and drawing. XSZ: conceptualization, methodology, and drawing. YWW: drawing. CCX: supervision, investigation, and writing. HWD: supervision, conceptualization, and methodology.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Informed consent
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Zhang, X., Wu, Y. et al. Challenges of mesenchymal stem cells in the clinical treatment of COVID-19. Cell Tissue Res 396, 293–312 (2024). https://doi.org/10.1007/s00441-024-03881-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-024-03881-y