Abstract
Rotator cuff tears (RCTs), the most common tendon injury, are always accompanied by muscle atrophy, which is characterized by excessive protein degradation. Autophagy–lysosome systems are the crucial proteolytic pathways and are activated in atrophying muscle. Thus, inhibition of the autophagy–lysosome pathway might be an alternative way to minimize skeletal muscle atrophy. In this present study, combined with a tendon transection-induced rat model of massive rotator cuff tears, HE staining and transmission electron microscopy methods, we found leucine supplementation effectively prevented muscle atrophy, muscle injury and autophagosome formation. Utilizing immunoblotting, we discovered that leucine supplementation is able to inhibit the rise in autophagy-related protein expression (including LC3, Atrogin-1, MuRF1, Bnip3 and FoxO3) driven by tendon transection. The PI3K/AKT/mTOR pathway that was essential in autophagosome formation and autophagy was blocked in atrophying muscle and reactivated in the presence of leucine. Once administrated with LY294002 (PI3K inhibitor) and Rapamycin (mTOR inhibitor), leucine mediated by the anti-atrophic effects was nearly blunted. These results suggest that leucine potentially attenuates tendon transection-induced muscle atrophy through autophagy inhibition via activating the PI3K/AKT/mTOR pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rotator cuff consists of four types of muscles (supraspinatus, tears minor, subscapularis and infraspinatus muscle) and their tendons that are important in maintaining shoulder joint and shoulder movements stability (Morag et al. 2006). Repeated overhead motions or forceful pulling motions can cause rotator cuff injuries. Among these injuries, rotator cuff tears (RCTs) are the most common conditions causing shoulder pain, muscle atrophy and tendon rupture (Williams Jr. et al. 2004). Patients with chronic rotator cuff tears show significant changes in the muscle, such as atrophy, fat accumulation and fibrosis; these changes can be used as the predictors of poor outcome following surgical repair (Gladstone et al. 2007; Shen et al. 2008). Tear size and concomitant nerve injury on the rotator cuff muscle contribute to the fatty degeneration in a rat model of rotator cuff tears (Kim et al. 2012). The prevalence of massive RCTs has been up to 40% in all rotator cuff tears (Bedi et al. 2010). Patients with massive RCTs have a poor outcome and a higher rate of recurrent tearing after surgery compared to patients with smaller rotator cuff tears (Cofield et al. 2001; Galatz et al. 2004). Even though a majority of studies have focused on improved repair techniques, new found biologic factors, or other therapeutic drugs to improve tendon to bone healing, there is no established classification system to solve the difficulty in treating clients with massive rotator cuff tears. Thus, finding a therapeutic strategy or a special substance that could ameliorate the outcomes of patients with massive RCTs has a great clinical significance.
Muscle atrophy is the main complication of chronic massive RCTs (Gerber et al. 2007). Muscle atrophy can result in decreased muscle weight and muscle length. It is a decisive factor in affecting the outcome of rotator cuff repair and is irreversible even in the microenvironment of a successful repair (Goutallier et al. 2003). The hallmarks of atrophy are enhanced protein degradation and diminished protein synthesis that blunt the muscle force-producing capacity (Schoenfeld 2012). Excessive muscle protein degradation could lead to muscle necrosis or body death. The autophagy–lysosome system is the major proteolytic pathway of the cell and is coordinately activated in the process of muscle atrophy (Furuno et al. 1990). Studies have identified that some designated atrophy-related genes also belong to the autophagy–lysosome system, such as LC3 and Gabarap, indicating that autophagic flux is involved in the process of atrophy (Mammucari et al. 2007; Zhao et al. 2007). Autophagy in skeletal muscle can last for many days and prolongs autophagic induction LC3 and Gabarap supplementation (Mizushima et al. 2004). Autophagy suppressor Runt-related transcription factor 1 (Runx1) could modulate Forkhead Box O3 (FoxO3) action, which is responsible for the transcription of LC3, Gabarap and Bnip3 genes (Tracy and Macleod 2007). FoxO3 is required to activate lysosomal-dependent protein breakdown and acts as the key regulator for autophagy control in muscles. Knockdown of Bnip3, which is a central player downstream of FoxO3, attenuates FoxO3 upregulation-evoked autophagy (Mammucari et al. 2007). Activation of AKT/mTOR pathway in muscle cell also inhibits FoxO3-mediated autophagosome formation and protein degradation during fasting. As we all know, fasting can induce atrophy, implying that the activated the AKT signaling pathway potentially improves muscle atrophy through inhibition of FoxO3-mediated autophagy (Mammucari et al. 2008; Zhao et al. 2008). This means that the autophagy system-mediated protein breakdown shows a certain intersection with muscle loss, muscle atrophy and subsequently chronic massive RCTs.
Leucine is an essential branched chain amino acid that functions in accelerating protein synthesis and minimizing protein degradation (Baptista et al. 2010; Baptista et al. 2013). It is confirmed that leucine supplementation quickens the progress of muscle recovery suffering from multiple injuries (Anthony et al. 1999; Koopman et al. 2006). FoxO3a suppression is essential to the anti-atrophic effects of leucine in skeletal muscle (Baptista et al. 2017). Supplementation with leucine affects the proteolysis pathway by inhibiting relevant transcription factors such as FoxO3a (Pereira et al. 2014). Overexpression of FoxO3a is sufficient to accelerate transcription of the muscle-specific E3 ligases muscle atrophy F-box (MAFbx/Atrogin-1) and muscle-specific ring finger protein 1 (MuRF1) (Reed et al. 2012; Senf et al. 2011). FoxO3 transcriptional factor acts as a decisive skeletal muscle mass controller mainly through the proteasomal and autophagic systems (Mammucari et al. 2007). Activation of the IGF-I/phosphatidylinositol-3-kinase (PI3K)/AKT/mTOR pathway suppresses the cytosolic-retained FoxO factors. More importantly, the AKT/mTOR pathway contributes to the anti-atrophic effect of skeletal muscle (Bacurau et al. 2016), which suggests that the AKT/mTOR/FoxO signaling pathway might participate in the progress of muscle atrophy prevention. Studies have identified that leucine alone can induce phosphorylation of AKT and mTOR in skeletal muscle accompanied by the phosphorylation of 70-kDa ribosomal protein S6 kinase (p70S6K) and eukaryotic initiation factor (eIF) 4E-binding protein 1 (4E-BP1), which are the downstream molecules of mTOR (Anthony et al. 2000; Bolster et al. 2004). Even though researchers have showed that the anti-atrophic effects of leucine were not accompanied by activation of the PI3K/AKT/mTOR signaling pathway in the regenerating soleus muscles (Pereira et al. 2014), the precise molecular anti-atrophic effects and mechanisms driven by leucine in rotator cuff muscle tears are still not fully known.
Herein, we evaluated the anti-atrophic effects of leucine in a rat model of massive rotator cuff tears. Our findings suggest that leucine supplementation significantly improves the tendon transection-induced rotator cuff muscle injury, muscle atrophy and the formation of autophagosome in rotator cuff supraspinatus. The PI3K/AKT/mTOR signaling pathway was required in the anti-atrophic progress of leucine in the RCT rat model. These observations provide a novel theoretical insight into the anti-atrophic role of leucine on the rotator cuff and leucine might be an adjuvant therapy following surgical repair of chronic RCTs.
Materials and methods
Animal treatment
Adult male (8 weeks old) Sprague–Dawley (SD) rats were used and all animal care procedures were approved by laboratory animals of the Fifth Affiliated Hospital of Wenzhou Medical College. SD rats were fed under a stable raising condition and then divided into three groups randomly. For sham control, rats were anesthetized with 3% pentobarbital natrium (0.1 mL/10 g, Sigma, P3761). Then, a 1-cm deltoid incision was performed and the rotator cuff was identified. After that, the deltoid muscle and skin were closed. In the tendon transection surgery group, which was defined as the muscle cutoff group in this study, tendons of supraspinatus were completely transected and 5-mm-long tendons at each supraspinatus were removed in order to prevent tendon reattachment. Then, the incision of muscle and skin was closed. There were at least eight rats in each group. Postoperatively, the sham control was fed with standard chow diet and the tendon transection surgery group was fed with standard chow diet and 5% leucine-containing diet (Sigma, 61,820) respectively for 2 weeks. Both the standard chow and leucine-rich diet that were prepared according to previous studies were semi-purified and isocaloric diets (Cruz and Gomes-Marcondes 2014; Viana and Gomes-Marcondes 2013). Standard chow diets contained 18% protein and leucine-rich diets contained 18% protein plus 5% l-leucine, which represented a high percentage of this amino acid. Besides, both diets contained 70% carbohydrates (sucrose, starch and dextrin), 7% fat (soybean oil) and 5% fiber (micro-cellulose purified) accompanied by a mixture of vitamins, minerals, cysteine and choline. The standard chow and the leucine-rich diets contained 2% and 5% l-leucine, respectively. For inhibitor treatment, the established RCT rat model was treated with a tail intravenous injection of LY294002 (0.25 mg/100 g, PI3K inhibitor, Beyotime, S1737) and Rapamycin (0.1 mg/100 g, mTOR inhibitor, MCE, HY-10219) for 2 weeks. LY294002 and Rapamycin were diluted by PBS and the other three groups were treated with a tail intravenous injection of isometric vehicle (PBS).
Muscle atrophy detection
Rats were anesthetized with an intraperitoneal injection of 3% chloral hydrate (0.1 mL/10 g) and placed on 7.0T Magnetic Resonance Imaging (BioSpec 70/20USR, Germany). Based on the MRI system, the surface coil for the shoulder joint was used and the respiration rate of rats was monitored to ensure high image quality. The images were then analyzed and processed using ImageJ and Adobe Illustrator CS4.
Muscle harvest
Two weeks after surgery and treatment, SD rats were anesthetized and decapitated. Then, a 3–4-cm incision was performed longitudinally and the supraspinatus was harvested carefully. The wet weight of the supraspinatus was processed immediately after removing the tendon and scar tissue. Supraspinatus tissues were divided into three parts and frozen for the follow-on experiments.
Histologic analysis
Muscle tissues were cut into a reasonable size (0.5–1 cm) and processed 4% phosphate-buffered paraformaldehyde-fixed for more than 24 h. After paraffin embedding, a 4-μm paraffin section was stained with hematoxylin and eosin stain (Solarbio, A8090) according to a previous study (Kim et al. 2012). The morphology and histology of the supraspinatus were identified through a microscope and analyzed utilizing ImageJ and Adobe Illustrator CS4 software.
Cell apoptosis assay
Apoptosis assay was performed according to the manufacture’s instruction. In brief, a 4-μm paraffin section of supraspinatus was dewaxed using xylene and hydrated. Then, the section was processed with antigen retrieval using the high pressure method. After blocking with 5% BSA for 20 min, the tissue section was incubated with terminal transferase reaction solution at 37 °C for 60 min in darkness. One hundred microliters of HRP was added on the section after washing twice with TBS. Staining results were obtained when the section was stained utilizing DAB working solution. The number of positive staining was analyzed utilizing ImageJ software.
Autophagosome measurement
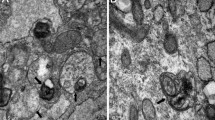
Supraspinati were cut into 1-mm3 tissue blocks at 4 °C and fixed with stationary liquid for 24 h at 4 °C. Then, tissue samples were fixed for another 2 h in 1% osmic acid. After uranyl acetate (Merck, 10011) staining for 3 h under room temperature and dark conditions, samples were washed with redistilled water and dehydrated using graded ethanol. Dehydrated tissues were placed at a mixture of ethanol and epoxypropane (Aladdin, P109309) (1:2) for 10 min, epoxypropane twice for 10 min, mixture of epoxypropane and embedding medium (1:1) for 40 min, mixture of epoxypropane and embedding medium (1:4) for 3 h and then tissues were embedded using embedding medium at 4 °C overnight and subsequently polymerized at 60 °C for 48 h. After making histological sections, the tissue sections were stained with uranyl acetate and lead citrate and autophagosome was observed under a transmission electron microscope and photographed.
Immunoblotting
Total protein of supraspinatus extraction and immunoblotting were performed in line with a previous study (Baptista et al. 2010). Protein levels were detected using primary antibody against LC3, Atrogin-1, MuRF1, Bnip3, FoxO3, PI3K, AKT, p-AKT, mTOR, p-mTOR, p70S6K, p-p70S6K, 4EBP1, and p-4EBP1 respectively. GAPDH was served as the internal control. LC3 (12135-1-AP), PI3K (20584-1-AP), mTOR (20657-1-AP), p70S6K (14485-1-AP), 4EBP1 (60246-1-Ig), and GAPDH (10494-1-AP) were obtained from Proteintech. p-mTOR (9964) and p-p70S6K (7617) were purchased from Cell Signaling Technology. Antibodies against AKT (bs-0115R) and p-AKT (bs-2720R) were from Bioss and p-EBP1 was from Abcam (ab111421). The relative quantitative analysis of protein expression was based on Quantity One software.
Statistical analysis
Muscle wet weight was analyzed among groups using an unpaired t test. Histologic analysis and apoptosis detection were based on ImageJ and Adobe Illustrator CS4 software. Quantitative analysis of protein level was executed by Quantity One software. Results were presented as mean + standard deviation (SD). Statistical analysis was compared using ANOVA and t test methods. P < 0.05 was defined as statistical significance.
Results
Leucine attenuates supraspinatus atrophy in tendon transection-induced RCT disease
To evaluate the effect of leucine on rotator cuff injury, we established tendon transection-induced rotator cuff tears in rats fed with standard chow and leucine-containing diet. After surgery, food intake was increased over time in the sham control group, whereas drastic reduction in food intake was found in the RCT model with normal and leucine-containing diet. Food consumption returned to the preoperative level at day 4 and day 3 after surgery in the RCT group with normal and leucine diet, respectively. But food intake in the leucine diet group returned to the level of the sham control group starting from day 5 (Table 1). Mouse weight also appeared to rise in the sham control group that decreased at first and then increased in both RCT groups but leucine treatment regained their weight more quickly compared to normal diet mice in RCT models (Fig. 1(a)). Obvious gross morphometric changes were also found in this model. Utilizing magnetic resonance imaging, we observed that there was injured/torn rotator cuff in RCT with normal diet compared to the sham control group implying tendon transection surgery had indeed led to rotator cuff tears in supraspinatus muscles. However, leucine administration repressed tendon transection-induced rotator cuff tears and muscle atrophy though showing moderate tissue edema (highlighted-signal indicated by white arrow) (Fig. 1b). Additionally, the average wet weight of supraspinatus muscle was reduced in the tendon transection group, whereas it was returned to normal level after leucine administration (Fig. 1c). These results indicate that leucine supplementation effectively prevents the whole disease process of tendon transection-induced RCT.
(a) The growth curve of body weight. (b–b”) MRI of supraspinatus in sham control, tendon transection group feeding with normal and leucine-containing diet. White arrow indicates the signal of muscle atrophy. (c) The average wet weight of supraspinatus muscle. Double number sign indicates the comparison between sham control group and tendon transection group feeding with normal and leucine-containing diet. ##p < 0.01; **p < 0.01
Leucine rescues tendon transection-induced muscle pathological changes and cell apoptosis
Muscle pathological changes were evaluated with H&E-stained histology; atrophic muscle fibers, fatty infiltration and inflammatory cell infiltration were observed in the tendon transection group feeding with normal diet (Fig. 2a, b). When administrated with leucine in the tendon transection group, there was a significant decrease in the number of inflammatory cells, fat accumulation, the degree of muscle fiber degeneration and fiber cross-sectional area (Fig. 2a–c). Since tendon transection could cause myopathy, we speculated there should have been an occurrence of muscle cell apoptosis. As shown in Fig. 2(d, e), tendon transection-induced muscle cell apoptosis was noticeably inhibited by feeding with leucine (Fig. 2d, e). The increased number of TUNEL-positive staining cells was also repressed in the presence of leucine (Fig. 2f). These findings reveal the protective effect of leucine on tendon transection-induced muscle injury and cell apoptosis.
(a–b”) HE staining of supraspinatus sections in sham control, tendon transection group feeding with normal and leucine-containing diet. (c) Quantitative analysis of fiber cross-sectional area in the above HE staining assay. (d–e”) TUNEL staining of different supraspinati in the three groups. (f) The muscle cell apoptosis rate with or without leucine treatment. **p < 0.01
Administration with leucine inhibits autophagy levels in atrophying muscles
A previous study has confirmed that autophagy in skeletal muscle presents sustained generation of autophagosomes that can maintain for days (Mizushima et al. 2004). Thus, we performed transmission electron microscopy analysis to assess the production of autophagosomes in these models and found that there was no damage on histological structure and no autophagosome vesicles in the sham control group. But cutting off the muscle obviously destroyed muscular tissue structure and promoted the numbers of autophagosomes and autophagolysosomes. Once treated with leucine, the destroyed muscular tissue was restored to normal level and the production of autophagosomes and autophagolysosomes was inhibited (Fig. 3a, b). Next, we performed immunoblotting to further verify the autophagy inhibition effect of leucine on atrophying muscles. We discovered that tendon transection induced the increase of Atrogin-1 and MuRF1 expressions that were repressed in the condition of leucine-containing diet treatment (Fig. 3c), which suggested that leucine supplement weakened the level of Atrogin-1/MuRF1 and subsequently improved the process of supraspinatus atrophy. We also evaluated the expression of key regulators for autophagy in muscle and detected that transcription factor FoxO3 and its downstream signals LC3 and Bnip3 that were important for the process of autophagy in muscle were upregulated in the tendon transection group but leucine treatment made them recover to the normal level (Fig. 3c). Quantitative analysis of these protein expressions also showed that leucine supplementation significantly blunted tendon transection-evoked muscle atrophy and the formation of autophagosomes (Fig. 3d).
(a–a”) Transmission electron microscopy scanning of the autophagosomes vesicles in atrophying supraspinatus. Red arrow indicates autophagosomes and white arrow indicates autophagolysosomes. (b) Quantitative analysis of the number of vesicles in TEM scanning assay. (c) Expression of LC3, Atrogin-1, MuRF1, Bnip3, and FoxO3 was evaluated by immunoblotting in different supraspinatus tissues. GAPDH was served as the internal control. A: sham + normal diet; B: muscle cutoff + normal diet; C: muscle cutoff + leu diet. (d–d””) Relative quantitative analysis of LC3, Atrogin-1, MuRF1, Bnip3, and FoxO3. *p < 0.05; **p < 0.01
Inhibitors of PI3K/AKT/mTOR pathway blunt the anti-atrophic effects of leucine
Numerous evidences have verified that AKT is the potent autophagy inhibitor in skeletal muscle and that the PI3K/AKT/mTOR pathway is the crucial element in muscle atrophy but whether leucine-mediated anti-atrophic effects were correlated with PI3K/AKT/mTOR signaling transduction remains unclear. As shown in Fig. 4(a–a””), tendon transection-mediated supraspinatus muscle atrophy was crippled by leucine administration. However, through MRI scanning, we observed that injection with LY294002 (PI3K inhibitor) and Rapamycin (mTOR inhibitor) distinctly blocked the anti-atrophic effects of leucine (Fig. 4a–a””). The promotion effect of leucine on supraspinatus muscle was also blunted in the presence of LY294002 and Rapamycin through measuring muscle wet weight (Fig. 4b). Besides, the protective role of leucine on pathological changes of atrophying muscle was significantly repressed in the treatment with PI3K and mTOR inhibitors (Fig. 5a–b””). As shown in Fig. 5a–a, we found that the area of inflammation infiltration, fat accumulation, muscle fibrosis and fiber cross-sectional area was obviously enhanced in the inhibitor group compared to the leucine alone-treated group (Fig. 5a–c). PI3K and mTOR inhibitors also blunted leucine mediated by the decreased characterization of autophagy in the RCT group accompanied by a bigger amount of autophagosomes and autophagolysosomes in comparison with the leucine-treated group but slightly less than the RCT group fed with standard chow diet (Fig. 5d–e). Our findings indicate that the PI3K/AKT/mTOR pathway participated in the process of leucine-mediated improvement of muscle atrophy and autophagosome formation.
(a–b””) HE staining of supraspinatus sections in sham control and RCT group treated with normal, leucine, leucine + LY294002, and leucine + Rapamycin, respectively. (c) Quantitative analysis of fiber cross sectional area. (d–d””) Transmission electron microscopy scanning of the autophagosomes vesicles in supraspinatus in the five groups. Red arrow indicates autophagosomes, and white arrow indicates autophagolysosomes. (e) Quantitative analysis of the number of vesicles in TEM scanning assay. *p < 0.05; **p < 0.01
Leucine-mediated improvement of supraspinatus atrophy is dependent on the PI3K/AKT/mTOR pathway
To directly prove whether PI3K/AKT/mTOR was the integrant signaling pathway in the process of leucine-mediated anti-atrophic effects, we observed the changes of the related signal protein. Similar to the previous results, LC3II/LC3I level was upregulated after tendon transection surgery, which was significantly reduced in the leucine-treatment group. In LY294002 and the Rapamycin treatment group, LC3II/LC3I returned to the same level as the tendon transection surgery group (Fig. 6a), implying that PI3K/AKT/mTOR was required for the leucine-mediated inhibition role on autophagosome generation. The impaired PI3K/AKT/mTOR/p70S6K/4-EBP1 signal axis in the tendon transection group was reactivated by leucine supplementation. Nevertheless, blockade of the key signal molecule using LY294002 and Rapamycin could effectively eliminate the protective effect of leucine on the destroyed signal transduction (Fig. 6a). With the quantitative analysis of the signal axis (Fig. 6b–b””’), we confirmed that PI3K/AKT/mTOR was the crucial signal transduction pathway in the process of anti-atrophic effects induced by leucine.
(a) Protein levels of LC3, PI3K, AKT, phospho-AKT, mTOR, phospho-mTOR, p70S6K, phosphop70S6K, 4E-BP1, and phospho-4E-BP1. (b–b’’’’’) Relative protein levels of LC3II/LC3I, PI3K, phospho-AKT, phospho-mTOR, phospho-p70S6K, and phospho-4E-BP1 were measured by using Quantity One software. GAPDH was used as the reference control. A: sham + normal diet; B: muscle cutoff + normal diet; C: muscle cutoff + leu diet; D: muscle cutoff + leu diet + LY294002; E: muscle cutoff + leu diet + Rapamycin. **p < 0.01
Discussion
Chronic rotator cuff tears are considered irreparable and often associated with an uncertain prognosis (Sevivas et al. 2016). The presenting symptoms are usually a painful and pseudoparalytic shoulder along with atrophy and fatty infiltration of the muscles (Fuchs et al. 1999; Sevivas et al. 2017). Muscle atrophy is one main obstacle to solve the poor clinical outcomes of RCTs following surgical repair. Therefore, improved muscle atrophy condition possibly brings about good outcomes for chronic RCT patients after surgical repair. In the present study, we found leucine was able to ameliorate supraspinatus muscle atrophy via the activating PI3K/AKT/mTOR signal pathway and modulating muscle cell autophagy in the rat model of chronic RCTs disease. Possibly, these findings provide a new insight into the anti-atrophic effects of leucine on rotator cuff muscle tears disease.
The clinical features of RCTs show severe muscle atrophy, fatty infiltration and inflammatory cells infiltration (Maher et al. 2017). Muscle atrophy is displayed by decreased muscle weight and length at an early time point in the supraspinatus and infraspinatus and fatty accumulation appears at a later time point in the infraspinatus only in a rat model of massive RCTs (Liu et al. 2011). In our established RCT model, we found that tendon transection-induced chronic RCTs severely impacted food intake and body weight of rats accompanied by muscle atrophy and decreased wet weight of supraspinatus muscle. Muscle atrophy and fat infiltration were observed in the rotator cuff muscles after 2 weeks of rotator cuff tendon transection, which was similar to the rat model of massive rotator cuff tears reported by others (Liu et al. 2012; Liu et al. 2011). Because our model was based on tendon transection but not nerve cutoff, it reflected a specific condition of the rotator cuff but not a model of generalized tendon injury.
Muscle atrophy in the RCT model involves a complex protein network and molecular mechanisms. Atrogin-1 (a muscle-specific F-box protein), Bnip3 (BCL2/adenovirus E1B 19 kDa protein-interacting protein 3) and E3 ubiquitin-ligase MuRF-1 have been shown to be implicated in the regulation process of skeletal muscle atrophy (Benson et al. 2010; Gomes et al. 2001; Rom and Reznick 2016). LC3-II, which is the most widely used marker of autophagosomes formation, is identified participating in the progress of rotator cuff tendon disease (Kim et al. 2014). These genes are regulated by FoxO transcription factors, which are a critical controller in the ubiquitin–proteasome and autophagy–lysosome systems (Sandri 2010), implying autophagy–lysosome systems should play an important role in the progression of skeletal muscle atrophy. In fact, emerging studies have confirmed that autophagy is a significant process in the development of rotator cuff injury, which was also identified in our research. The expressions of atrophy associated protein including LC3, Atrogin-1, Bnip3 and MuRF-1 were more/bigger in the RCT model than in the sham control group. However, leucine supplementation significantly repressed the production of autophagosome in the RCT group. It has been confirmed that leucine is sufficient to improve regeneration of skeletal muscles from old rats (Pereira et al. 2015) but no literature reports the muscle rebuilding effect of leucine on rotator cuff tears disease and how the autophagy signaling pathway functions in that progression. Our presented data potentially filled the void in this field.
Once stimulated by common upstream signals such as growth factors and amino acid availability, protein synthesis in skeletal muscle is mainly regulated by the phosphoinositide 3-kinase (PI3K)/AKT/mTOR pathway (Drummond et al. 2009; Wu et al. 2011). During the translation, p70S6K and 4E-BP1, downstream of mTOR kinase, are the crucial determinants and phosphorylated mTOR mediated the activation of p70S6K and 4E-BP1 facilitated the start of codon scanning and the complete ribosome assembly (Hornberger et al. 2006). Additionally, the AKT/mTOR pathway is the key negative regulator of autophagy in skeletal muscle. Rapid activation of AKT and its downstream signal mTOR contribute to protein synthesis, autophagosome inhibition and cell growth in adult mice or muscle cell cultures (Zhao et al. 2008). This suggests that the PI3K/AKT/mTOR signaling pathway negatively regulates the process of autophagosome formation and protein degradation in muscle cell. Here, we discovered that leucine administration rescued the inhibition of PI3K/AKT/mTOR/p70S6K/4E-BP1 signaling in tendon transection mediated by the destroyed skeletal muscle. Both PI3K and mTOR inhibitors blunt the anti-atrophic effects of leucine on skeletal muscle, implying that leucine administration ameliorated muscle atrophy potentially through the inhibition of autophagy by activating PI3KAKT/mTOR signaling during rotator cuff tears disease.
In summary, we determined the underlying mechanism of leucine mediated by the anti-atrophic effects in rotator cuff tear disease. Herein, the aspect of leucine might be more relevant with an anti-atrophic effect. Considering the amounts of consumed leucine of rats (2.5 g/1 kg/day), 23.8 g/day (2.5 g/6.3 × 60 kg) of leucine should be consumed in a person’s body weight (60 kg). Actually, it is very difficult for a man to eat so much leucine. However, the ingredients of a leucine-rich diet in our study were based on previous reports. Our data provide a rationale for further exploring the role of leucine supplementation in the prevention and treatment of RCT disease. Clinically, most RC muscle atrophy in patients could possibly be improved with the help of a leucine-rich diet supplement. To summarize, our findings provide a new therapeutic strategy for the treatment of RCT disease that can be widely applied in a clinic environment.
References
Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS (2000) Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 130:139–145
Anthony JC, Anthony TG, Layman DK (1999) Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J Nutr 129:1102–1106
Bacurau AV, Jannig PR, de Moraes WM, Cunha TF, Medeiros A, Barberi L, Coelho MA, Bacurau RF, Ugrinowitsch C, Musaro A, Brum PC (2016) Akt/mTOR pathway contributes to skeletal muscle anti-atrophic effect of aerobic exercise training in heart failure mice. Int J Cardiol 214:137–147
Baptista IL, Leal ML, Artioli GG, Aoki MS, Fiamoncini J, Turri AO, Curi R, Miyabara EH, Moriscot AS (2010) Leucine attenuates skeletal muscle wasting via inhibition of ubiquitin ligases. Muscle Nerve 41:800–808
Baptista IL, Silva WJ, Artioli GG, Guilherme JP, Leal ML, Aoki MS, Miyabara EH, Moriscot AS (2013) Leucine and HMB differentially modulate proteasome system in skeletal muscle under different sarcopenic conditions. PLoS One 8:e76752
Baptista IL, Silvestre JG, Silva WJ, Labeit S, Moriscot AS (2017) FoxO3a suppression and VPS34 activity are essential to anti-atrophic effects of leucine in skeletal muscle. Cell Tissue Res 369:381–394
Bedi A, Dines J, Warren RF, Dines DM (2010) Massive tears of the rotator cuff. J Bone Joint Surg Am 92:1894–1908
Benson RT, McDonnell SM, Knowles HJ, Rees JL, Carr AJ, Hulley PA (2010) Tendinopathy and tears of the rotator cuff are associated with hypoxia and apoptosis. J Bone Joint Surg Br 92:448–453
Bolster DR, Vary TC, Kimball SR, Jefferson LS (2004) Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J Nutr 134:1704–1710
Cofield RH, Parvizi J, Hoffmeyer PJ, Lanzer WL, Ilstrup DM, Rowland CM (2001) Surgical repair of chronic rotator cuff tears. A prospective long-term study. J Bone Joint Surg Am 83-A:71–77
Cruz B, Gomes-Marcondes MC (2014) Leucine-rich diet supplementation modulates foetal muscle protein metabolism impaired by Walker-256 tumour. Reprod Biol Endocrinol 12:2
Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB (2009) Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol (1985) 106:1374–1384
Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C (1999) Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elb Surg 8:599–605
Furuno K, Goodman MN, Goldberg AL (1990) Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J Biol Chem 265:8550–8557
Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K (2004) The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 86-A:219–224
Gerber C, Schneeberger AG, Hoppeler H, Meyer DC (2007) Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elb Surg 16:691–696
Gladstone JN, Bishop JY, Lo IK, Flatow EL (2007) Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 35:719–728
Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A 98:14440–14445
Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S (2003) Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elb Surg 12:550–554
Hornberger TA, Sukhija KB, Chien S (2006) Regulation of mTOR by mechanically induced signaling events in skeletal muscle. Cell Cycle 5:1391–1396
Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S (2012) The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elb Surg 21:847–858
Kim RJ, Hah YS, Sung CM, Kang JR, Park HB (2014) Do antioxidants inhibit oxidative-stress-induced autophagy of tenofibroblasts? J Orthop Res 32:937–943
Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, Wagenmakers AJ, van Loon LJ (2006) Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr 84:623–632
Liu X, Joshi SK, Samagh SP, Dang YX, Laron D, Lovett DH, Bodine SC, Kim HT, Feeley BT (2012) Evaluation of Akt/mTOR activity in muscle atrophy after rotator cuff tears in a rat model. J Orthop Res 30:1440–1446
Liu X, Manzano G, Kim HT, Feeley BT (2011) A rat model of massive rotator cuff tears. J Orthop Res 29:588–595
Maher A, Leigh W, Brick M, Young S, Caughey M (2017) Causes of pain and loss of function in rotator cuff disease: analysis of 1383 cases. ANZ J Surg 87:488–492
Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M (2007) FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6:458–471
Mammucari C, Schiaffino S, Sandri M (2008) Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy 4:524–526
Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111
Morag Y, Jacobson JA, Miller B, De Maeseneer M, Girish G, Jamadar D (2006) MR imaging of rotator cuff injury: what the clinician needs to know. Radiographics 26:1045–1065
Pereira MG, Baptista IL, Carlassara EO, Moriscot AS, Aoki MS, Miyabara EH (2014) Leucine supplementation improves skeletal muscle regeneration after cryolesion in rats. PLoS One 9:e85283
Pereira MG, Silva MT, da Cunha FM, Moriscot AS, Aoki MS, Miyabara EH (2015) Leucine supplementation improves regeneration of skeletal muscles from old rats. Exp Gerontol 72:269–277
Reed SA, Sandesara PB, Senf SM, Judge AR (2012) Inhibition of FoxO transcriptional activity prevents muscle fiber atrophy during cachexia and induces hypertrophy. FASEB J 26:987–1000
Rom O, Reznick AZ (2016) The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass. Free Radic Biol Med 98:218–230
Sandri M (2010) Autophagy in skeletal muscle. FEBS Lett 584:1411–1416
Schoenfeld BJ (2012) Does exercise-induced muscle damage play a role in skeletal muscle hypertrophy? J Strength Cond Res 26:1441–1453
Senf SM, Sandesara PB, Reed SA, Judge AR (2011) p300 Acetyltransferase activity differentially regulates the localization and activity of the FOXO homologues in skeletal muscle. Am J Physiol Cell Physiol 300:C1490–C1501
Sevivas N, Teixeira FG, Portugal R, Araújo L, Carriço LF, Ferreira N, Vieira da Silva M, Espregueira-Mendes J, Anjo S, Manadas B, Sousa N, Salgado AJ (2016) Mesenchymal stem cell secretome: a potential tool for the prevention of muscle degenerative changes associated with chronic rotator cuff tears. Am J Sports Med 45:179–188
Sevivas N, Ferreira N, Andrade R, Moreira P, Portugal R, Alves D, Vieira da Silva M, Sousa N, Salgado AJ, Espregueira-Mendes J (2017) Reverse shoulder arthroplasty for irreparable massive rotator cuff tears: a systematic review with meta-analysis and meta-regression. J Shoulder Elb Surg 26:e265–e277
Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC (2008) Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elb Surg 17:1S–7S
Tracy K, Macleod KF (2007) Regulation of mitochondrial integrity, autophagy and cell survival by BNIP3. Autophagy 3:616–619
Viana LR, Gomes-Marcondes MC (2013) Leucine-rich diet improves the serum amino acid profile and body composition of fetuses from tumor-bearing pregnant mice. Biol Reprod 88:121
Williams GR Jr, Rockwood CA Jr, Bigliani LU, Iannotti JP, Stanwood W (2004) Rotator cuff tears: why do we repair them? J Bone Joint Surg Am 86-A:2764–2776
Wu M, Falasca M, Blough ER (2011) Akt/protein kinase B in skeletal muscle physiology and pathology. J Cell Physiol 226:29–36
Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL (2007) FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6:472–483
Zhao J, Brault JJ, Schild A, Goldberg AL (2008) Coordinate activation of autophagy and the proteasome pathway by FoxO transcription factor. Autophagy 4:378–380
Funding
This study is supported by Zhejiang Provincial Natural Science Foundation (grant number: Y16H060008, PR China) and Medical and Health Research Program of Zhejiang Province (grant number: 2012KYB249, 2017KY725, PR China).
Author information
Authors and Affiliations
Contributions
Dr. Rongzong Zheng (MD), the director of orthopedics, sports medicine and joint surgery sub-specialty conceived,designed and launched this research.
Dr. Shuming Huang (MD) performed the experiments and analyzed data, taking the responsibility of the chief instructor of orthopedics trauma sub-specialty, and shares the privilege of co-first authors and corresponding author.
Dr. Junkun Zhu (MD), the director of orthopedics rehabilitation sub-specialty, submitted the proposal and composed the manuscript and shares the privilege of co-first authors.
Wei Lin, Huan Xu and Xiang Zheng participated and collected the experiment data as well as completing statistics analysis.
Corresponding author
Ethics declarations
All animal care procedures were approved by laboratory animals of the Fifth Affiliated Hospital of Wenzhou Medical College in this study.
Disclaimer
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, R., Huang, S., Zhu, J. et al. Leucine attenuates muscle atrophy and autophagosome formation by activating PI3K/AKT/mTOR signaling pathway in rotator cuff tears. Cell Tissue Res 378, 113–125 (2019). https://doi.org/10.1007/s00441-019-03021-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03021-x