Abstract
The present study reports the feasibility and successful production of rabbit cG-CAOMECS, designed to reconstruct corneal epithelium of patients with bilateral limbal stem cell deficiency. To produce a safe, chemically defined and FDA compliant cG-CAOMECS, oral mucosal epithelial cells were isolated from a biopsy of rabbit buccal tissue and seeded on a cGMP-certified cell culture surface coated with GMP-grade extracellular matrix. A newly designed clinical-grade medium (KaFa™ medium) was utilized to carry out cell expansion. Detachment and harvesting of the produced cell sheet was accomplished using collagenase treatment. Live cell imaging and morphological analysis techniques were used to examine cell growth. Cells attached onto the surface and self-assembled into colony-forming units (CFUs). Microscopic examination showed that CFUs formed during the first 5 days, and basal monolayer cell sheet formed in less than 10 days. Cells expanded to form a multilayered epithelial cell sheet that was harvested after 17–19 days in culture. Immunostaining and Western blot analyses showed that deltaNp63 was expressed in the basal cells and K3/K12 was expressed in the apical cells, indicating the presence of corneal epithelial-like cells in the produced cell sheet. Adhesion molecules, E-cadherin, beta-catenin, and Cnx43 were also expressed and exhibited the epithelial integrity of the cell sheet. The expression of integrin-beta1 and beta4 confirmed that the collagenase treatment used for detaching and harvesting the cell sheet did not have adverse effects. Our results showed that the utilization of clinical-grade and FDA-approved reagents successfully supported the production of cG-CAMECS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment of corneal surface with oral mucosal epithelial stem cells was initially reported in the twentieth century (Gipson et al. 1986) (Denig 1912) (Ballen 1963). These studies documented the possibility of reconstructing corneal surface with epithelial cells from a non-ocular surface. In 2003, Dr. Kinoshita’s group reported the expansion and engineering of oral mucosal epithelial cell sheet using cell culture (Nakamura et al. 2003). Several other groups reported the use of oral mucosal epithelial cell sheet to reconstruct corneal surface and treat corneal diseases (Hayashida et al. 2005) (Nakamura et al. 2003) (Kinoshita et al. 2004) (Higa and Shimazaki 2008) (Nishida et al. 2004) (Burillon et al. 2012) (Ma et al. 2009). The therapeutic application of oral mucosal epithelial cells is well documented in reconstructing the corneal surface. Oral mucosal epithelial stem cells are somatic stem cells distributed all over the buccal tissue. Upon cultivation and grafting, the cells mimic the corneal epithelium as they maintain their stemness at the ectopic site, and replace the lost corneal epithelial cells of the host with limbal stem cell deficiency (LSCD). The cells in the resulting differentiated cell sheet also express corneal-specific progenitor cell markers such as deltaNp63, as well as others corneal epithelium markers such as cytokeratins CK3 and K12 (Bardag-Gorce, et al. 2015) (Hassan NT and AbdelAziz 2018) (Hirayama et al. 2012). The regenerative spectrum of cultured oral mucosal epithelial cell sheet (CAOMECS) is not limited to reconstruct corneal surface. CAOMECS can be used in various epithelial disorders, such as severe burns (Lee et al. 2018) (Gallico et al. 1984), giant congenital nevi (Gallico et al. 1989), and esophagus regeneration (Yamaguchi et al. 2017).
However, with all the above-listed successful literature reporting the therapeutic role that oral mucosal epithelial cell sheets offer, cell culture methods used to produce these cell sheets are to date utilizing products of animal origin. Xenogeneic products present risks of immunologic rejection and potential introduction of infections across species barriers (Roumiana et al. 2001). The inherent possibility of infection or unknown pathogen transmission from animal-derived materials and reagents needs to be addressed.
The biggest challenge that cell sheet technology is facing is to produce a multilayered epithelium-like cell sheet without the use of fetal bovine serum and murine 3T3 fibroblasts feeder cells. The use of murine feeder cells and animal-derived products is a major concern in regenerative medicine. These xenogeneic products can cause pathogen transmissions and immune reactions in humans (Uthei et al. 2016). Adhering to FDA regulations of xeno-free cell culture conditions for the isolation and expansion of OMECS without any feeder cells, without fetal bovine serum, and with a clinical-grade medium will make the clinical application of oral mucosal epithelial cell sheet safe and transplantable for human use. Existing cell culture media contain some form or derivative of xenogeneic products that are not approved by US regulatory agencies for human clinical applications. Cholera toxin, animal-derived cytokines, and digestive enzymes are examples of such reagents that need to be phased out and replaced with human-derived equivalents. Furthermore, recombinant proteins and growth factors used to produce multilayered oral mucosal epithelium cell sheets need to be manufactured in a cGMP facility and certified for clinical use. The entire process including reagents, consumables, and cultureware needs to be cGMP-certified for clinical application with regard to enhancing the efficacy of the graft pertaining to the patient’s safety.
Our present study reports the successful production of rabbit multilayered oral mucosal cell sheet without the use of murine feeder cells and without fetal bovine serum. Only FDA-approved GMP-grade cell culture reagents were utilized. Cell sheets were cultured with a newly designed clinical-grade medium (KaFa™ medium), and seeded on a GMP-grade extracellular matrix, using a cGMP-grade cell cultureware. GMP-grade collagenase was used to detach and harvest the cell sheet. Cell sheet production was deemed successful when a multilayered cell sheet was achieved. Confluent monolayer of cells formed in about a week to 10 days, and colony-forming units were visible in the monolayer, which eventually disappeared to form a multilayered cell sheet. Differentiation into corneal epithelial-like cells was investigated, as the future goal is to reconstruct corneal surface of patients with limbal stem cell deficiency. Basal progenitor cells were detected as well as corneal epithelial cell markers. The most promising source of cells not derived from eye tissue, but nonetheless able to function as a replacement to corneal stem cells, is the oral mucosal epithelial cells. Clinical-grade cultured autologous oral mucosal epithelial cell sheet (cG-CAOMECS) technology can be the solution for bilateral total loss of limbal stem cells as it poses no problem of immunosuppression and graft failure. Moreover, oral mucosal epithelium can be easily harvested with a small biopsy. The derived epithelial cells proliferate relatively rapidly as this population of cells is rich in basal regenerative cells/epithelial progenitor cells (Jones and Klein 2013).
The present study reports for the first time, the successful production of a multilayered oral mucosal epithelial cell sheet, cG-CAOMECS, using clinical-grade medium, without a feeder and with cGMP-certified cell cultureware and reagents. Our designed clinical-grade cell culture conditions successfully produced differentiated corneal epithelial cell sheets that satisfy current cGMP regulations and qualify for safe grafting onto corneal surface of patients with limbal stem cell deficiency.
Material and methods
Animals
New Zealand white rabbits weighing between 2.5 and 3 kg were used. The animals were maintained according to the Guidelines of Animal Care, as described by the National Academy of Sciences and published by the Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council. Buccal tissue biopsy was performed on healthy control rabbits from an existing protocol, approved by the IACUC. The rabbits used for the oral biopsy were deceased from surgical complications (sacrificed for a different experiment). This adopted approach prevents animal purchase and sacrifice, just for securing a small buccal biopsy.
Oral mucosa buccal biopsy
A buccal biopsy was performed on the oral cavity of the rabbits after disinfection using diluted povidone-iodine. After sanitization of the oral cavity, buccal mucosa biopsy was performed below the occlusal plane in the back of the cheek. The progenitor cells are more concentrated in this area. The mucosa was grasped with tissue forceps, and tissue piece was removed using dissecting scissors. The biopsied tissue was transported in cell culture basal medium at 4 °C from the vivarium to cell culture room for processing.
Feeder cell preparation
The effect of co-culture with feeder cells was investigated on the generation of CAOMECS by using two types of feeder cells. The two types of feeder cells used for comparison were the cell line NIH3T3 mouse embryonic fibroblasts (ATCC) and the FibroGRO human fibroblasts (Millipore, St. Louis, MO). Both the types of feeder cells were mitotically inactivated with Mitomycin C (Bardag-Gorce et al. 2013). Both the types of feeder cells were plated 24 h before OMECS seeding.
Oral mucosal epithelial cell isolation and cell culture
Tissue biopsy was washed in sterile saline, placed in 5% povidone-iodine solution for 1 min, washed again in sterile saline, and then in cell culture medium KODMEMF12. The tissue biopsy was cut into smaller pieces after being cleaned from any excess of fat and muscle tissue. Tissue pieces were incubated with Dispase® I (neutral protease, grade I. Roche Diagnostics GmbH, Mannheim, Germany) for 1 h at 37 °C, which allowed the epithelium to be separated from the lamina propria. Next, these epithelium pieces were subjected to trypsin digestion to extract and isolate the oral mucosal epithelial cells. Trypsin digestion was inactivated using our newly designed clinical-grade KaFa™ medium. Isolated cells were re-suspended in a small volume of KaFa™ medium and were seeded at a density of 0.3–0.5 × 105cells/cm2 on GMP-certified 6-multiwell plate (6MWP) previously coated with GMP-grade extracellular matrix substrate CELLstart™.
The latest is a defined xeno-free substrate for stem cell culture because it contains only components of human origin (xeno-free): (https://www.thermofisher.com/order/catalog/product/A1014201?SID=srch-hj-A1014201#/A1014201?SID=srch-hj-A1014201). CELLstart™ is cGMP-manufactured to help ensure manufacturability and traceability.
The cells were cultured for about 3 weeks at 37 °C in a humidified atmosphere containing 5% CO2. Cell culture medium change was scheduled for every other day in the first week, and every day in the last 2 weeks. After a multilayered cell sheet was seen under the microscope, harvesting was performed using collagenase treatment at 0.5 mg/mL final concentration (Collagenase NB-6; Amsbio, Cambridge, MA https://www.nordmark-pharma.de/biochemicals/products/gmp-collagenase/collagenase-nb-6-gmp-grade). Collagenase originated from Clostridium histolyticum and is certified by the supplier to be GMP-grade and qualifies as ancillary product suitable for clinical application by FDA.
Immunohistochemistry
Harvested cell sheets were fixed using 10% neutral buffered formalin and histologically processed via dehydration to be finally paraffin embedded. Cross sections of cell sheet were cut and tissue sections were stained for morphological analysis. Tissue sections were immunofluorescent-stained using DeltaNp63 (Biocare Medical, Concord, CA), PCNA (DAKO, Carpinteria, CA), K3 (ImmuQuest, Cleveland, UK), K4 and K12 (Santa Cruz Inc., Santa Cruz CA), E-cadherin, and beta-catenin (BD bioscience, San Jose, CA) to examine the expression levels of junctional complexes. Alexa Fluor® 488 and Alexa Fluo® 568 donkey anti-rabbit/mouse/goat fluorophore conjugated secondary antibodies (Santa Cruz Biotechnology) were used. DAPI or propidium iodide (Invitrogen, Eugene, OR) was used for nuclear staining of the cell nuclei. A Nikon 400 fluorescent microscope was used to analyze the slides. The images were analyzed and processed using Adobe Photoshop CS5.
Western blot analysis
The harvested cell sheet was homogenized in PBS and the protein concentration was measured. Two micrograms of total protein from sample homogenates was separated by SDS-PAGE gels and transferred to a PVDF membrane (Bio-Rad, Hercules, CA) for 1 h in 25 mM Tris-HC1 (pH = 8.3), 192 mM glycine, and 20% methanol. Membranes were probed with primary antibodies against deltaNp63, K3, K12, E-cadherin, beta-catenin, Cnx43 (Abcam, Cambridge, MA), and beta-actin (Millipore, St. Louis, MO). The levels of expression of phosphorylated focal adhesion kinase (FAK from Millipore, St. Louis, MO) as well as integrins beta1 and beta4 (R&D system Minneapolis, MN) were measured.
Statistics
Statistical data was calculated using at least three separate set of experiments. Bars represent mean values ± SEM. P values are determined by one-way ANOVA and Student-Newman Keuls for multiple group comparisons (Sigma-Stat softdish, San Francisco, CA). Statistical significance is set at p = or < 0.05. Bar graphs were shown as mean ± SEM, n = 12–15.
Results
Oral mucosal epithelial cell isolation and seeding overview
Safety of cell culture products remains the most important criterion for translational applications. For human translational studies, the FDA regulations encourage the use of xeno-free cell culture conditions to minimize the risk of transmitting pathogens or causing human immune reactions. The goal of this study was to isolate oral mucosal epithelial cells (OMECS) from a buccal biopsy and expand them under clinical-grade condition for use in future translational applications.
In our previous study (Bardag-Gorce et al. 2015), we successfully reconstructed the ocular surface of rabbit with limbal stem cell deficiency by grafting CAOMECS. In order to take CAOMECS to the bedside, our lab took the challenge to produce CAOMECS with reagents that are approved by the FDA. The rabbit oral mucosal epithelial cells were isolated from a biopsy of buccal tissue. The harvested buccal tissue biopsies were transported to the biological safety cabinet to be rinsed, cleaned from connective tissue, and minced to be subjected to enzymatic digestion for cell isolation. The isolated cells were seeded right after cell counting and cell viability check. This procedure is a routine experiment in our lab yielding isolated cells with high survival rate. Cells are then cultured at 37 °C in 5% CO2 in a high-humidity environment to grow a multilayered cell sheet.
Multiple experiments were conducted to optimize the cell culture medium—KaFa medium. Our goal was to develop a medium that will fully support the production of cG-CAOMECS—a multilayered cell sheet without feeder cells, without fetal bovine serum, without Cholera toxin, and without animal-derived cytokines and growth factors. The reagents employed to prepare KaFa medium were carefully chosen to ensure that each component was certified for clinical application.
Another factor that plays a major role in cell attachment and cell growth is the extracellular matrix (ECM) substrate. Currently, the only available ECM substrates that are GMP-grade and certified for clinical use are CELLstart™ and Laminins. KaFa™ medium composition was formulated and proved efficient in maintaining rabbit OMECS attachment and growth when used with CELLstart™ as the matrix (Fig. 1). CELLstartTM CS is commercially available, xeno-free, chemically defined, and cGMP-produced (Sorkio et al. 2014). Cell culture surfaces were also investigated by testing several clinical-grade certified cell culture surfaces. Certified GMP-grade 6-multiwell plate (6MWP) were pre-coated with CELLstart™ before OMECS seeding.
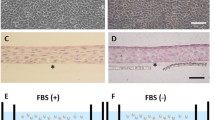
Rabbit oral mucosal epithelial cell sheets cG-CAOMECS. a, b, and c are the same cell sheet pictured respectively at D5 and D17 of culture and after harvesting. d, e, and f are the same cell sheet pictured respectively at D9 and D18 of culture and after harvesting. g, h, and I are the same cell sheet examined respectively at D10 and D17 of culture and after harvesting. These three cell sheets were produced with KaFa medium on 6MWP coated with CELLstart™ and harvested on days 17, 18, and 17, respectively. Colony-forming units (CFU) were visible in less than day 5 (arrow). Detachment of cell sheet was accomplished using collagenase treatment. KaFa medium supported cell growth with homogeneous typical epithelial polygonal morphology. j, k, and l are the positive control cell sheet grown with animal origin medium (AOM) and harvested on D11 and D16, respectively. Magnification is 5 ×
After cell seeding at a density of 3 to 5 × 105, cell culture medium was changed every 2 days during the initial week, and every day henceforth until the cell sheet was completely formed. Live cell imaging and visual morphological analysis were used to examine cell growth, cell morphology, and cell sheet formation. It was shown that the cells attached onto the surface and self-assembled into colony-forming units (CFUs). These CFUs formed during the first 5 days (Fig. 1a), and a monolayer cell sheet formed in less than 10 days (Fig. 1b and c). These cultured cells differentiated and formed a multilayered epithelial cell sheet that was harvested after 17–19 days (Fig. 1d–i).
Harvest of a multilayered cell sheet, cG-CAOMECS
Once the microscopic examination showed fully grown and tissue-like multilayered cell sheet features, collagenase solution was incubated with cG-CAOMECS at 37 °C for 45 min to harvest the cell sheet. Collagenase is considered a cell culture ancillary product that is rinsed off before cG-CAOMECS grafting. It is a clinical-grade certified product and was used to hydrolyze collagen molecules that attached basal cells to the ECM substrate (Rovere et al. 2018).
The harvested cell sheets were processed and paraffin embedded for immunofluorescent staining and analysis. Figure 2 shows that DeltaNp63 was expressed in the basal cells nucleus of cell sheet, indicating the positive expression of progenitor cells (Fig. 2a). PCNA staining performed also indicated the level of proliferative capacity of the cells and showed that in yellow (superposition of red and green), the nucleus basal and supra-basal cells were positive for PCNA, indicating that cells were entering S phase of cell cycle (Fig. 2b).
Figure 2c shows that cell sheet stained positive for K3, a corneal epithelial cell–specific keratin that pairs with K12 to form keratin filaments specific to corneal epithelial cells. These results indicated that KaFa™ medium and our cell culture conditions successfully differentiated oral mucosal epithelial cells into corneal epithelial cells. Figure 2d shows that few apical cells were still positive for K4, an oral mucosal epithelial cell–specific keratin that pairs with K13
The results from the immunofluorescent staining showed that KaFaTM medium was efficient in fully supporting the growth of the multilayered cG-CAOMECS. It also showed that the produced cG-CAOMECS included the epithelial polarity reflected by the existence of basal cell responsible for cell renewal and apical squamous cells positive for mature and differentiation markers such as the keratins.
Comparative analysis
In the last decade, cell sheets were produced with feeder cells, fetal bovine serum, and animal-derived cytokines and growth factors. These cell sheets were used as a positive control for the newly produced cG-CAOMECS. In addition, in order to examine the impact of cell culture media, we compared cG-CAOMECS grown with KaFa™ medium to those grown with AOM (Animal Origin Media), with and without mitotically inactivated feeder cells (NIH3T3 and/or HuFFs—human foreskin fibroblasts).
Quantitative experiments were conducted to measure the levels of DeltaNp63, and K3 expression, comparing cell sheet grown with KaFa™ medium to those grown with AOM containing FBS (fetal bovine serum) and animal origin reagents. Results showed a similar positive detection of DeltaNp63 in all conditions (Fig. 3a), indicating the preserved expression of progenitor cell markers in the cell sheets. cG-CAOMECS stained positive for DeltaNp63, similar to cell sheets grown with AOM and feeder, indicating that KaFa™ medium alone perfectly maintained the renewal capacity of the basal cells in the cell sheet.
Cell sheets grown on CELLstart™ ECM substrate and with KaFa™ medium showed similar levels of expression of progenitor cells when compared to cell sheets grown with AOM and feeder cells (Fig. 3a).
These results confirmed our hypothesis that a comprehensively designed compositionally complete chemically defined cell culture medium can support the production of an epithelium-like and multilayered cG-CAOMECS without the use of feeder cells or fetal bovine serum or any other animal origin supplements.
The results also showed that cell sheet grown with KaFa™ medium similarly expressed K3 (Fig. 3b) as compared to cell sheet grown with AOM and feeder cells. K3 expression was significantly higher in KaFa™ cell sheets compared to those in AOM cell sheets (Fig. 3b). K3 positive expression indicated that the produced cell sheet with KaFa™ had differentiated into corneal epithelial-like cells. The expression of K12 (Fig. 3c) was higher in KaFa™ than in the other conditions but the difference was insignificant.
Junctional complexes were also investigated to examine the epithelial integrity of cG-CAOMECS. The levels of E-cadherin, beta-catenin, and Cnx43 expression were measured, and results showed that E-cadherin was significantly high in cell sheets produced with KaFa™ medium compared to those grown on feeder cells or with AOM alone (Fig. 3d). E-cadherin recruits beta-catenin in downstream signaling to promote cell adhesion and epithelial integrity. Figure 3e shows that total beta-catenin is low in cell sheets grown with KaFa™ medium in comparison to cells grown with AOM and feeder cells with no significant difference. However, similar to E-cadherin, Cnx-43—a gap junction protein—was significantly high in cell sheets grown with KaFa™ medium (Fig. 3f) compared to all other conditions, indicating a strong adhesion and integrity of the epithelium-like tissue of cG-CAOMECS.
Analysis of effect of collagenase during cG-CAOMECS harvesting
Every time AOM was used, the need to use collagenase to detach the cell sheet did not arise. A pre-cut PVDF ring membrane and a plastic spatula to gently manipulate and mechanically detach and lift the cell sheet was utilized. When KaFa™ medium was used, it was not possible to manually detach an intact cell sheet from any surface of cell culture. Collagenase treatment had to be used to release cG-CAOMECS from the surface of cell culture dish. The expression levels of extracellular matrix components in cG-CAOMECS produced with KaFa™ medium were then compared to those in cell sheets produced with AOM. Specifically, the effects of collagenase on the extracellular matrix of cell sheets grown with KaFa™ medium was investigated.
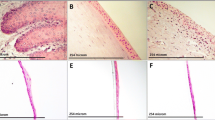
Figure 4a shows a cell sheet produced with KaFa™ medium and treated with collagenase for less than 1 h at 37 °C. The edges of the cell sheet were free and detached. Pre-cut PVDF membrane was used to fold over the free edges and gently lift the cell sheet (Fig. 4b). In the final step, the cell sheet was released from the PVDF membrane (Fig. 4c) to be processed for immunofluorescent staining and proteomic analysis
The effect of collagenase treatment was investigated by examining the morphology of the focal adhesion of cG-CAOMECS. Figure 4d, e, and f show a cross section of cG-CAOMECS staining positive for phosphorylated focal adhesion kinase (FAK) (Fig. 4d) and integrin betas 1 and 4 (Fig. 4e and f), respectively. Results show that in the cell sheets harvested with collagenase, phosphorylated FAK was detected in the basal side of the cell sheet (Fig. 4d, arrow). FAK phosphorylation indicates that focal adhesion was functional and that integrin beta 1 was recruited/activated via the cytoplasmic membrane, which promote better adhesion with extra cellular matrix molecules such as collagen, fibronectin, and laminin. Figure 4e and f show that both integrin beta 1 and integrin beta 4 were positively expressed on the cell membrane.
Collagenase was used to detach the cell sheet. The PVDF membrane used to lift the cell sheet after incubation with collagenase a, b, and c. Cross sections of harvested cell sheet stained positive for phosphorylated FAK d and integrin betas 1 and 4 e and f. The levels of expression of intergrins are shown in g and h. Beta-actin for loading control in the semi-quantitative measurements i
The analysis was also extended to quantitatively measure the levels of expression of these ECM proteins. The semi-quantitative measurements showed that both integrins were highly expressed in the cell sheets produced with KaFa™ medium and harvested with collagenase, as compared to cell sheets harvested without collagenase treatment (Fig. 4g and h).
Discussion
Currently, limbal stem cell deficiency (LSCD) is treated with donor corneal graft tissue. Oral mucosal epithelial cells (OMEC) have successfully been used as a non-limbal cell source to treat unilateral and bilateral LSCD. However, the animal-derived growth supplements utilized for CAOMECS (cultured autologous oral mucosal epithelial cell sheet) manufacturing may lead to clinical complications. The goal of this study was to design a clinical-grade cell culture medium that completely supports the growth of the cell sheet without any type of feeder cells and without any animal-derived growth supplements. Development of a chemically defined cell culture medium reduces the risk of transmitting inherent organism-specific carrier diseases and improves the consistency of the results. KaFa™ medium was formulated and developed, solely to produce clinical-grade corneal epithelial cell sheets. Consequently, eliminating inherent biological variability due to the use of feeder cells and animal origin reagents.
Experiments were designed to test the effects of clinical-grade reagents. Every reagent added in the preparation of KaFa™ cell culture medium had to be animal-free and produced under GMP guidelines. Each supplement used in the developed medium had to be certified for clinical application and included the MDF number listed by the FDA to ensure the clinical application compatible quality of the produced cell sheet.
To eliminate the mouse NIH3T3 feeder cells, we tested our newly designed clinical-grade KaFa™ medium with human foreskin fibroblast (HuFFs) as a feeder cell (data not shown) for comparison with the previously produced cell sheet with mouse NIH3T3 feeder and animal-origin medium (AOM). The results showed that both feeder cells supported cell sheet growth in a similar way. However, the effect of the type of media used made a major impact on cell sheet growth. Regardless of feeder cell type, cell sheets grown with AOM quickly formed a multilayered graft and were sturdy for easy manipulation. Cell sheets produced with KaFa™ medium needed more time (24–72 h more) to grow and to form a multilayered cell sheet. This discovery geared our efforts towards further focusing on designing the cell culture medium. Specifically, we needed to supplement the medium with chemically defined FDA/GMP-grade substitutes to fetal bovine serum (FBS) that could sustain cell growth, and for CAOMECS to qualify as an autologous graft compliant with FDA regulations for human clinical application. We also investigated each reagents’ suitability with respect to oral mucosal epithelial stem cells to direct them to differentiate towards corneal epithelial cells. Epithelial stem cells intrinsically require a matrix to attach in the absence of feeder cells. We chose CELLstart™ matrix for cell attachment as it is GMP-certified. The surface of the 6MWP was treated for an hour with CELLstart™ coating prior to OMECS seeding. Cells were seeded using KaFa™ medium, and the medium was changed every 2 days during the first week and every day in the following weeks.
Colony-forming units (CFU) formed in the first 3–6 days of culture, reflecting the existence of epithelial stem cells and their expansion (Nishida et al. 2004) (Priya et al. 2011) (Burillon et al. 2012) (Bardag Gorce et al. 2015).
Towards the end of week two of cell culture, the CFUs disappeared and integrated in the differentiated multilayered epithelial cell sheet. This result was important as it suggested that the newly designed KaFa™ medium was sufficient to support the growth of the cell sheet—cG-CAOMECS. This experiment was successfully reproduced, and the cultured cell sheet was harvested intact. Collagenase was used for cell sheet harvesting. A completely grown cell sheet was harvested and processed for morphology and proteomic analysis. Microscopic imaging analysis showed that the harvested cell sheet grown with KaFa™ medium was a multilayered cell sheet similar to cell sheets grown with feeder cells and AOM medium. Tissue processing and immunofluorescent staining of cell sheet cross sections confirmed that the cell sheets produced with KaFa™ medium were multilayered with at least three layers of epithelial cells.
The presence of deltaNp63-positive cells in a corneal graft is essential for a successful clinical outcome for the treatment of LSCD (Rama et al. 2010) (Nishida et al. 2004). Similar to limbal stem cells, our cG-CAOMECS showed a great number of nuclei positive for delataNp63 in the basal layer of the cell sheet, reflecting the existence of progenitor cells and the proliferative capacity of these cells for future grafting onto the Bowman’s membrane of denuded corneas. PCNA staining was also used to examine the proliferative capacity of cG-CAOMECS.
Morphological analysis also showed a positive expression of corneal epithelial biomarkers keratin 3 (K3) reflecting the cultured OMECS differentiated into corneal epithelial cells. Our proprietary designed cell culture conditions proved to be adequate for differentiation of OMECS into corneal epithelial progenitor cells.
The persistency of oral mucosal epithelial keratin pattern was documented using K4 staining. Results show a positive staining in the apical squamous mature cells. It is possible that after grafting back onto cornea and after a period of time; the K4-positive cells will shed off the surface (Bardag Gorce et al. 2015). Similar to conjunctival epithelial cells, oral mucosal epithelia cells are characterized by the expression of keratins K4 and K13 (Bardag Gorce et al. 2018). The effects of corneal injuries on the expression of these keratins as well as the effects of clinical-grade reagents are yet to be determined in the preclinical model of LSCD.
Proteomic semi-quantitative analysis was conducted to compare cG-CAOMECS to CAOMECS produced with AOM and feeder cells. Results showed that cG-CAOMECS expressed similar levels with no significant differences of epithelial progenitor cells (deltaNp63) and corneal marker K3/K12 when compared to CAOMECS grown with feeder cells. Furthermore, cG-CAOMECS expressed significantly high levels of K3 when compared to CAOMECS grown with only AOM and no feeder cells, validating the efficacy of our newly designed cell culture conditions.
In addition, the expression of E-cadherin was significantly decreased in cell sheet produced with AOM and no feeder, while E-cadherin expression in cG-CAOMECS was similar to cell sheets grown with feeder cells and AOM. Total beta-catenin expression decreased in cG-CAOMECS, which may reflect a low level of beta-catenin translocation to the nucleus for canonical WNT/TCF activation and low levels of pro-survival gene expression (Melotti et al. 2014). The expression levels of Cnx43, a gap junction protein, were significantly greater in cG-CAOMECS, which corroborated the results obtained from E-cadherin and beta-catenin analysis.
cG-CAOMECS produced with KaFa™ medium is a multilayered epithelial cell sheet that expressed epithelial markers required for normal corneal epithelial barrier function (Bardag Gorce et al. 2016). Results indicate that KaFa™ medium with GMP-grade extracellular matrix replaces and exceeds the efficiency of feeder cells and AOM supplements in producing cG-CAOMECS.
However, our newly designed clinical-grade cell culture conditions did not allow us to produce a cell sheet that detached easily from the cell culture surface. Collagenase was necessary to harvest the cell sheet. The activity of collagenase is specific towards collagen molecules cleaving it at specific sites and releasing multiple small peptides. This action did not affect the expression of integrin beta 1 and beta 4, as shown in the “Results” section of immunofluorescent staining. These cell membrane proteins were highly expressed in cG-CAOMECS as compared to the levels in cell sheets harvested without collagenase treatment.
In conclusion, our experiments show that the combination of the newly designed clinical-grade cell culture conditions and KaFa™ medium resulted in the successful production of cG-CAOMECS, which in the clinical setting, using autologous human cells shall qualify as an efficient and safe clinically feasible tissue suitable for grafting back onto an LSCD patient’s corneal surface.
References
Ballen PH (1963) Mucous membrane grafts in chemical (lye) burns. Am J Ophthalmol 55:302–312. https://doi.org/10.1016/0002-9394(63)92687-4
Burillon C, Huot L, Justin V, Nataf S, Chapuis F, Decullier E, Damour O (2012) Cultured autologous oral mucosal epithelial cell sheet (CACAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci 53(3):1325–1331. https://doi.org/10.1167/iovs.11-7744
Denig R (1912) A surgical treatment for alkali burns of the eye. Munchen Med Wochenschr 1:579–590
Bardag-Gorce F, Makalinao A, Meepe I, Hoft RH, Cortez D, Oliva J, Niihara Y (2018) Corneal keratin aggresome (CKAGG) formation and clearance by proteasome activation. Heliyon 4(12):e01012. https://doi.org/10.1016/j.heliyon.2018.e01012.
Bardag-Gorce F, Oliva J, Wood A, Hoft R, Pan D, Thropay J, Makalinao A, French SW, Niihara Y (2015) Carrier-free cultured autologous oral mucosa epithelial cell sheet (CAOMECS) for corneal epithelium reconstruction: a histological study. Ocul Surf Apr 13(2):150–163. https://doi.org/10.1016/j.jtos.2014.12.003
Bardag-Gorce F, Oliva J, Wood A, Niihara H, Makalinao A, Sabino S, Niihara Y (2013) Microarray analysis of oral mucosal epithelial cell sheet. Tissue Eng Regen Med 10:362-370
Bardag-Gorce F, Hoft RH, Wood A, Oliva J, Niihara H, Makalinao, A, Niihara Y (2016) The role of E-cadherin in maintaining the barrier function of corneal epithelium after treatment with cultured autologous oral mucosa epithelial cell sheet grafts for limbal stem deficiency. J Ophthalmol 4805986. https://doi.org/10.1155/2016/4805986
Gallico GG 3rd, O’Connor NE, Compton CC, Kehinde O, Green H (1984) Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med 311:448–451
Gallico GG 3rd, O’Connor NE, Compton CC, Remensnyder JP, Kehinde O, Green H (1989) Cultured epithelial auto grafts for giant congenital nevi. Plast Reconstr Surg 84:1–9
Gipson IK, Geggel HS, Spurr-Michaud SJ (1986) Transplant of oral mucosal epithelium to rabbit ocular surface wounds in vivo. Arch Ophthalmol Oct 104(10):1529–1533 (PMID: 3767686)
Hassan NT, AbdelAziz NA (2018) Oral mucosal stem cells, human immature dental pulp stem cells and hair follicle bulge stem cells as adult stem cells able to correct limbal stem cell deficiency. Curr Stem Cell Res Ther 13(5):356–361. https://doi.org/10.2174/1574888X13666180223124936
Hayashida Y, Nishida K, Yamato M, Watanabe K, Maeda N, Watanabe H, Kikuchi A, Okano T, Tano Y (2005) Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature-responsive culture surface.. Invest Ophthalmol Vis Sci 46(5):1632–1639. PMID: 15851562
Higa K, Shimazaki J (2008) Recent advances in cultivated epithelial transplantation. Cornea Sep 27(Suppl 1):S41–S47
Hirayama M, Satake Y, Higa K, Yamaguchi T, Shimazaki J (2012) Transplantation of cultivated oral mucosal epithelium prepared in fibrin-coated culture dishes. Invest Ophthalmol Vis Sci 53(3):1602–1609
Kinoshita S, Koizumi N, Nakamura T (2004) Transplantable cultivated mucosal epithelial sheet for ocular surface reconstruction. Exp Eye Res Mar 78(3):483–491
Jones KB, Klein OD (2013) Oral epithelial stem cells in tissue maintenance and disease: the first steps in a long journey. Int J Oral Sci Sep 5(3):121–129. https://doi.org/10.1038/ijos.2013.46
Lee J, Shin D, Roh JL (2018) Use of a pre-vascularised oral mucosal cell sheet for promoting cutaneous burn wound healing. Theranostics Eight 20:5703–5712. https://doi.org/10.7150/thno.28754
Ma DH, Kuo MT, Tsai YJ, Chen HC, Chen XL, Wang SF, Li L, Hsiao CH, Lin KK (2009) Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye (Lond) 23(6):1442–1450. https://doi.org/10.1038/eye.2009.60. PMID: 19373264
Melotti A, Mas C, Kuciak M, Lorente-Trigos A, Borges I, Altaba RI, A, (2014) The river blindness drug Ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol Med Oct Six 10:1263–1278. https://doi.org/10.15252/emmm.201404084
Nakamura T, Endo K, Cooper LJ, Fullwood NJ, Tanifuji N, Tsuzuki M, Koizumi N, Inatomi T, Sano Y, Kinoshita S (2003) The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol vis Sci Jan 44(1):106–116
Nakamura T, Kinoshita S (2003) Ocular surface reconstruction using cultivated mucosal epithelial stem cells. Cornea 22(7 Suppl):S75–S80
Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y (2004) Corneal reconstruction with tissue engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 351(12):1187–1196
Priya CG, Arpitha P, Vaishali S, Prajna NV, Usha K, Sheetal K, Muthukkaruppan V (2011) Adult human buccal epithelial stem cells: identification, ex-vivo expansion, and transplantation for corneal surface reconstruction. Eye (lond) 25(12):1641–1649
Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G (2010) Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 363(2):147–155
Boneva RS, Folk TM, Chapman LE (2001) Infectious disease issues in xenotransplantation. Clin Microbiol Rev 14(1):1–14. https://doi.org/10.1128/CMR.14.1.1-14.2001
Rovere MR, Rousselle P, Haftek M, Charleux B, Kocaba V, Auxenfans, C, Nataf S, Damour O (2018) Preserving basement membranes during detachment of cultivated oral mucosal epithelial cell sheets for the treatment of total bilateral limbal stem cell deficiency Cell Transplant 27(2):264-274 https://doi.org/10.1177/0963689717741140
Sorkio A, Hongisto H, Kaarniranta K, Uusitalo H, Juuti-Uusitalo K, Skottman H (2014) Structure and barrier properties of human embryonic stem cell–derived retinal pigment epithelial cells are affected by extracellular matrix protein coating. Tissue Eng Part A 20(3–4):622–634. https://doi.org/10.1089/ten.tea.2013.0049
Utheim TP, Utheim ØA, Khan Q-E-S, Sehic A (2016) Culture of oral mucosal epithelial cells for the purpose of treating limbal stem cell deficiency. J Funct Biomater 1;7(1)
Yamaguchi N, Isomoto H, Kobayashi S, Kanai N, Kanetaka K, Sakai Y, Kasai Y, Takagi R, Ohki T, Fukuda H, Kanda T, Nagai K, Asahina I, Nakao K, Yamato M, Okano T, Eguchi S (2017). Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep 7(1):17460. https://doi.org/10.1038/s41598-017-17663-w
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC).
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
Grant support: Research is fully supported by Emmaus Medical Inc. Dr. Fawzia Bardag Gorce and Mrs. Kavita Narwani salaries were fully supported by Emmaus Medical Inc., and Dr. Yutaka Niihara is the CEO and the Chairman of Emmaus Medical, Inc. The rest of the authors have no support from Emmaus Medical, Inc. and no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Narwani, K., Stark, J., Cortez, D. et al. cG-CAOMECS—clinical-grade cultured autologous oral mucosal epithelial cell sheet. Cell Tissue Res 386, 47–57 (2021). https://doi.org/10.1007/s00441-021-03507-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03507-7