Abstract
Glycosylation changes occur widely in colon tumours, suggesting glycosylated molecules as potential biomarkers for colon cancer diagnostics. In this study, proteoglycans (PGs) expression levels and their transcriptional patterns are investigated in human colon tumours in vivo and carcinoma cells in vitro. According to RT-PCR analysis, normal and cancer colon tissues expressed a specific set of PGs (syndecan-1, perlecan, decorin, biglycan, versican, NG2/CSPG4, serglycin, lumican, CD44), while the expression of glypican-1, brevican and aggrecan was almost undetectable. Overall transcriptional activity of the PGs in normal and cancer tissues was similar, although expression patterns were different. Expression of decorin and perlecan was down-regulated 2-fold in colon tumours, while biglycan and versican expression was significantly up-regulated (6-fold and 3-fold, respectively). Expression of collagen1A1 was also increased 6-fold in colon tumours. However, conventional HCT-116 colon carcinoma and AG2 colon cancer-initiating cells did not express biglycan and decorin and were versican-positive and -negative, respectively, demonstrating an extracellular origin of the PGs in cancer tissue. Selective expression of heparan sulfate (HS) proteoglycans syndecan-1 and perlecan in the AG2 colon cancer-initiating cell line suggests these PGs as potential biomarkers for cancer stem cells. Overall transcriptional activity of the HS biosynthetic system was similar in normal and cancer tissues, although significant up-regulation of extracellular sulfatases SULF1/2 argues for a possible distortion of HS sulfation patterns in colon tumours. Taken together, the obtained results suggest versican, biglycan, collagen1A1 and SULF1/2 expression as potential microenvironmental biomarkers and/or targets for colon cancer diagnostics and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An understanding of molecular changes in colon carcinogenesis is an important baseline for revealing new biomarkers and targets for personalised/companion diagnostics and treatment of the disease. Along with proteins and nucleic acids, glycosylated molecules (proteoglycans, PGs) take part in normal cell physiological processes and also in malignant transformation (Iozzo and Sanderson 2011; Theocharis et al. 2010), including colon cancer (Joo et al. 2014; Nikitovic et al. 2012). An important functional contribution of proteoglycans and glycosaminoglycans (GAGs) in colon carcinogenesis is supported by the effects of exogenious GAGs on colon carcinoma cells: exogenously added heparin but not endogenous GAGs/PGs, exclusively stimulates colon cancer cell growth in a sulfation pattern-related manner (Chatzinikolaou et al. 2008); proteoglycan (P1) purified from Phellinus linteus has an antiproliferative effect in the HT-29 human colon adenocarcinoma cell line in vitro and an anti-tumour effect in vivo (Li et al. 2011).
At the moment, a number of studies have shown an involvement of individual PGs in colon carcinogenesis. Expression of syndecan-1 is decreasing throughout colon carcinoma progression; de novo carcinomas have lower syndecan-1 expression compared with ex adenoma carcinomas (Pap et al. 2009); syndecan-1 and glypican-3 are down-regulated in colon cancer compared with normal tissues (Joo et al. 2014); and low epithelial syndecan-1 expression is associated with higher histological grade of colorectal tumours and more advanced clinical stages of the disease (Lundin et al. 2005). Expression of tumorigenic syndecan-2 in a highly metastatic colorectal HCT-116 cancer cell line is enhanced by fibroblast ECM, while the expression of other anti-tumorigenic syndecans is decreased (Vicente et al. 2013); the extracellular domain of syndecan-2 regulates the interaction of HCT 116 human colon carcinoma cells with fibronectin (Kwon et al. 2013) and affects adhesion and migration of cancer cells through cooperation with integrin alpha2 (Choi et al. 2009), Tiam1-dependent Rac activation (Choi et al. 2010) and interaction with syntenin-1 (Lee et al. 2011); syndecan-2 enhances both expression and secretion of matrix metalloproteinase-7 (MMP-7) and directly interacts with pro-MMP-7, activating its processing into an active MMP-7 (Ryu et al. 2009); perlecan is expressed in Caco2 human colon carcinoma cells (Molist et al. 1998) and the WiDr human colon carcinoma cell line (Knox et al. 2001) and markedly attenuates growth of colon carcinoma cells in vitro and induces tumour growth and angiogenesis in vivo (Sharma et al. 1998); lumican expression and its presence in ECM has an impact on colon cancer cells motility and may modulate invasiveness of colon cancer (Radwanska et al. 2012); and biglycan expression is increased in human colorectal carcinoma (Galamb et al. 2009; Mikula et al. 2011; Gu et al. 2012).
Controversial data concern the expression of decorin, a leucine-rich proteoglycan involved in the regulation of matrix assembly and cell proliferation and versican in colon cells and tissues, especially taking into account cellular and/or stromal localisation of the PGs. Decorin expression is dramatically reduced in paired colorectal cancer tissues compared with the differentiated area of human normal colonic mucosa (Bi et al. 2008); decorin was suggested as a tumour suppressor gene, which inhibits colorectal cancer growth and migration through the interaction with and stabilisation of E-cadherin (Bi et al. 2012). On the other hand, analysis of mRNA encoding PG-40 (decorin), the main chondroitin sulfate proteoglycan of colon tissue, revealed a 7-fold increase in the two transcripts encoding this gene product (Adany et al. 1990); decorin levels are markedly elevated in the stroma of colon carcinoma (Iozzo and Cohen 1993); human colon cancer is characterised by a significant increase of decorin and versican (8- and 13-fold in terms of protein, respectively) (Theocharis 2002); versican expression is significantly up-regulated in colon tumour tissues according to RT-PCR analysis (Pitule et al. 2013); and versican mRNA levels do not significantly vary between normal and colon carcinoma tissues (Adany and Iozzo 1990). Furthermore, along with the core protein expression changes, these PGs are significantly modified at the post-translational level (the type, length and sulfation pattern of their GAG chains) and these specific structural alterations of versican and decorin may influence the biology of cancer cells in HCC (Theocharis 2002).
All these data suggest an important role of proteoglycans in colon carcinogenesis, both at protein core levels and polysaccharide GAG chains (which are generated by the complex PG biosynthetic system in a non-template manner). So, a coordinated expression of core proteins—and GAG biosynthetic enzymes—coding genes is vital for the appearance of functionally active matured PGs on the cell surface and in the cell microenvironment. In this study, we investigate common expression patterns of key proteoglycans and their biosynthetic enzymes in normal and colon cancer cells and tissues.
Materials and methods
Patients and tissue samples
All tissue samples were obtained from primary tumours and surrounding tissue during radical surgery at the National Institute of Cancer Research (Kiev, Ukraine). For histological and immunohistochemical studies, clinical tissue samples were fixed by buffered formalin and paraffin-embedded tissue sections were stained by hematoxylin and eosin. For molecular studies, tissues were “snap-frozen” in liquid nitrogen and stored at –70 °C. Regions were manually dissected from the tissue samples to provide a consistent tumour cell content of more than 70 % for analysis. The prevalent histological type of the tumours was adenocarcinoma, with different grades of malignancy. Most patients were at the fourth stage of malignancy progression according to the TNM formula. All patients provided written informed consent and the study protocol was approved by the Local Ethics Committee in accordance with the Helsinki Declaration of 1975.
Cell lines and cell culture
The HCT-116 human colon carcinoma cell line and AG2 colon cancer-initiating cell line were obtained from MTC (Karolinska Institute, Stockholm, Sweden). All cell lines were maintained in Iscove’s Modified Dulbecco’s medium (IMDM) supplemented with glutamine, 100 units ml−1 penicillin, 100 μgml−1 streptomycin and 10 % FBS at 37 °C in a humidified 5 % CO2 incubator. Cells were harvested for passaging or analysis using trypsin/EDTA. Each experiment was repeated three times.
RT-PCR analysis
Total RNA was extracted from the cells using the PureLink Total RNA Purification System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA was synthesised from 1–2 μg of total RNA using a First Strand cDNA Synthesis kit (Fermentas, Hanover, MD, USA) and 1/10th of the product was subjected to PCR analysis.
Quantitative real-time RT-PCR (qRT-PCR) was performed using the ABI PRISM 7500 Sequence Detector (AppliedBiosystems, USA) and the Maxima SYBR Green/RO master mix (Thermo Scientific) under the following conditions: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles at 95 °C for 105 s and 60 °C for 1 min. The total reaction volume was 25 μl. GAPDH was used as the housekeeping gene. The PCR primers and conditions are listed in Table 1. Three replicates were performed for each experiment.
Immunostaining
For immunohistochemistry, the Histostain-Plus 3rd Gen IHC Detection Kit (Invitrogen) was used according to the manufacturer’s instructions. Briefly, 4- to 5-μm sections of formalin-fixed, paraffin-embedded tissue sections were deparaffinised and antigen was retrieved by treatment with unmasking solution at 95–98 °C for 20 min. Endogenous peroxidase was blocked with peroxidase quenching solution, unspecific staining was blocked with blocking solution and the primary anti-versican mouse monoclonal antibody (1:100; Santa Cruz, USA) or the anti-decorin monoclonal antibody (1:500; Abcam, UK) was used for immunostaining. Negative (no primary antibodies) and positive (tissue samples with proved antigen presence) controls were done to confirm a specificity of the antibodies. Staining patterns were visualised by incubation with the biotinylated secondary antibody, then streptavidin-peroxidase conjugate and DAB chromogen, counterstained with hematoxylin and observed by light microscopy with magnification 400 (Axio Scope.A1, camera AxioCam MRc 5; Carl Zeiss).
Statistical analysis
Statistical analyses were performed using a computer program ORIGIN Pro 8.1; a value of p < 0.05 was considered to indicate a statistically significant difference. Data are expressed as the means ± SEM and the median values were calculated to take into account a possible dispersion of the samples.
Results
Proteoglycans expression in human colon tumours
SYBR Green-based quantitative Real-Time RT-PCR analysis was used to determine expression of main PGs (syndecan-1, glypican-1, perlecan, decorin, biglycan, versican, aggrecan, serglycin, NG2/CSPG4, brevican, lumican, CD44) in human colon tumours with GAPDH as the reference gene. Tumour and control samples were matched pairs for each patient with malignancy, from the central part of the tumour and a more distant part of the colon, accordingly (Fig. 1).
Proteoglycans expression in human colon tumours and normal colon tissues. a Transcriptional patterns of total proteoglycans expression in matched normal and colon cancer tissue pairs. Real-time RT–PCR analysis, intensity of the amplified DNA fragments for each gene normalised to that of GAPDH. The stacked columns compare the contribution of each value to a total across the categories. b–e Individual proteoglycans expression: b decorin, c biglycan, d perlecan, e versican. The graphs show the mean expression levels from triplicate experiments (±SD) (OriginPro 8.1), 33–126 patients
According to the RT-PCR analysis, the overall transcriptional activities of the PGs were similar in normal and cancer colon tissues (165 ± 64 vs. 175 ± 134, respectively), although the expression levels were more variable in colon tumours than in control tissues (median values 167.6 vs. 143.38, respectively), suggesting a common deregulation of PGs expression in colon cancer. As to the individual proteoglycans, glypican-1, brevican and aggrecan were almost undetectable in both normal and malignant human colon tissues. Syndecan-1, perlecan, decorin, biglycan, versican, NG2/CSPG4, serglycin, lumican and CD44 were expressed, resulting in a complex transcriptional pattern of PGs expression in colon tissue (Fig. 1a; Table 2).
To analyse the obtained data, an expression change more than 2-fold was taken as reliable in comparative analysis of the individual PGs in tumour and control colon tissues. The expression levels of syndecan-1, serglycin, NG2/CSPG4, lumican and CD44 were not changed in colon tumours compared with the matched control colon tissues. The most significant changes were detected for biglycan (Fig. 1c) and versican (Fig. 1e), the expression levels of which were increased 6-fold and 3-fold in colon tumours, respectively; decorin (Fig. 1b) and perlecan (Fig. 1d) expression levels had a tendency to be decreased (1.5-fold) in colon tumours (although still being actively expressed). Taking into account enhanced heterogeneity of the colon tumours in terms of proteoglycans expression levels, the mean values were calculated for the individual PGs. The obtained results confirmed the significant up-regulation of biglycan and versican (5-fold and 2.5-fold, respectively) and down-regulation of decorin and perlecan (2-fold) in colon tumours.
To study the expression of the proteoglycans at the protein level, we performed immunohistochemical analysis for the presence and localisation of their protein cores in colon tumours with different clinical characteristics (low- and high-grade colon adenocarcinomas) (Fig. 2).
Surprisingly, decorin expression was significantly higher in poor-differentiated high-grade colon adenocarcinoma (especially in cancer-associated stroma) (Fig. 2e) than in moderately-differentiated low-grade adenocarcinoma (Fig. 2b), in spite of a 1.5- to 2-fold decrease of its mRNA levels in colon tumours compared with matched normal tissues. Versican expression was also increased in high-grade carcinomas, where it was localised preferentially in cancer-associated stroma (Fig. 2f). Possibly, the visible increase of decorin and versican staining in the poor-differentiated adenocarcinoma stromal compartment could be explained by a higher density of the cancer cells in the tissues and a condensed extracellular matrix.
The results show that both the transcriptional activity of PGs and their localisation in tissue compartments are differently affected in colon cancer and these changes could be associated with heterogeneity of individual colon tumours and their clinical characteristics.
Expression of proteoglycans in colon carcinoma cells in vitro
To check the hypothesis that the morphological and clinical heterogeneity of colon tumours could be related to different PGs composition in a heterogeneous cancer cell population, proteoglycans expression was determined in a conventional HCT116 colon carcinoma cell line and AG2 colorectal carcinoma-derived cancer-initiating cells (CICs) (Tallerico et al. 2013).
Proteoglycans expression levels in these cell lines were determined by RT-PCR analysis with the GAPDH gene as an internal control (Fig. 3). The CIC status of the AG2 cells was confirmed by CD44 expression analysis, which is a known molecular marker for cancer stem cells (Haraguchi et al 2008). A six-fold higher CD44 expression level was shown in AG2 cells compared with HCT116 cells.
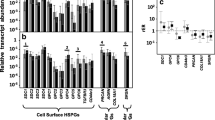
The colon carcinoma cell lines showed different total expression levels and transcriptional patterns of PG-coding genes. HCT116 carcinoma cells expressed mainly chondroitin sulfate (CS) proteoglycan versican (Fig. 3a), while AG2 cancer-initiating cells preferentially expressed heparan sulfate (HS) proteoglycans syndecan-1 and perlecan (Fig. 3b). NG2 was expressed in both cell lines at a similar level. Interestingly, no biglycan and decorin expression was detected in either cell line in vitro, in spite of high decorin expression and significant biglycan up-regulation in colon tumours in vivo (Fig. 1b, c), supporting a stromal origin of the molecules in malignant colon tissues.
Taken together, the obtained data demonstrate that the HCT116 and AG2 colon cancer cell lines with different pro-carcinogenic potential express different proteoglycans types (CS vs. HS). It suggests proteoglycans as potential players in colon cancer initiation and development and pays special attention to syndecan-1 and perlecan as new potential molecular markers of colon cancer-initiating cells.
Expression of the HS metabolic system in colon tumours in vivo and cancer cells in vitro
Heparan sulfate proteoglycans (HSPGs) are complex protein–polysaccharide macromolecules and structural changes could occur not only at their protein level (expression of HSPGs core proteins) but also at the polysaccharide (glycosaminoglycan, GAG) level. Biosynthesis and post-synthetic modifications of the GAG chains are governed by a complex system of specific enzymes, where proper expression and activity are principally important for biosynthesis of functionally active PGs.
To analyse a transcriptional activity of the heparan sulfate biosynthetic system, we determined expression levels of key HS biosynthesis-involved genes in normal and cancer colon tissues in vivo (Fig. 4) and HCT116 and AG2 colon carcinoma cell lines in vitro (Fig. 5).
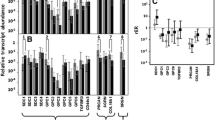
Expression of the HS biosynthetic system in human normal colon tissues and colon tumours in vivo. a Transcriptional patterns of HS metabolism-involved genes in matched normal and cancer colon tissue pairs. Real-time RT–PCR analysis, intensity of the amplified DNA fragments for each gene normalised to that of GAPDH. The stacked columns compare the contribution of each value to a total across the categories. b–e Expression levels of the most changed individual genes: b decorin, c biglycan, d perlecan, e versican. The graphs show the mean expression levels from triplicate experiments (±SD) (OriginPro 8.1), 33–126 patients
Expression of the HS metabolism-involved genes in human colon cancer cell lines in vitro. a HCT116 and b AG2 colon carcinoma cell lines. Real-time RT–PCR analysis, intensity of the amplified DNA fragments normalised to that of GAPDH. The graphs show the mean expression levels from triplicate experiments (±SD) (OriginPro 8.1)
According to the RT-PCR data, the overall transcriptional activity of the HS-metabolic system was similar in normal and cancer colon tissues (Fig. 4a). No evident changes were observed in the expression levels of the genes, involved in the elongation and early modification steps of HS biosynthesis (EXT1/2, NDST1/2, GLCE). Formally, 2-fold changes in the expression of some sultotransferases (3OST1/HS3ST1, 6OST2/HS6ST2) were detected in colon tumours (Fig. 4b, d) but their very low expression levels in both tissues did not let to speculate on it. The most significant changes in the transcriptional activity of the HS biosynthetic machinery were shown for such a GAG modification step as post-synthetic desulfatation. A 3- to 4-fold up-regulation of sulfatase-1 (SULF1) expression was determined in colon tumours compared with matched normal colon tissues (Fig. 4c), along with a 1.5-fold increase of the sulfatase-2 (SULF2) expression level (Fig. 4e). Interestingly, the genes are almost non-expressed in colon carcinoma cells (Fig. 5), suggesting a stromal origin of the SULF1/2 expression and more active desulfatation of HS in the colon cancer microenvironment compared with normal colon tissue.
The conventional HTC116 and cancer-initiating AG2 colon carcinoma cells both showed similar expression patterns for HS metabolism-involved genes, although an activation of HS elongation and early modification steps occurs in AG2 cancer-initiating cells (Fig. 5b). The results logically correspond to up-regulation of syndecan-1 and perlecan expressions in AG2 cells (Fig. 3) and suggest that the colon cancer stem cells are characterised by both increased expression of syndecan-1 and perlecan core proteins and simultaneous activation of their HS chains biosynthetic system. At the same time, HCT116 cells expressed no HS proteoglycans core proteins and displayed low transcriptional activity of HS biosynthesis-involved genes (Figs. 3, 5a).
Extracellular components expression in human colon tumours
To further analyse colon cancer-associated stromal components, expression of collagen 1A1 (COL1A1), fibronectin and elastin were determined in colon carcinoma cell lines in vitro and normal and cancer colon tissues (matched pairs for each patient with malignancy) in vivo.
Collagen 1A1 and fibronectin were expressed in normal colon tissues in vivo, with a significant up-regulation of COL1A expression (6- to 7-fold) in colon tumours (Fig. 6a, b). However, no collagen 1A1 and fibronectin expression was detected in HCT116 and AG2 colon carcinoma cells, suggesting COL1A1 as a potential stromal biomarker for colon carcinogenesis.
Collagen and fibronectin expression in human normal colon tissues and colon tumours. a collagen 1A1, b fibronectin. Real-time RT–PCR analysis, intensity of the amplified DNA fragments normalised to that of GAPDH. The graphs show the mean expression levels from triplicate experiments (±SD) (OriginPro 8.1), 33–126 patients
Taken together, the obtained data show similar total transcriptional activities of proteoglycan core protein genes in normal and cancer colon tissues. The total expression level of HS biosynthesis-involved genes in colon tumours is also not changed compared with normal colon tissue. At the same time, higher variability of the colon tumours for both PGs and HS biosynthesis-involved genes expression and changes in the transcriptional patterns of the expressed genes were revealed. In colon tumours, significant up-regulation of biglycan, versican and collagen1A1 occurred, although no biglycan, versican and collagen1A1 expression was detected in colon carcinoma cell lines in vitro. Comparative analysis of the conventional HCT116 colon carcinoma cell line with AG2 colon cancer-initiating cells revealed mainly different proteoglycan expression patterns (chondroitin sulfate proteoglycan versican vs. heparan sulfates proteoglycans syndecan-1 and perlecan, respectively), hypothesising syndecan-1 and perlecan as new potential biomarkers for colon cancer stem cells.
The demonstrated disbalance in the expression of different ECM components (proteoglycans, collagen1A1, fibronectin) and heparan sulfate modifying enzyme(s) (SULF1/2) in colon tumours seem to be associated with some structural characteristic of cancer-associated stroma and suggest biglycan, versican, collagen1A1 and SULF1 as potential microenvironmental biomarkers in colon cancer.
Discussion
The main purpose of the study was to reveal specific total expression pattern(s) of different proteoglycans, ECM molecules (collagen, fibronectin, elastin) and HS metabolism-involved genes in normal and cancer colon tissues in vivo and colon cancer cells in vitro and provide an additional information to controversial points concerning the expression of some individual proteoglycans (decorin, versican, syndecan-1) in colon cancer.
Proteoglycans expression is deregulated in colon tumours in vivo
In spite of numerous publications on the involvement of individual PGs in carcinogenesis, complex patterns of PGs expression in different tissues remain obscure. For the moment, different sets of PGs have been studied in different tumours: expression of syndecan, glypican, perlecan and CD44 was analysed in human lung cancer cells (Nackaerts et al. 1997); syndecans-1,2,4, glypicans-1,3,5 and CD44 in human ovarian carcinoma cells (Kokenyesi 2001); syndecan-1,2,4, biglycan, versican in malignant mesothelioma (Gulyás and Hjerpe 2003); aggrecan, versican, decorin and biglycan in advanced laryngeal squamous cell carcinoma (Skandalis et al. 2004); decorin, lumican, aggrecan, syndecan-1 and glypican-1 in human breast cancer (Eshchenko et al. 2007); decorin, lumican, aggrecan, versican, NG2, brevican, syndecan-1, glypican-1 and perlecan in human prostate cancer (Suhovskih et al. 2013); and glypican-1,5 and syndecan-2 in human neuroendocrine tumors (García-Suárez et al. 2014). As to colon cancer, simultaneous expression of decorin, glypican-1,3 and syndecan-1,2 (in decreasing order of magnitude) is shown in normal colon tissue and all the PGs (especially syndecan-1 and glypican-3) are down-regulated in colon carcinomas, with evident heterogeneity of the colon tumours for these parameters (Joo et al. 2014). Also, lumican, biglycan, decorin, versican and perlecan expression is shown in colon cancer cells and/or tissues according to numerous studies (see “Introduction”).
Our results are in good agreement with the published data and the expression levels of all previously identified genes were detected in colon normal and cancer tissues. Additionally, for the first time, serglycin, perlecan and NG2/CSPG4 expression was shown in normal tissue, being moderately decreased in colon tumours. Taken together, all the proteoglycans create a specific transcriptional pattern of PGs in normal colon tissue and no major changes in the expressed set of PGs were detected in colon tissues. The main quantitative changes were related to the increase of biglycan and versican and decrease of decorin expression levels, as well as significant up-regulation of collagen1A1, resulting in changed PGs expression patterns (but not overall transcriptional activity) in colon tumours.
The data suppose an existence of a special regulatory mechanism for the balanced expression of different PGs in colon tissue. The mechanism seems control and coordinate transcriptional activity of proteoglycan genes, possibly through some growth factors or ECM components (Kinsella et al. 2004). It was shown that transforming growth factor-beta (TGF-beta) 1 differentially regulates PG genes expression in fibroblasts: it markedly inhibits the expression and secretion of decorin and increases the expression and secretion of versican, biglycan and type I procollagen in the same cultures (Kähäri et al. 1991); IL-10 is able to both regulate decorin, collagen I, fibronectin mRNAs expression in normal skin fibroblasts and reverse TGF-beta effects on the genes (Yamamoto et al. 2001); and co-culturing of human colonic carcinoma cells Caco-2 or HT-29 with stromal cells results in increased versican expression in stromal cells, which appeared to be mediated by TGF-beta and platelet-derived growth factor (PDGF) and promotes transdifferentiation of stromal cells into myofibroblasts (Mukaratirwa et al. 2005). The supposed mechanism seems to be distorted in colon tumours, resulting in high variability of colon tumours according to PGs expression.
Syndecan-1 and perlecan as potential biomarkers for colon cancer-initiating cells
To check a hypothesis that the common deregulation of PGs expression in colon tissue could be involved in increasing of cell population heterogeneity and appearing as potential cancer-initiating cells in the tissue, we performed a comparative analysis of proteoglycans expression in conventional HCT116 cells and primary AG2 colon carcinoma cancer-initiating cells (CICs) (Tallerico et al. 2013). Surprisingly, completely different proteoglycans sets were identified in the cells, where heparan sulfates syndecan-1 and perlecan were dominant in AG2 cells versus chondroitin sulfate versican in HCT116 cells. The high syndecan-1 expression level in AG2 cancer-initiating cells and its absence in HCT116 cells confirm a high heterogeneity of colon cancer cells for syndecan-1 expression (where the main subpopulation of colon cancer cells do not express the proteoglycan) and suggest syndecan-1 as a novel molecular marker for CICs.
The result fits well with some published data on the functional role of syndecans in carcinogenesis (Choi et al. 2013; Barbouri et al. 2014) and is further supported by the recent finding that syndecan-1 is a key molecule maintaining the stability of prostate cancer-initiating cells (Shimada et al. 2013). Selected single cell-derived CD133(+)/CD44(+) PC3 cell clones over-express syndecan-1, the down-regulation of which strongly reduces the number of CD133(+)/CD44(+) prostate cancer cells in culture. In a transgenic mouse prostate adenocarcinoma (TRAMP) model of prostate cancer, early intervention with a syndecan-1 inhibitor (OGT2115) or syndecan-1 RNAi reduces the incidence of adenocarcinoma and the number of c-kit(+)/CD44(+) cells in cancer foci (Shimada et al. 2013). Taken together, the data suggest that the high expression of syndecan-1 in AG2 colon cancer stem cells, in spite of the decrease of its expression in conventional colon carcinoma cells, could “mask” the cancer-initiating cells, helping them to escape a controlling mechanism of anticancer defense and facilitate their invasion and metastasis.
According to the results, another potential companion biomarker for CICs could be perlecan. Although pro-carcinogenic properties of the proteoglycan in colon cancer are shown (Molist et al. 1998; Knox et al. 2001; Sharma et al. 1998), no data on its relationship to CICs were reported. In this study, for the first time perlecan has been revealed as a potential molecular marker for colon cancer-initiating cells. Interestingly, both identified potential markers are heparan sulfate proteoglycans (HSPGs).
So, along with the core proteins expression changes, the correct structure of their heparan sulfate chains could be under question. Because of un-template HS biosynthesis, the complex system of interacting enzymes (“GAGosome”) must operate for proper post-translational modification of syndecan-1 and perlecan core proteins. We showed that the system is more transcriptionally active in AG2 cancer-initiating cells, especially its HS elongation (EXT1/2) and initial post-synthetic modification (NDST1/2, GLCE) steps. At the same time, O-sulfatation (HS3ST1/2, HS6ST1/2) and desulfatation (SULF1/2) steps were almost inactivated in both cell lines. The result supposes that HS chains of syndecan-1 and perlecan in colon cancer stem cells have specific sulfation patterns and that their interaction with physiological ligands and receptors could be distorted. This perfectly fits previously published observations that the overall sulfation and HS chains length were similar in the normal epidermal cell line (JB6) and its transformed counterpart (RT101) but the HS chains of tumour cell-derived perlecan were less sulfated. This resulted from reduced 2-O- and 6-O-sulfation but not N-sulfation and an increase in the proportion of unsulfated disaccharides (Tapanadechopone et al. 2001).
Finally, all the results suggest that colon cancer-initiating cells express syndecan-1 and perlecan proteoglycans with undersulfated (especially 6-O-sulfation) HS chains, in contrast to the conventional HCT116 colon carcinoma cells, expressing predominantly CSPG versican.
Transcriptional patterns of HS metabolism-involved genes in normal and cancer colon tissues
The disbalance of the HS metabolism-involved genes expression pattern was shown in colon cancer tissues compared with normal colon tissue. Whereas no changes in transcriptional activity of elongation- and post-synthetic modification-involved genes were detected in colon tumours, significant up-regulation of sulfatases (SULF1/2) was revealed. The absence of SULF1/2 expression in colon carcinoma cells in vitro suggest their extracellular origin and correspond to previously published data (Hammond et al. 2014). Sulfatases play very important roles in normal cell physiology and malignant transformation of cells (Vivès et al. 2014; Bret et al. 2011) and our results support their important role in colon carcinogenesis. As to the HS biosynthetic system as a whole, there are few published data with which to compare our results. It has been shown that the overall transcriptional activity of the HS biosynthetic system is specifically impaired in benign prostate hyperplasia and prostate cancer (Suhovskih et al. 2014), where expression levels of individual genes in the system were proportionally decreased. However, colon tumours show only qualitative changes in the HS metabolic machinery expression and transcriptional pattern, supposing a distortion of sulfation patterns of HS chains rather than overall impairment of HS biosynthesis in colon cancer.
References
Adany R, Iozzo RV (1990) Altered methylation of versican proteoglycan gene in human colon carcinoma. Biochem Biophys Res Commun 171:1402–1413
Adany R, Heimer R, Caterson B, Sorrell JM, Iozzo RV (1990) Altered expression of chondroitin sulfate proteoglycan in the stroma of human colon carcinoma. Hypomethylation of PG-40 gene correlates with increased PG-40 content and mRNA levels. J Biol Chem 265:11389–11396
Barbouri D, Afratis N, Gialeli C, Vynios DH, Theocharis AD, Karamanos NK (2014) Syndecans as modulators and potential pharmacological targets in cancer progression. Front Oncol 4:4, eCollection
Bi X, Tong C, Dockendorff A, Bancroft L, Gallagher L, Guzman G, Iozzo RV, Augenlicht LH, Yang W (2008) Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis 29:1435–1440
Bi X, Pohl NM, Qian Z, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A, Iozzo RV, Yang W (2012) Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis 33:326–330
Bret C, Moreaux J, Schved JF, Hose D, Klein B (2011) SULFs in human neoplasia: implication as progression and prognosis factors. J Transl Med 9:72
Chatzinikolaou G, Nikitovic D, Asimakopoulou A, Tsatsakis A, Karamanos NK, Tzanakakis GN (2008) Heparin–a unique stimulator of human colon cancer cells’ growth. IUBMB Life 60:333–340
Choi S, Kim Y, Park H, Han IO, Chung E, Lee SY, Kim YB, Lee JW, Oh ES, Yi JY (2009) Syndecan-2 overexpression regulates adhesion and migration through cooperation with integrin alpha2. Biochem Biophys Res Commun 384:231–235
Choi Y, Kim H, Chung H, Hwang JS, Shin JA, Han IO, Oh ES (2010) Syndecan-2 regulates cell migration in colon cancer cells through Tiam1-mediated Rac activation. Biochem Biophys Res Commun 391:921–925
Choi S, Kang DH, Oh ES (2013) Targeting syndecans: a promising strategy for the treatment of cancer. Expert Opin Ther Targets 17:695–705
Eshchenko TY, Rykova VI, Chernakov AE, Sidorov SV, Grigorieva EV (2007) Expression of different proteoglycans in human breast tumors. Biochemistry (Mosc) 72:1016–1020
Galamb O, Sipos F, Spisák S, Galamb B, Krenács T, Valcz G, Tulassay Z, Molnár B (2009) Potential biomarkers of colorectal adenoma-dysplasia-carcinoma progression: mRNA expression profiling and in situ protein detection on TMAs reveal 15 sequentially upregulated and 2 downregulated genes. Cell Oncol 31:19–29
García-Suárez O, García B, Fernández-Vega I, Astudillo A, Quirós LM (2014) Neuroendocrine tumors show altered expression of chondroitin sulfate, glypican 1, glypican 5, and syndecan 2 depending on their differentiation grade. Front Oncol 4:15. doi:10.3389/fonc.2014.00015. eCollection 2014
Gu X, Ma Y, Xiao J, Zheng H, Song C, Gong Y, Xing X (2012) Up-regulated biglycan expression correlates with the malignancy in human colorectal cancers. Clin Exp Med 12:195–199
Gulyás M, Hjerpe A (2003) Proteoglycans and WT1 as markers for distinguishing adenocarcinoma, epithelioid mesothelioma, and benign mesothelium. J Pathol 199:479–487
Hammond E, Khurana A, Shridhar V, Dredge K (2014) The role of heparanase and sulfatases in the modification of heparan sulfate proteoglycans within the tumor microenvironment and opportunities for novel cancer therapeutics. Front Oncol 4:195, eCollection
Haraguchi N, Ohkuma M, Sakashita H, Matsuzaki S, Tanaka F, Mimori K, Kamohara Y, Inoue H, Mori M (2008) CD133+ CD44+ population efficiently enriches colon cancer initiating cells. Ann Surg Oncol 15:2927–2933
Iozzo RV, Cohen I (1993) Altered proteoglycan gene expression and the tumor stroma. Experientia 49:447–455
Iozzo RV, Sanderson RD (2011) Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med 15:1013–1031
Joo EJ, Weyers A, Li G, Gasimli L, Li L, Choi WJ, Lee KB, Linhardt RJ (2014) Carbohydrate-containing molecules as potential biomarkers in colon cancer. OMICS 18:231–241
Kähäri VM, Larjava H, Uitto J (1991) Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem 266:10608–10615
Kinsella MG, Bressler SL, Wight TN (2004) The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr 14:203–234
Knox S, Melrose J, Whitelock J (2001) Electrophoretic, biosensor, and bioactivity analyses of perlecans of different cellular origins. Proteomics 1:1534–1541
Kokenyesi R (2001) Ovarian carcinoma cells synthesize both chondroitin sulfate and heparan sulfate cell surface proteoglycans that mediate cell adhesion to interstitial matrix. J Cell Biochem 83:259–270
Kwon MJ, Kim Y, Choi Y, Kim SH, Park S, Han I, Kang DH, Oh ES (2013) The extracellular domain of syndecan-2 regulates the interaction of HCT116 human colon carcinoma cells with fibronectin. Biochem Biophys Res Commun 431:415–420
Lee H, Kim Y, Choi Y, Choi S, Hong E, Oh ES (2011) Syndecan-2 cytoplasmic domain regulates colon cancer cell migration via interaction with syntenin-1. Biochem Biophys Res Commun 409:148–153
Li YG, Ji DF, Zhong S, Zhu JX, Chen S, Hu GY (2011) Anti-tumor effects of proteoglycan from Phellinus linteus by immunomodulating and inhibiting Reg IV/EGFR/Akt signaling pathway in colorectal carcinoma. Int J Biol Macromol 48:511–517
Lundin M, Nordling S, Lundin J, Isola J, Wiksten JP, Haglund C (2005) Epithelial syndecan-1 expression is associated with stage and grade in colorectal cancer. Oncology 68:306–313
Mikula M, Rubel T, Karczmarski J, Goryca K, Dadlez M, Ostrowski J (2011) Integrating proteomic and transcriptomic high-throughput surveys for search of new biomarkers of colon tumors. Funct Integr Genomics 11:215–224
Molist A, Romarís M, Lindahl U, Villena J, Touab M, Bassols A (1998) Changes in glycosaminoglycan structure and composition of the main heparan sulphate proteoglycan from human colon carcinoma cells (perlecan) during cell differentiation. Eur J Biochem 254:371–377
Mukaratirwa S, Koninkx JF, Gruys E, Nederbragt H (2005) Mutual paracrine effects of colorectal tumour cells and stromal cells: modulation of tumour and stromal cell differentiation and extracellular matrix component production in culture. Int J Exp Pathol 86:219–229
Nackaerts K, Verbeken E, Deneffe G, Vanderschueren B, Demedts M, David G (1997) Heparan sulfate proteoglycan expression in human lung-cancer cells. Int J Cancer 74:335–345
Nikitovic D, Chatzinikolaou G, Tsiaoussis J, Tsatsakis A, Karamanos NK, Tzanakakis GN (2012) Insights into targeting colon cancer cell fate at the level of proteoglycans / glycosaminoglycans. Curr Med Chem 19:4247–4258
Pap Z, Pávai Z, Dénes L, Kovalszky I, Jung J (2009) An immunohistochemical study of colon adenomas and carcinomas: E-cadherin, Syndecan-1, Ets-1. Pathol Oncol Res 15:579–587
Pitule P, Vycital O, Bruha J, Novak P, Hosek P, Treska V, Hlavata I, Soucek P, Kralickova M, Liska V (2013) Differential expression and prognostic role of selected genes in colorectal cancer patients. Anticancer Res 33:4855–4865
Radwanska A, Litwin M, Nowak D, Baczynska D, Wegrowski Y, Maquart FX, Malicka-Blaszkiewicz M (2012) Overexpression of lumican affects the migration of human colon cancer cells through up-regulation of gelsolin and filamentous actin reorganization. Exp Cell Res 318:2312–2323
Ryu HY, Lee J, Yang S, Park H, Choi S, Jung KC, Lee ST, Seong JK, Han IO, Oh ES (2009) Syndecan-2 functions as a docking receptor for pro-matrix metalloproteinase-7 in human colon cancer cells. J Biol Chem 284:35692–35701
Sharma B, Handler M, Eichstetter I, Whitelock JM, Nugent MA, Iozzo RV (1998) Antisense targeting of perlecan blocks tumor growth and angiogenesis in vivo. J Clin Invest 102:1599–1608
Shimada K, Anai S, Fujii T, Tanaka N, Fujimoto K, Konishi N (2013) Syndecan-1 (CD138) contributes to prostate cancer progression by stabilizing tumour-initiating cells. J Pathol 231:495–504
Skandalis SS, Theocharis AD, Theocharis DA, Papadas T, Vynios DH, Papageorgakopoulou N (2004) Matrix proteoglycans are markedly affected in advanced laryngeal squamous cell carcinoma. Biochim Biophys Acta 1689:152–161
Suhovskih AV, Mostovich LA, Kunin IS, Boboev MM, Nepomnyashchikh GI, Aidagulova SV, Grigorieva EV (2013) Proteoglycan expression in normal human prostate tissue and prostate cancer. ISRN Oncol 2013:680136
Suhovskih AV, Tsidulko AY, Kutsenko OS, Kovner AV, Aidagulova SV, Ernberg I, Grigorieva EV (2014) Transcriptional activity of heparan sulfate biosynthetic machinery is specifically impaired in benign prostate hyperplasia and prostate cancer. Front Oncol 4:79, eCollection
Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, Palmieri C, Tirinato L, Pangigadde PN, La Rocca R, Mandelboim O, Stassi G, Di Fabrizio E, Parmiani G, Moretta A, Dieli F, Kärre K, Carbone E (2013) Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol 190:2381–2390
Tapanadechopone P, Tumova S, Jiang X, Couchman JR (2001) Epidermal transformation leads to increased perlecan synthesis with heparin-binding-growth-factor affinity. Biochem J 355:517–527
Theocharis AD (2002) Human colon adenocarcinoma is associated with specific post-translational modifications of versican and decorin. Biochim Biophys Acta 1588:165–172
Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK (2010) Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J 277:3904–3923
Vicente CM, Ricci R, Nader HB, Toma L (2013) Syndecan-2 is upregulated in colorectal cancer cells through interactions with extracellular matrix produced by stromal fibroblasts. BMC Cell Biol 14:25
Vivès RR, Seffouh A, Lortat-Jacob H (2014) Post-synthetic regulation of hs structure: the yin and yang of the sulfs in cancer. Front Oncol 3:331, eCollection
Yamamoto T, Eckes B, Krieg T (2001) Effect of interleukin-10 on the gene expression of type I collagen, fibronectin, and decorin in human skin fibroblasts: differential regulation by transforming growth factor-beta and monocyte chemoattractant protein-1. Biochem Biophys Res Commun 281:200–205
Acknowledgments
The work was supported by a research grant from the Russian Foundation for Basic Research (RFBR 12-04-01657a) and the Ministry of Education and Science of the Russian Federation.
Conflict interest
The authors declare that they have no competing interests or financial disclosure.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suhovskih, A.V., Aidagulova, S.V., Kashuba, V.I. et al. Proteoglycans as potential microenvironmental biomarkers for colon cancer. Cell Tissue Res 361, 833–844 (2015). https://doi.org/10.1007/s00441-015-2141-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2141-8