Abstract
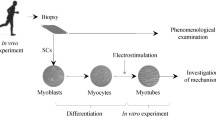
Satellite cells can be isolated from skeletal muscle biopsies, activated to proliferating myoblasts and differentiated into multinuclear myotubes in culture. These cell cultures represent a model system for intact human skeletal muscle and can be modulated ex vivo. The advantages of this system are that the most relevant genetic background is available for the investigation of human disease (as opposed to rodent cell cultures), the extracellular environment can be precisely controlled and the cells are not immortalized, thereby offering the possibility of studying innate characteristics of the donor. Limitations in differentiation status (fiber type) of the cells and energy metabolism can be improved by proper treatment, such as electrical pulse stimulation to mimic exercise. This review focuses on the way that human myotubes can be employed as a tool for studying metabolism in skeletal muscles, with special attention to changes in muscle energy metabolism in obesity and type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Satellite cells can be isolated from skeletal muscle biopsies, activated to proliferating myoblasts and differentiated into multinuclear myotubes in culture. These cell cultures represent an essential model system for intact human skeletal muscle and can be modulated ex vivo. The advantages of this system include: (1) the most relevant genetic background for the investigation of human disease (as opposed to rodent cell cultures), (2) the extracellular environment can be precisely controlled and (3) the cells are not immortalized, thereby offering the possibility of studying innate characteristics of the donor. This review will focus on the way that human myotubes can be used as a tool to study metabolism in skeletal muscles, with special attention to changes in muscle energy metabolism in obesity and type 2 diabetes. Limitations of this cell system and possible approaches for improving the current model will also be discussed.
Characteristics of human myotubes in vitro
Satellite cells were discovered in 1961 by electron microscopy (Mauro 1961). The cells can be isolated from muscle samples by two principally different mechanisms: by enzymatic digestion (Yasin et al. 1977) or by cell migration (Bonavaud et al. 2002; Rosenblatt et al. 1995). In adult skeletal muscle, satellite cells are in a quiescent state but are activated in response to muscle growth or injury (Blau and Webster 1981). Proliferation and differentiation of human muscle satellite cells can occur in vitro, where they form multinucleated myotubes (Blau and Webster 1981). Over the past 30 years, these cells have been extensively used to study glucose and lipid metabolism. The myotubes display morphological, metabolic and biochemical similarities to adult skeletal muscle (Gaster et al. 2001; Henry et al. 1995; Thompson et al. 1996) and thus offer a unique and powerful model for distinguishing between genetic and environmental factors, for instance, in the etiology of insulin resistance. Examples of protocols for maintaining muscle cells in culture are given by Gaster et al. (2001) and Henry et al. (1995).
Activated proliferating satellite cells, also denoted as myoblasts, express myogenic markers including MyoD (Hawke and Garry 2001). After further proliferation, myoblasts either fuse with existing muscle fibers or fuse with other myoblasts and form multinucleated myotubes (Schultz and McCormick 1994; Zammit et al. 2006). The expression of key proteins for both glucose (Al-Khalili et al. 2003) and lipid (Muoio et al. 2002) metabolism increase during the differentiation of myoblasts into myotubes. Compared with myoblasts, the protein expression pattern of myotubes exhibits closer resemblance to adult skeletal muscle and myotubes are therefore preferred for experimental use (Berggren et al. 2007).
Primary human myotubes are generally characterized by low mitochondrial oxidative capacity and their fuel preference is for carbohydrates over lipids (Aas et al. 2011). This might be attributable to the predominant production of ATP by aerobic glycolysis, rather than mitochondrial oxidative phosphorylation (OXPHOS; Warburg effect; Benard et al. 2010) and is possibly the result of a lack of proliferation of mitochondria and OXPHOS activation in the absence of appropriate environmental signals in vitro. The cells are normally grown in energy-rich media with excess glucose concentrations and energy demand is low.
In addition to satellite cells, other progenitor cells have myogenic potential, such as pericyte-derived cells, which can differentiate into myotubes in vitro (Dellavalle et al. 2007). These myotube cultures differ from satellite cell cultures and less is known about the metabolic characteristics of pericyte-derived myotubes.
Glucose and lipid metabolism
In addition to the glucose transporters GLUT1 and GLUT4, other hexose transporters such as GLUT3, GLUT5 and GLUT12 are present in skeletal muscles (Stuart et al. 2006). GLUT1 is thought to be involved mainly in basal glucose transport in both rodent (Hansen et al. 1998) and human (Ciaraldi et al. 2005) skeletal muscle. In response to insulin stimulation, GLUT4 but not GLUT1, is translocated to the cell surface of the skeletal muscle cells, resulting in an increase in glucose uptake (Ploug and Ralston 1998). However, the ratio of GLUT1:GLUT4 is higher in human myotubes compared with adult skeletal muscle (Sarabia et al. 1992), resulting in a lower insulin responsiveness of glucose transport (Al-Khalili et al. 2003; Sarabia et al. 1992). Insulin typically increases glucose uptake by 40%–50% in myotubes (Aas et al. 2002; Al-Khalili et al. 2003; McIntyre et al. 2004; Montell et al. 2001). Even though the effect of insulin is lower in myotubes than in intact skeletal muscle, the responsible molecular mechanisms of glucose transport remain the same (Al-Khalili et al. 2003). Because of a decreased insulin responsiveness of human myotubes, insulin concentrations in the range of 0.1–1 μM are often applied in studies of human myotubes (Aas et al. 2004; Aguer et al. 2011; Al-Khalili et al. 2003; Bell et al. 2010; Gaster et al. 2004; Jackson et al. 2000; Rune et al. 2009a). This insulin concentration is markedly above the physiological concentration, which lies in the picomolar range. Insulin stimulation increases glycogen synthesis about two-fold in human myotubes, similar to in vivo observations (Aas et al. 2004; Al-Khalili et al. 2003, 2005). In intact muscle, glycogen content is linked to glucose uptake and reduced glycogen content increases glucose uptake (J. Jensen et al. 1997). Importantly, low glycogen content also increases insulin-stimulated glucose uptake (J. Jensen et al. 1997) and reduced glycogen content after exercise is a major reason for the improved insulin sensitivity observed under these conditions (J. Jensen et al. 2011). In intact muscles, glycogen content also regulates glucose metabolism and muscles with high glycogen content have reduced glycogen synthesis during insulin stimulation and channel more glucose into glycolysis (J. Jensen et al. 2006). In myotubes, we have not observed increased glycogen content after incubation with high glucose, although basal and insulin-stimulated glucose uptake decreases (Aas et al. 2004). Although the relationship between glycogen and glucose uptake has not yet been clearly established, cultured human muscle satellite cells might provide a suitable model for the study of glucose metabolism, both in the basal and insulin-stimulated states.
The capacity for long-chain fatty acid oxidation has been compared between homogenates of donor muscle biopsies and the derived skeletal muscle cell cultures (Jacobs et al. 1987; Zuurveld et al. 1985). The palmitate oxidation rate (Jacobs et al. 1987; Zuurveld et al. 1985) and the activity of citrate synthase (Jacobs et al. 1987) are comparable between human myotubes and those measured in homogenates of the donor muscle (m. quadriceps). Based on these findings, cultured human muscle cells are concluded to represent a suitable model for studies of mitochondrial oxidative metabolism of muscle.
Muscle contraction
Mature myotubes are quiescent and do not typically contract spontaneously. Muscle contraction stimulates the translocation of GLUT4 and increases glucose uptake (Rose et al. 2009). The mechanism for contraction-stimulated glucose uptake in the myotube is not clear but contraction activates AMP-activated protein kinase (AMPK) and AMPK activation stimulates glucose uptake. However, studies with AMPK knockdown mice have shown that contraction can stimulate glucose uptake, even in muscles in which AMPK is kinase-dead (Maarbjerg et al. 2009). Ca2+/calmodulin-dependent kinase also seems to be involved in contraction-stimulated glucose uptake and a link between muscle glycogen content and contraction-stimulated glucose uptake has been suggested (Aslesen et al. 2001). Recently, human myotubes have been shown to respond to “exercise”, induced by electrical pulse stimulation, in a similar way to exercising muscle in vivo (Lambernd et al. 2012; Nikolic et al. 2012a) and this model might be important for clarifying the mechanisms for contraction-stimulated glucose uptake. Chronic low-frequency stimulation (CLF) increases glucose uptake and oxidation, oleic acid oxidation, mitochondrial content, AMPK activation and interleukin-6 (IL-6) production (Lambernd et al. 2012; Nikolic et al. 2012a). Indeed, insulin resistance induced by adipocyte-conditioned media, chemerin and monocyte chemotactic protein-1 (MCP-1) is abrogated by electrical pulse stimulation (Lambernd et al. 2012). Together, these observations imply that glucose metabolism, insulin signaling and responses to exercise are similar in human myotubes and in skeletal muscle in vivo.
Fiber type comparison
Human muscle fibers express three isoforms of myosin heavy chain (MyHC): MyHC-β, MyHC-2A and MyHC-2X (with their respective genes MYH7, MYH2 and MYH1; Schiaffino and Reggiani 2011). The fourth isoform MHC-2B (MYH4) is not detected in humans. The expression of the MyHC isoforms is associated with muscle fiber phenotype. The muscle fibers that express MyHC-β are characterized as slow, fatigue resistant and oxidative (type 1 muscle fibers), whereas the fibers that express MyHC-2A are fast oxidative (type 2A muscle fibers), and the MyHC-2X fibers are fast glycolytic (type 2X muscle fibers). Most human muscles consist in all three muscle fiber types in varying degrees, depending on the functional demands of the muscle (Harridge et al. 1996), age and gender (Schiaffino and Reggiani 2011). Studies performed in rodents have demonstrated that MyHC isoforms expressed in differentiated myotubes are identical to the MyHC expression pattern in the muscle fibers from which the satellite cells were isolated (Dusterhoft and Pette 1993; Rosenblatt et al. 1996). In human muscle cell cultures, all myotubes in 8-day differentiated cell cultures have been found to be immunoreactive for fast MyHC, regardless of donor muscles having mixed MyHC expression in vivo (Gaster et al. 2001) . In our experiments, myotube cultures stained at day 8 after differentiation also contain a noteworthy amount of slow muscle fibers (Fig. 1) and the cell content of MyHC-β proteins increases after chronic electrical pulse stimulation of the cells, showing plasticity potential (Nikolic et al. 2012a). When satellite cells are isolated from pools of single human skeletal muscle fibers exclusively expressing either the fast or slow MyHC isoform, the isolated cells differentiate into myotubes that co-express both fast and slow MyHC isoforms (Bonavaud et al. 2001). In addition to the fast and slow MyHC isoforms present in adult skeletal muscle in vivo, several developmental MyHC isoforms are expressed in myotubes (Bonavaud et al. 2001).
a Myotubes were fixed, permeabilized and stained for myosin heavy chain 1 (mouse anti-slow muscle myosin monoclonal antibody, red), lipid droplets (BODIPY, green) and nuclei (2,6-diamidino-2-phenylindole, blue). b Human muscle biopsy samples were frozen, sectioned and stained for myosin heavy chain 1 (MAB 1628, Millipore, Mass., USA; red) and lipids (BODIPY 493/503, Invitrogen Molecular Probes; green)
Despite the finding that myotubes can be of different types, debate has been heated as to whether muscle satellite cells are of multiple origin and conflicting results have been reported in the literature depending on the markers used to identify cell type differences, the species used to obtain cells and the culturing conditions (for a review, see Schultz and McCormick 1994). Satellite cells can be heterogenous and we do not know whether distinct populations of fast-twitch (type 2) and slow-twitch (type 1) cells exist. However, when grown in culture, human satellite cells mature into myotubes that express MYH1, MYH2 and MYH7 (Nikolic et al. 2012a). In accordance with our observations in human myotubes, murine satellite cells isolated from various muscles are uniform regardless of muscle origin and the dominant muscle fiber type both in primary murine myoblast and in the murine muscle cell lines is the intermediate MyHC-2A (LaFramboise et al. 2003). MyHC-2X has been suggested to be the default form of the protein in human skeletal muscle (Harridge 2007). An important additional observation is that the expression pattern of MyHC seems to be the same in myotubes from male and female donors (Bonavaud et al. 2001). Taken together, these findings demonstrate that myotubes differ from donor muscle with respect to MyHC expression.
Retention of metabolic characteristics of muscle cell donor
Even though cultured human skeletal muscle cells differ from their donor in MyHC expression, some metabolic properties of the donor muscle are maintained in the derived cell cultures. Several studies have shown that the diabetic phenotype is conserved in myotubes established from type 2 diabetic (T2D) subjects (Gaster et al. 2002; Henry et al. 1996; Thompson et al. 1996) and several potential intrinsic deficiencies in myotubes from individuals with T2D have been reported (Gaster et al. 2002, 2004; Henry et al. 1995; Thompson et al. 1996). For instance, primary defects have been observed in glucose metabolism, such as decreased glucose transport and glycogen synthase activity in myotubes from diabetic individuals (Gaster et al. 2002; Henry et al. 1995, 1996; McIntyre et al. 2004), decreased insulin-stimulated citrate synthase activities (Ortenblad et al. 2005) and impaired glucose oxidation (Wensaas et al. 2009). With respect to lipid metabolism, decreases in both complete palmitate oxidation and fatty acid beta-oxidation have been observed in myotubes from individuals with T2D when compared with control cells from obese but normoglycemic individuals (Cha et al. 2005; Gaster et al. 2004; Wensaas et al. 2009). Moreover, an increased formation of fatty acid beta-oxidation products in diabetic cells when chronically exposed to fatty acids (Wensaas et al. 2009) and a reduced tricarboxylic acid (TCA) cycle flux have also been demonstrated when compared with myotubes established from obese subjects (Gaster 2009). Additionally, myotubes from individuals with T2D show increased lipogenesis and lipid accumulation when chronically treated with a liver X receptor (LXR) agonist (Kase et al. 2005) or in the presence of the polyunsaturated fatty acid eicosapentaenoic acid (Wensaas et al. 2009) when compared with lean and/or obese control cells. Notably, the metabolic differences between myotubes from lean donors and myotubes from obese T2D donors are striking but metabolic differences in myotubes from obese donors and myotubes from obese T2D donors are not always as obvious (Gaster 2007a).
In myotubes established from young healthy subjects, the capacity to increase lipid oxidation in response to increased fatty acid availability in vitro (adaptability) is positively related to insulin sensitivity in vivo (Ukropcova et al. 2005). In a recent study, we have observed that intracellular lipid oxidation and adaptability are reduced in myotubes established from T2D individuals (Corpeleijn et al. 2010). The variation in intracellular lipid oxidation in vitro is significantly related to the in vivo fasting respiratory quotient of the subjects (Corpeleijn et al. 2010). This suggests that skeletal muscle fatty acid handling is intrinsically impaired in T2D individuals (Gaster et al. 2004; Wensaas et al. 2009).
Metabolic switching, representing dynamic changes in fatty acid oxidation in skeletal muscle cells, is also correlated with clinical phenotypes of cell donors. Myotubes retain the characteristics of the donor indicating that metabolic switching is an intrinsic characteristic of skeletal muscle (Gaster 2007b; Ukropcova et al. 2005). This suggests that defects in metabolic switching are one of the primary events in the development of obesity and insulin resistance. We have also found that the lipid content in myotubes is strongly associated with in vivo maximal oxidative capacity, insulin sensitivity, physical activity level and the proportion of type 1 muscle fibers (S. Bajpeyi, unpublished data).
Insulin-stimulated activation of Akt (PKB) is impaired in skeletal muscle of T2D individuals in vivo (Krook et al. 1998). This defect also seems to be maintained in cultures (Cozzone et al. 2008). However, insulin-stimulated phosphorylation of Akt is not reduced in muscles from 72-h-fasted subjects, although severe insulin resistance occurs (Vendelbo et al. 2012). Other components in the insulin-signaling cascade, such as the activation of Akt substrate of 160 kDa (AS160), are also impaired in muscles from T2D subjects (Karlsson et al. 2005). Interestingly, AS160 phosphorylation is also reduced after 72-h fasting and AS160 might be a better intracellular signaling molecule reflecting insulin-stimulated glucose uptake (Vendelbo et al. 2012). After the induction of insulin resistance by palmitic acid in myotubes, phosphorylation of AS160 and other signaling proteins is correspondingly reduced (Bikman et al. 2010). These are only a few examples of the reported signaling defects in myotubes from obese and insulin-resistant subjects showing that results from myotubes reflect the in vivo situation.
Gender is definitely a factor affecting energy metabolism in vivo; however, this difference does not seem to be maintained in myotube cultures (Rune et al. 2009b; Salehzadeh et al. 2011). Both glucose and palmitic acid metabolism are similar in myotubes from male and female donors. Gene (mRNA) expression of several genes involved in glucose and lipid metabolism has been observed to be higher in skeletal muscle biopsies from female vs. male donors but the expression is similar in cultured myotubes from the same donors (Rune et al. 2009b; Salehzadeh et al. 2011).
These observations suggest that the characteristics of the in vivo phenotype are, to a certain extent, carried over to the myotubes in vitro. The precise means by which myotubes are able to retain the in vivo characteristics are not known; however, a combination of genetic and epigenetic mechanisms are probably involved. Epigenetic regulation of skeletal muscle stem cells and skeletal muscle differentiation are well known (see, for example, Bharathy et al. 2012; Sousa-Victor et al. 2011). Exercise (Barres et al. 2012; Nitert et al. 2012), diet (Jacobsen et al. 2012) and a family history of type 2 diabetes (Nitert et al. 2012) are all described to influence DNA methylation in human skeletal muscle, traits that might accompany the isolated satellite cells into their corresponding cultured myotubes. Thus, acute exercise has been found to induce hypomethylation of promoters of several metabolic genes (Barres et al. 2012) and a 6-month exercise intervention induces decreased methylation of genes involved in, for example, retinol metabolism, the calcium-signaling pathway, starch and sucrose metabolism and the insulin-signaling pathway (Nitert et al. 2012). Short-term (5 days) high-fat overfeeding introduces widespread DNA-methylation changes, which are only partly reversed after 6–8 weeks (Jacobsen et al. 2012).
Mutations are of course carried over from the in vivo to the in vitro situation. One example is the naturally occurring R225W mutation in the skeletal-muscle-specific γ3 subunit of the cellular energy sensor AMPK. This mutation, identified in humans in 2007 (Costford et al. 2007), has been shown to result in increased muscle glycogen and decreased intramuscular triacylglycerol in R225W carriers and is linked to increased AMPK activity in R225W cultured myotubes. Further studies have shown that the fuel storage phenotype is maintained in the R225W myotubes and that R225W myotubes also have increased mitochondrial content, oxidative capacity and glucose uptake, the latter of which is consistent with the finding that R225W carriers show a trend for increased skeletal muscle glucose uptake in vivo, as measured by [18F]-fluorodeoxyglucose positron emission tomography (Crawford et al. 2010). The maintenance of the R225W carrier phenotype in R225W myotubes is another example of the donor-myotube similarities that make primary human myotube culture systems an extremely useful tool for metabolic research.
Limitations of myotube model
Cultured myotubes lack the in vivo microenvironment and the communication that exist with other cells through direct contact and via bioactive substances. Indeed, co-cultures with adipocytes might overcome some of these limitations (Trayhurn et al. 2011). Importantly, the uptake of glucose in vivo is regulated by its delivery, transport and metabolism (Rose and Richter 2005), whereas cultured myotubes only allow the studies of its transport and metabolism. In these studies, another limitation of the myotube model is that, compared with in vivo, the insulin-stimulated glucose uptake in primary human myotubes is relatively low (about 1.5-fold increased; Aas et al. 2004; Al-Khalili et al. 2003; McIntyre et al. 2004; Montell et al. 2001), possibly caused by a low expression of the insulin-responsive glucose transporter GLUT4 (Al-Khalili et al. 2003; Sarabia et al. 1992). In vivo, GLUT4 is more highly expressed in type 1 oxidative muscle fibers than in type 2 fibers (Daugaard et al. 2000; Gaster et al. 2000; Stuart et al. 2006) and insulin sensitivity correlates with the proportion of slow-twitch oxidative fibers in the muscle (Lillioja et al. 1987). Insulin-stimulated glucose uptake is generally greater in slow-twitch-enriched muscles than fast-twitch muscles (Zierath and Hawley 2004). For example, white gastrocnemius in rat containing mostly type 2 fibers shows a seven-fold response to insulin, whereas glucose uptake increases 11-fold in red gastrocnemius (James et al. 1985). Therefore, ways to change the phenotype to a more oxidative type would improve insulin sensitivity. W shoulde also note that the denervation of skeletal muscles reduces GLUT4 expression and causes insulin resistance (E.B. Jensen et al. 2009). Indeed, the innervation of human myotubes is possible in vitro by co-culturing with rat motor neurons and such innervation increases GLUT4 expression (Chowdhury et al. 2005). However, more convenient methodologies are required.
Clear differences have also been found in gene expression between muscle biopsies and myotubes. A direct comparison of mRNA expression between skeletal muscle biopsy and cultures from human myotube has shown that most genes that we study are expressed to a higher extent in biopsies than in cells (Fig. 2). The only exception is the lipid droplet protein PLIN3. This implies that fewer active cells are present, that they are possibly not completely matured to muscle fibers, or simply that the cells are more quiescent. Indeed, muscles strips from human adult differentiated muscles can be used to study glucose uptake and GLUT4 translocation in vitro (Lund et al. 1997). This methodology has provided important information but the muscle strips also have their limitations: gene transcription cannot be manipulated and the strips have limited viability.
Myotube to muscle tissue mRNA expression ratio of selected genes. Total RNA was isolated, cDNA was synthesized and real-time quantitative polymerase chain reaction was performed. Expression of the selected genes was quantified according to the average of two housekeeping genes (D-glyceraldehyde-3-phosphate dehydrogenase and acidic ribosomal phosphoprotein P0). The average ratio of the expression in myotubes to muscle tissue from the same donor is given ± SEM (n=4–18; PGC-1 peroxisome proliferator-activated receptor γ coactivator-1, PPAR peroxisome proliferator-activated receptor, ERR estrogen-related receptor, GABPA GA-binding protein transcription factor alpha-subunit 60 kDa, MCAD medium-chain acyl-CoA dehydrogenase, CPT1b carnitine palmitoyltransferase 1b, PDK4 pyruvate dehydrogenase kinase 4, COXIV cytochrome c oxidase IV, CD36 cluster of differentiation 36/fatty acid translocase, PLIN perilipin, MYH myosin heavy chain, ATGL adipose triglyceride lipase)
Adult stem cells are known to have a finite replication potential. Human myotubes from myoblast cultures undergoing senescence exhibit defects in glucose and lipid metabolism, as shown by Nehlin et al. (2011). In their study, muscle-biopsy-derived human satellite cells were grown at various passages and were differentiated into human myotubes in culture in order to analyze the functional state of the various carbohydrate and lipid metabolic pathways. A number of cellular functions were altered as the proliferative potential of myoblasts decreased with increasing passage number. The capacity of the myoblasts to fuse and differentiate into myotubes was reduced and metabolic processes in the myotubes, such as glucose uptake, glycogen synthesis, glucose oxidation and fatty acid beta-oxidation, became gradually impaired. Late-passage non-proliferating myoblast cultures showed strong senescence-associated β-galactosidase activity, indicating that the observed metabolic defects accompanied the induction of a senescent state (Nehlin et al. 2011).
Strategies to improve human myotube model
Several parameters of the cell culture model can be improved by using various approaches and we have, in this review, focused on some ways of improving oxidative capacity (Fig. 3). The muscle phenotype can be changed; as has long been known, muscle contractile activity impacts the muscle phenotype and the phenotype can change when the motor nerve activity is altered (Vrbova 1963). In humans, the muscle fiber type composition varies among individuals depending on age, gender, genetic background and activity level. The gastrocnemius of sprint athletes, for instance, is dominated by fast-twitch type 2 fibers and that of top endurance athletes by slow-twitch type 1 fibers (Costill et al. 1976). The transition from slow- to fast-twitch muscle fiber occurs after denervation and by disuse of the muscles, such as after spinal cord injury (Andersen et al. 1996). Increasing age has also been associated with an increased proportion of fast fibers (D'Antona et al. 2003). However, this is controversial, since loss of type 2 fibers with age has also been shown (Lexell 1995), as have changes in biochemical parameters without changes in fiber type composition (He et al. 2001). Type 2 diabetes is also associated with an increased proportion of fast, glycolytic type 2 fibers (Marin et al. 1994; Oberbach et al. 2006). The transition from fast to slow fibers is more difficult to achieve in humans than the other way round. The experimental model of CLF in animals has shown such transitions in rabbits and rats (for a review, see Pette and Vrbova 1999). The plasticity of skeletal muscle in general has been thoroughly reviewed by others (Fluck and Hoppeler 2003; Gundersen 2011; Schiaffino and Reggiani 2011).
Suggested strategies to improve oxidative capacity of myotubes and some mechanisms involved. Muscle fiber type switch and improved oxidative capacity might be achieved by chronic low-frequency stimulation (CLF). Stimulation of AMP-dependent kinase (AMPK) by 5-amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide (AICAR) and GW501516, via activation peroxisome proliferator-activated receptor (PPAR) δ, increase fatty acid oxidation. Other nuclear receptors, such as the other PPARs and liver X receptor (LXR), might improve myotube metabolism and metformin increases energy uptake. Palmitate, forskolin and ionomycin (PFI) treatment improves lipid metabolism and insulin sensitivity. The activator of SIRT (silent mating type information regulation 2 homolog), SRT1720, increases lipid oxidation probably via PPAR γ coactivator 1α (PGC-1α). Incubation of myotubes with certain nutrients such as galactose, fatty acids and lactate can increase oxidative energy metabolism (PKA protein kinase A)
Since myotubes in culture seem to be rather glycolytic, strategies to improve oxidative capacity, i.e., by inducing fiber type switching from fast type 2 to slow type 1 fibers, would improve the model. The mechanisms involved in fiber type switching are not known in detail but clearly, gene expression must be changed in some way. Prolonged increase in intracellular calcium, activation of the calcineurin pathway and dephosphorylation of the transcription factor nuclear factor of activated T-cells (NFAT) have been shown to initiate the slow fiber type program (Chin et al. 1998). Slow fiber type genes have also been shown to be upregulated by the extracellular signal-regulated kinases (ERK) pathway in rats (Murgia et al. 2000) and by peroxisome-proliferator-activated receptor-gamma co-activator-1 alpha (PGC-1α) in mice (Lin et al. 2002). PGC-1β, on the other hand, is involved in fast-twitch (type 2X) fiber transition (Arany et al. 2007). In human skeletal muscle in vivo, a correlation seems to exist between the expression of the peroxisome proliferator-activated receptors (PPAR) α and δ and the proportion of type 1 fibers (Kramer et al. 2006; Russell et al. 2003). Overexpression of a constitutive active PPARδ in adult rat muscles induces slow muscle properties (Lunde et al. 2007). PGC-1α overexpression in primary human myotubes promotes increased lipid oxidative capacity, increases the expression of genes involved in the regulation of mitochondrial function and biogenesis and decreases the expression of a fast fiber type gene marker (MyHC-2A) when compared with cells infected with an empty retrovirus-vector (Nikolic et al. 2012). We have also observed that PGC-1α overexpression increases the expression of several genes that are markedly down-regulated during the process of cell isolation and culturing when compared with skeletal muscle in vivo. After treatment of PGC-1α-transfected cells with the selective PPARδ agonist GW501516, palmitate oxidation is further increased. GW501516 also increases mRNA levels for carnitine palmitoyltransferase-1 (CPT-1). Additionally, a dose-dependent increase in oleate oxidation to CO2 has been observed in myotubes pretreated for 3 days with this compound during the differentiation of the cells (Wensaas et al. 2007). The PPARδ agonist has also been shown to increase basal and insulin-mediated glucose transport and to lead to the phosphorylation of AMPK and p38 mitogen-activated protein kinase (MAPK) in cultured human myotubes (Kramer et al. 2007).
We have recently shown that chronic low-frequency electrical pulse stimulation of human myotubes increases glucose and fatty acid oxidation and mitochondrial content (Nikolic et al. 2012a). The protein content of MyHC-β increases, indicating a fiber type switch. Another recent study by Lambernd et al. (2012) has demonstrated that insulin-stimulated glucose uptake is increased and that insulin resistance can be prevented by chronic electrical pulse stimulation. In murine C2C12 cells exposed to electrical pulse stimulation (40 V/60 mm, 24 ms, 1 Hz), a decrease in type 2 fibers and an increase in type 1 fibers occur. In addition, ERK1/2, ERK5 and c-Jun N-terminal kinase (JNK) are activated and glucose uptake is improved (Nedachi et al. 2008). GLUT4 mRNA expression is unaltered but GLUT4 recycling increases, both under basal and insulin-stimulated conditions (Nedachi et al. 2008). In animal studies, the elevation in PGC-1α is associated with fiber-type switch from glycolytic fast-twitch type 2B/2X to oxidative slow-twitch type 2A and 1 muscle fibers (Handschin et al. 2007; Lin et al. 2002). The expression of PGC-1α also increases in primary rat skeletal muscle cells after electric stimulation for 90 min a day for 5 days (Silveira et al. 2006) and in C2C12 cells stimulated for 90 min a day for 4 days or after CLF for 24 h (Burch et al. 2010). In this latter study, the expression of several genes involved in oxidative phosphorylation and in glucose and fatty acid metabolism increases concomitantly, although not followed by a clear fiber type switch, since both MyHC-β and MyHC-2X increase (Burch et al. 2010). All these studies indicate that oxidative capacity can be improved by electrical pulse stimulation (exercise mimetic) of the myotubes.
In adult rat muscle, PPARδ expression has been shown to be influenced by muscle contractile activity and to induce slow muscle fiber properties after somatic gene transfer (Lunde et al. 2007). Thus, strategies to enhance PPARδ activity, by either agonist treatment or gene transfer (not yet shown in human myotubes), might increase the oxidative potential of myotubes and promote fiber type switching. However, other PPARs might also be targets for increased oxidative capacity. Muoio et al. (2002) have demonstrated that PPARα protein expression is induced during myocyte differentiation and its activation stimulates lipid oxidation (Djouadi et al. 2005; Muoio et al. 2002) and decreases triacylglycerol accumulation (Muoio et al. 2002) in primary human myotubes. Treatment with the PPARγ agonist troglitazone reverses impaired fatty acid metabolism in skeletal muscle cells from individuals with T2D (Cha et al. 2005), whereas all the thiazolidinediones (troglitazone, rosiglitazone and pioglitazone) normalize the impaired fatty acid uptake (Wilmsen et al. 2003).
Other pharmacological approaches, such as the treatment of differentiated myotubes with the pan-PPAR agonist tetradecylthioacetic acid, improves complete palmitate oxidation in diabetic myotubes, opposes increased lipid accumulation and increases glucose oxidation (Wensaas et al. 2009). The activation of cAMP/PKA and calcium signaling pathways is known to promote mitochondrial biogenesis and increase lipid oxidation capacity in vivo (Baar et al. 2002; Hood 2001). We have hypothesized that the activation of these signaling pathways in human primary myotubes will induce functional changes in lipid and glucose metabolism. The activation of cAMP/PKA and calcium signaling pathways by PFI-treatment (palmitic acid, forskolin and ionomycin) increases lipid oxidation, storage and lipolysis and improves insulin responsiveness in human primary myotubes (Sparks et al. 2011).
Activation of LXR in myotubes by an LXR agonist (T0901317) increases glucose uptake and oxidation but also promotes lipogenesis and increases lipid storage (Kase et al. 2005, 2007). This is further enhanced in myotubes established from T2D donors. However, the increased lipid storage in T2D myotubes does not impair insulin-stimulated glucose metabolism during the 4-day treatment of the cells with an LXR agonist (Kase et al. 2005, 2007). A new strategy is to grow the myoblasts in nutrient-limited culturing media to increase the pressure for metabolic remodeling. The OXPHOS vs. glycolytic capacity is possibly changed to increase the ATP yield (Vander Heiden et al. 2009). As is well known, cancer cells in particular but also other cells, become highly glycolytic when grown in high-glucose concentrations. Glucose inhibits oxidative phosphorylation, a phenomenon known as the Crabtree effect (Diaz-Ruiz et al. 2011; Ibsen 1961). This effect can possibly be avoided by growing cells in media with other nutrients, such as amino acids, lipids, or lactate. A problem with this approach is that proliferating cells deficient in ATP undergo apoptosis or cell cycle arrest. However, the replacement of glucose with galactose during the differentiation of myoblasts to myotubes increases the basal mitochondrial oxygen consumption rate (Aguer et al. 2011). Mitochondrial biogenesis is not increased but some mitochondrial enzymes are increased, as is AMPK activity (Aguer et al. 2011). We have recently confirmed an increased oxidative capacity in myotubes grown and differentiated in galactose-containing media, assessed as the increased oxidation of glucose and oleic acid (Kase et al. 2013). We have also shown that the suppression of oleic acid oxidation by glucose is improved. Preliminary data indicate that the removal of glucose (no glucose DMEM with 10% fetal calf serum) and the replacement of glucose with lactate (5 mM) have similar effects on oxidative capacity (unpublished observations).
Like AMPK, the sirtuins (e.g., silent mating type information regulation 2 homologue 1, SIRT1) are also regarded as fuel-sensing enzymes. AMPK and SIRT1 have a long-standing partnership, whereby they can regulate each other (Ruderman et al. 2010). Although the effect is less than the effect from PPARδ activation, the treatment of human myotubes for 4 days with the AMPK-activator 5-amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide (AICAR) or the SIRT-activator SRT1720 leads to about 60% increased oleic acid oxidation (Fig. 4).
Oleic acid oxidation in human myotubes after pretreatment with a PPARδ agonist (GW501516), the AMPK-activator 5-amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide (AICAR) and a SIRT-activator (SRT1720). Myotubes were pretreated with GW501516, AICAR, or SRT1720 for 24 h, before 100 μM 14C-labeled oleic acid was added and CO2 was trapped for 4 h. Average amount of CO2 ± SEM is given (n=10). *Significantly increased from control (P<0.05)
The antidiabetic drug metformin, which is an activator of AMPK, has been shown to increase glucose uptake (Sarabia et al. 1992) and insulin-stimulated glycogen synthesis in human myotubes (Al-Khalili et al. 2005). However, any stimulatory effect of metformin on oxidative capacity has, to our knowledge, not been shown. Neither have we been able to increase substrate oxidation by metformin (10-100 nM for 4–8 days). Glucose uptake increases significantly by about 25% (±3) and oleic acid uptake by 19% (±3), whereas oxidation of the same substrates does not increase equally (unpublished data).
In vitro, muscle cells fuse to multinucleated fibers that are randomly arranged, unlike the parallel fibers in intact muscles. Various attempts to affect myotube organization in vitro have been tried, such as culturing the cells on micro-patterned glass (Yamamoto et al. 2008), in three-dimensional gels, or in flow chambers. Co-cultures with adipocytes or neurons is another approach used to mimic the in vivo situation more closely. Sophistication of the culturing technique will possibly improve the muscle cell system.
Concluding remarks
To summarize, human myotubes seem to be a valuable model for the study of glucose and lipid metabolism. Some limitations have been noted in the differentiation status (fiber type) of the cells and energy metabolism but these can be improved by proper treatment, such as electrical pulse stimulation to mimic exercise. In vitro vs. in vivo translatability should be further studied, since epigenetic mechanisms are possibly translated to cultured myotubes.
References
Aas V, Torbla S, Andersen MH, Jensen J, Rustan AC (2002) Electrical stimulation improves insulin responses in a human skeletal muscle cell model of hyperglycemia. Ann N Y Acad Sci 967:506–515
Aas V, Kase ET, Solberg R, Jensen J, Rustan AC (2004) Chronic hyperglycaemia promotes lipogenesis and triacylglycerol accumulation in human skeletal muscle cells. Diabetologia 47:1452–1461
Aas V, Hessvik NP, Wettergreen M, Hvammen AW, Hallen S, Thoresen GH, Rustan A (2011) Chronic hyperglycemia reduces substrate oxidation and impairs metabolic switching of human myotubes. Biochim Biophys Acta Mol Basis Dis 1812:94–105
Aguer C, Gambarotta D, Mailloux RJ, Moffat C, Dent R, McPherson R, Harper ME (2011) Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PLoS One 6:e28536
Al-Khalili L, Chibalin AV, Kannisto K, Zhang BB, Permert J, Holman GD, Ehrenborg E, Ding VD, Zierath JR, Krook A (2003) Insulin action in cultured human skeletal muscle cells during differentiation: assessment of cell surface GLUT4 and GLUT1 content. Cell Mol Life Sci 60:991–998
Al-Khalili L, Forsgren M, Kannisto K, Zierath JR, Lonnqvist F, Krook A (2005) Enhanced insulin-stimulated glycogen synthesis in response to insulin, metformin or rosiglitazone is associated with increased mRNA expression of GLUT4 and peroxisomal proliferator activator receptor gamma co-activator 1. Diabetologia 48:1173–1179
Andersen JL, Mohr T, Biering-Sorensen F, Galbo H, Kjaer M (1996) Myosin heavy chain isoform transformation in single fibres from m. vastus lateralis in spinal cord injured individuals: effects of long-term functional electrical stimulation (FES). Pflugers Arch 431:513–518
Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM (2007) The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5:35–46
Aslesen R, Engebretsen EM, Franch J, Jensen J (2001) Glucose uptake and metabolic stress in rat muscles stimulated electrically with different protocols. J Appl Physiol 91:1237–1244
Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO (2002) Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16:1879–1886
Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O'Gorman DJ, Zierath JR (2012) Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 15:405–411
Bell JA, Reed MA, Consitt LA, Martin OJ, Haynie KR, Hulver MW, Muoio DM, Dohm GL (2010) Lipid partitioning, incomplete fatty acid oxidation, and insulin signal transduction in primary human muscle cells: effects of severe obesity, fatty acid incubation, and fatty acid translocase/CD36 overexpression. J Clin Endocrinol Metab 95:3400–3410
Benard G, Bellance N, Jose C, Melser S, Nouette-Gaulain K, Rossignol R (2010) Multi-site control and regulation of mitochondrial energy production. Biochim Biophys Acta 1797:698–709
Berggren JR, Tanner CJ, Houmard JA (2007) Primary cell cultures in the study of human muscle metabolism. Exerc Sport Sci Rev 35:56–61
Bharathy N, Ling BM, Taneja R (2012) Epigenetic regulation of skeletal muscle development and differentiation. Subcell Biochem 61:139–150
Bikman BT, Zheng D, Reed MA, Hickner RC, Houmard JA, Dohm GL (2010) Lipid-induced insulin resistance is prevented in lean and obese myotubes by AICAR treatment. Am J Physiol Regul Integr Comp Physiol 298:R1692–R1699
Blau H, Webster C (1981) Isolation and characterization of human muscle cells. Proc Natl Acad Sci USA 78:5623–5627
Bonavaud S, Agbulut O, Nizard R, D'Honneur G, Mouly V, Butler-Browne G (2001) A discrepancy resolved: human satellite cells are not preprogrammed to fast and slow lineages. Neuromuscul Disord 11:747–752
Bonavaud S, Agbulut O, D'Honneur G, Nizard R, Mouly V, Butler-Browne G (2002) Preparation of isolated human muscle fibers: a technical report. In Vitro Cell Dev Biol Anim 38:66–72
Burch N, Arnold AS, Item F, Summermatter S, Brochmann Santana Santos G, Christe M, Boutellier U, Toigo M, Handschin C (2010) Electric pulse stimulation of cultured murine muscle cells reproduces gene expression changes of trained mouse muscle. PLoS One 5:e10970
Cha BS, Ciaraldi TP, Park KS, Carter L, Mudaliar SR, Henry RR (2005) Impaired fatty acid metabolism in type 2 diabetic skeletal muscle cells is reversed by PPARgamma agonists. Am J Physiol Endocrinol Metab 289:E151–E159
Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS (1998) A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev 12:2499–2509
Chowdhury HH, Jevsek M, Kreft M, Mars T, Zorec R, Grubic Z (2005) Insulin-induced exocytosis in single, in vitro innervated human muscle fibres: a new approach. Pflugers Arch 450:131–135
Ciaraldi TP, Mudaliar S, Barzin A, Macievic JA, Edelman SV, Park KS, Henry RR (2005) Skeletal muscle GLUT1 transporter protein expression and basal leg glucose uptake are reduced in type 2 diabetes. J Clin Endocrinol Metab 90:352–358
Corpeleijn E, Hessvik NP, Bakke SS, Levin K, Blaak EE, Thoresen GH, Gaster M, Rustan AC (2010) Oxidation of intramyocellular lipids is dependent on mitochondrial function and the availability of extracellular fatty acids. Am J Physiol Endocrinol Metab 299:E14–E22
Costford SR, Kavaslar N, Ahituv N, Chaudhry SN, Schackwitz WS, Dent R, Pennacchio LA, McPherson R, Harper ME (2007) Gain-of-function R225W mutation in human AMPKgamma(3) causing increased glycogen and decreased triglyceride in skeletal muscle. PLoS One 2:e903
Costill DL, Daniels J, Evans W, Fink W, Krahenbuhl G, Saltin B (1976) Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol 40:149–154
Cozzone D, Frojdo S, Disse E, Debard C, Laville M, Pirola L, Vidal H (2008) Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from type 2 diabetic patients. Diabetologia 51:512–521
Crawford SA, Costford SR, Aguer C, Thomas SC, deKemp RA, DaSilva JN, Lafontaine D, Kendall M, Dent R, Beanlands RS, McPherson R, Harper ME (2010) Naturally occurring R225W mutation of the gene encoding AMP-activated protein kinase (AMPK)gamma(3) results in increased oxidative capacity and glucose uptake in human primary myotubes. Diabetologia 53:1986–1997
D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R (2003) The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol (Lond) 552:499–511
Daugaard JR, Nielsen JN, Kristiansen S, Andersen JL, Hargreaves M, Richter EA (2000) Fiber type-specific expression of GLUT4 in human skeletal muscle: influence of exercise training. Diabetes 49:1092–1095
Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G (2007) Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9:255–267
Diaz-Ruiz R, Rigoulet M, Devin A (2011) The Warburg and Crabtree effects: on the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim Biophys Acta 1807:568–576
Djouadi F, Aubey F, Schlemmer D, Bastin J (2005) Peroxisome proliferator activated receptor delta (PPARdelta) agonist but not PPARalpha corrects carnitine palmitoyl transferase 2 deficiency in human muscle cells. J Clin Endocrinol Metab 90:1791–1797
Dusterhoft S, Pette D (1993) Satellite cells from slow rat muscle express slow myosin under appropriate culture conditions. Differentiation 53:25–33
Fluck M, Hoppeler H (2003) Molecular basis of skeletal muscle plasticity—from gene to form and function. Rev Physiol Biochem Pharmacol 146:159–216
Gaster M (2007a) Insulin resistance and the mitochondrial link. Lessons from cultured human myotubes. Biochim Biophys Acta 1772:755–765
Gaster M (2007b) Metabolic flexibility is conserved in diabetic myotubes. J Lipid Res 48:207–217
Gaster M (2009) Reduced TCA flux in diabetic myotubes: a governing influence on the diabetic phenotype? Biochem Biophys Res Commun 387:651–655
Gaster M, Poulsen P, Handberg A, Schroder HD, BeckNielsen H (2000) Direct evidence of fiber type-dependent GLUT-4 expression in human skeletal muscle. Am J Physiol Endocrinol Metab 278:E910–E916
Gaster M, Kristensen SR, Beck-Nielsen H, Schroder HD (2001) A cellular model system of differentiated human myotubes. APMIS 109:735–744
Gaster M, Petersen I, Hojlund K, Poulsen P, Beck-Nielsen H (2002) The diabetic phenotype is conserved in myotubes established from diabetic subjects: evidence for primary defects in glucose transport and glycogen synthase activity. Diabetes 51:921–927
Gaster M, Rustan AC, Aas V, Beck-Nielsen H (2004) Reduced lipid oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin: evidence from cultured myotubes. Diabetes 53:542–548
Gundersen K (2011) Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev Camb Philos Soc 86:564-600
Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM (2007) Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem 282:30014–30021
Hansen PA, Wang W, Marshall BA, Holloszy JO, Mueckler M (1998) Dissociation of GLUT4 translocation and insulin-stimulated glucose transport in transgenic mice overexpressing GLUT1 in skeletal muscle. J Biol Chem 273:18173–18179
Harridge SD (2007) Plasticity of human skeletal muscle: gene expression to in vivo function. Exp Physiol 92:783–797
Harridge SD, Bottinelli R, Canepari M, Pellegrino MA, Reggiani C, Esbjornsson M, Saltin B (1996) Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch 432:913–920
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551
He J, Watkins S, Kelley DE (2001) Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 50:817–823
Henry RR, Abrams L, Nikoulina S, Ciaraldi TP (1995) Insulin action and glucose-metabolism in nondiabetic control and NIDDM subjects—comparison using human skeletal-muscle cell-cultures. Diabetes 44:936–946
Henry RR, Ciaraldi TP, AbramsCarter L, Mudaliar S, Park KS, Nikoulina SE (1996) Glycogen synthase activity is reduced in cultured skeletal muscle cells of non-insulin-dependent diabetes mellitus subjects—biochemical and molecular mechanisms. J Clin Invest 98:1231–1236
Hood DA (2001) Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 90:1137–1157
Ibsen KH (1961) The Crabtree effect: a review. Cancer Res 21:829–841
Jackson S, Bagstaff SM, Lynn S, Yeaman SJ, Turnbull DM, Walker M (2000) Decreased insulin responsiveness of glucose uptake in cultured human skeletal muscle cells from insulin-resistant nondiabetic relatives of type 2 diabetic families. Diabetes 49:1169–1177
Jacobs AE, Oosterhof A, Veerkamp JH (1987) Palmitate oxidation and some enzymes of energy metabolism in human muscles and cultured muscle cells. Int J Biochem 19:1049–1054
Jacobsen SC, Brons C, Bork-Jensen J, Ribel-Madsen R, Yang B, Lara E, Hall E, Calvanese V, Nilsson E, Jorgensen SW, Mandrup S, Ling C, Fernandez AF, Fraga MF, Poulsen P, Vaag A (2012) Effects of short-term high-fat overfeeding on genome-wide DNA methylation in the skeletal muscle of healthy young men. Diabetologia 55:3341–3349
James DE, Jenkins AB, Kraegen EW (1985) Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol 248:E567–E574
Jensen J, Aslesen R, Ivy JL, Brors O (1997) Role of glycogen concentration and epinephrine on glucose uptake in rat epitrochlearis muscle. Am J Physiol Endocrinol Metab 35:E649–E655
Jensen J, Jebens E, Brennesvik EO, Ruzzin J, Soos MA, Engebretsen EM, O'Rahilly S, Whitehead JP (2006) Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling. Am J Physiol Endocrinol Metab 290:E154–E162
Jensen EB, Zheng D, Russell RA, Bassel-Duby R, Williams RS, Olson AL, Dohm GL (2009) Regulation of GLUT4 expression in denervated skeletal muscle. Am J Physiol Regul Integr Comp Physiol 296:R1820–R1828
Jensen J, Rustad PI, Kolnes AJ, Lai YC (2011) The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol 2:112
Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H (2005) Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 54:1692–1697
Kase ET, Wensaas AJ, Aas V, Hojlund K, Levin K, Thoresen GH, Beck-Nielsen H, Rustan AC, Gaster M (2005) Skeletal muscle lipid accumulation in type 2 diabetes may involve the liver X receptor pathway. Diabetes 54:1108–1115
Kase ET, Thoresen GH, Westerlund S, Hojlund K, Rustan AC, Gaster M (2007) Liver X receptor antagonist reduces lipid formation and increases glucose metabolism in myotubes from lean, obese and type 2 diabetic individuals. Diabetologia 50:2171–2180
Kase ET, Nikolic N, Bogen KK, Bakke SS, Aas V, Thoresen GH, Rustan AC (2013) Remodeling of oxidative energy metabolism by galactose improves glucose handling and metabolic switching in human skeletal muscle cells. PLoS One 8:e59972
Kramer DK, Ahlsen M, Norrbom J, Jansson E, Hjeltnes N, Gustafsson T, Krook A (2006) Human skeletal muscle fibre type variations correlate with PPAR alpha, PPAR delta and PGC-1 alpha mRNA. Acta Physiol (Oxf) 188:207–216
Kramer DK, Al-Khalili L, Guigas B, Leng Y, Garcia-Roves PM, Krook A (2007) Role of AMP kinase and PPARdelta in the regulation of lipid and glucose metabolism in human skeletal muscle. J Biol Chem 282:19313–19320
Krook A, Roth RA, Jiang XJ, Zierath JR, Wallberg-Henriksson H (1998) Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes 47:1281–1286
LaFramboise WA, Guthrie RD, Scalise D, Elborne V, Bombach KL, Armanious CS, Magovern JA (2003) Effect of muscle origin and phenotype on satellite cell muscle-specific gene expression. J Mol Cell Cardiol 35:1307–1318
Lambernd S, Taube A, Schober A, Platzbecker B, Gorgens SW, Schlich R, Jeruschke K, Weiss J, Eckardt K, Eckel J (2012) Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia 55:1128–1139
Lexell J (1995) Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50 (Spec No):11–16
Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Jarvinen H, Christin L, Secomb TW, Bogardus C (1987) Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80:415–424
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797–801
Lund S, Holman GD, Zierath JR, Rincon J, Nolte LA, Clark AE, Schmitz O, Pedersen O, Wallberg-Henriksson H (1997) Effect of insulin on GLUT4 cell surface content and turnover rate in human skeletal muscle as measured by the exofacial bis-mannose photolabeling technique. Diabetes 46:1965–1969
Lunde IG, Ekmark M, Rana ZA, Buonanno A, Gundersen K (2007) PPARdelta expression is influenced by muscle activity and induces slow muscle properties in adult rat muscles after somatic gene transfer. J Physiol (Lond) 582:1277–1287
Maarbjerg SJ, Jorgensen SB, Rose AJ, Jeppesen J, Jensen TE, Treebak JT, Birk JB, Schjerling P, Wojtaszewski JF, Richter EA (2009) Genetic impairment of AMPKalpha2 signaling does not reduce muscle glucose uptake during treadmill exercise in mice. Am J Physiol Endocrinol Metab 297:E924–E934
Marin P, Andersson B, Krotkiewski M, Bjorntorp P (1994) Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 17:382–386
Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493–495
McIntyre EA, Halse R, Yeaman SJ, Walker M (2004) Cultured muscle cells from insulin-resistant type 2 diabetes patients have impaired insulin, but normal 5-amino-4-imidazolecarboxamide riboside-stimulated, glucose uptake. J Clin Endocrinol Metab 89:3440–3448
Montell E, Turini M, Marotta M, Roberts M, Noe V, Ciudad CJ, Mace K, Gomez-Foix AM (2001) DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am J Physiol Endocrinol Metab 280:E229–E237
Muoio DM, Way JM, Tanner CJ, Winegar DA, Kliewer SA, Houmard JA, Kraus WE, Dohm GL (2002) Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes 51:901–909
Murgia M, Serrano AL, Calabria E, Pallafacchina G, Lomo T, Schiaffino S (2000) Ras is involved in nerve-activity-dependent regulation of muscle genes. Nat Cell Biol 2:142–147
Nedachi T, Fujita H, Kanzaki M (2008) Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab 295:E1191–E1204
Nehlin JO, Just M, Rustan AC, Gaster M (2011) Human myotubes from myoblast cultures undergoing senescence exhibit defects in glucose and lipid metabolism. Biogerontology 12:349–365
Nikolic N, Bakke SS, Kase ET, Rudberg I, Flo Halle I, Rustan AC, Thoresen GH, Aas V (2012a) Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS One 7:e33203
Nikolic N, Rhedin M, Rustan AC, Storlien L, Thoresen GH, Stromstedt M (2012b) Overexpression of PGC-1alpha increases fatty acid oxidative capacity of human skeletal muscle cells. Biochem Res Int 2012:714074
Nitert MD, Dayeh T, Volkov P, Elgzyri T, Hall E, Nilsson E, Yang BT, Lang S, Parikh H, Wessman Y, Weishaupt H, Attema J, Abels M, Wierup N, Almgren P, Jansson PA, Ronn T, Hansson O, Eriksson KF, Groop L, Ling C (2012) Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes 61:3322–3332
Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schon MR, Bluher M, Punkt K (2006) Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29:895–900
Ortenblad N, Mogensen M, Petersen I, Hojlund K, Levin K, Sahlin K, Beck-Nielsen H, Gaster M (2005) Reduced insulin-mediated citrate synthase activity in cultured skeletal muscle cells from patients with type 2 diabetes: evidence for an intrinsic oxidative enzyme defect. Biochim Biophys Acta 1741:206–214
Pette D, Vrbova G (1999) What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve 22:666–677
Ploug T, Ralston E (1998) Anatomy of glucose transporters in skeletal muscle—effects of insulin and contractions. Adv Exp Med Biol 441:17–26
Rose AJ, Richter EA (2005) Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 20:260–270
Rose AJ, Jeppesen J, Kiens B, Richter EA (2009) Effects of contraction on localization of GLUT4 and v-SNARE isoforms in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 297:R1228–R1237
Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA (1995) Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim 31:773–779
Rosenblatt JD, Parry DJ, Partridge TA (1996) Phenotype of adult mouse muscle myoblasts reflects their fiber type of origin. Differentiation 60:39–45
Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y (2010) AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298:E751–E760
Rune A, Osler ME, Fritz T, Zierath JR (2009a) Regulation of skeletal muscle sucrose, non-fermenting 1/AMP-activated protein kinase-related kinase (SNARK) by metabolic stress and diabetes. Diabetologia 52:2182–2189
Rune A, Salehzadeh F, Szekeres F, Kuhn I, Osler ME, Al-Khalili L (2009b) Evidence against a sexual dimorphism in glucose and fatty acid metabolism in skeletal muscle cultures from age-matched men and post-menopausal women. Acta Physiol 197:207–215
Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Deriaz O (2003) Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52:2874–2881
Salehzadeh F, Rune A, Osler M, Al-Khalili L (2011) Testosterone or 17{beta}-estradiol exposure reveals sex-specific effects on glucose and lipid metabolism in human myotubes. J Endocrinol 210:219–229
Sarabia V, Lam L, Burdett E, Leiter LA, Klip A (1992) Glucose-transport in human skeletal-muscle cells in culture—stimulation by insulin and metformin. J Clin Invest 90:1386–1395
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91:1447–1531
Schultz E, McCormick KM (1994) Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol 123:213–257
Silveira LR, Pilegaard H, Kusuhara K, Curi R, Hellsten Y (2006) The contraction induced increase in gene expression of peroxisome proliferator-activated receptor (PPAR)-gamma coactivator 1alpha (PGC-1alpha), mitochondrial uncoupling protein 3 (UCP3) and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim Biophys Acta 1763:969–976
Sousa-Victor P, Munoz-Canoves P, Perdiguero E (2011) Regulation of skeletal muscle stem cells through epigenetic mechanisms. Toxicol Mech Methods 21:334–342
Sparks LM, Moro C, Ukropcova B, Bajpeyi S, Civitarese AE, Hulver MW, Thoresen GH, Rustan AC, Smith SR (2011) Remodeling lipid metabolism and improving insulin responsiveness in human primary myotubes. PLoS One 6:e21068
Stuart CA, Yin D, Howell ME, Dykes RJ, Laffan JJ, Ferrando AA (2006) Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab 291:E1067–E1073
Thompson DB, Pratley R, Ossowski V (1996) Human primary myoblast cell cultures from non-diabetic insulin resistant subjects retain defects in insulin action. J Clin Invest 98:2346–2350
Trayhurn P, Drevon CA, Eckel J (2011) Secreted proteins from adipose tissue and skeletal muscle—adipokines, myokines and adipose/muscle cross-talk. Arch Physiol Biochem 117:47–56
Ukropcova B, McNeil M, Sereda O, Jonge L de, Xie H, Bray GA, Smith SR (2005) Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest 115:1934–1941
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Vendelbo MH, Clasen BF, Treebak JT, Moller L, Krusenstjerna-Hafstrom T, Madsen M, Nielsen TS, Stodkilde-Jorgensen H, Pedersen SB, Jorgensen JO, Goodyear LJ, Wojtaszewski JF, Moller N, Jessen N (2012) Insulin resistance after a 72-h fast is associated with impaired AS160 phosphorylation and accumulation of lipid and glycogen in human skeletal muscle. Am J Physiol Endocrinol Metab 302:E190–E200
Vrbova G (1963) The effect of motoneurone activity on the speed of contraction of striated muscle. J Physiol (Lond) 169:513–526
Wensaas AJ, Rustan AC, Lovstedt K, Kull B, Wikstrom S, Drevon CA, Hallen S (2007) Cell-based multiwell assays for the detection of substrate accumulation and oxidation. J Lipid Res 48:961–967
Wensaas AJ, Rustan AC, Just M, Berge RK, Drevon CA, Gaster M (2009) Fatty acid incubation of myotubes from humans with type 2 diabetes leads to enhanced release of beta-oxidation products because of impaired fatty acid oxidation: effects of tetradecylthioacetic acid and eicosapentaenoic acid. Diabetes 58:527–535
Wilmsen HM, Ciaraldi TP, Carter L, Reehman N, Mudaliar SR, Henry RR (2003) Thiazolidinediones upregulate impaired fatty acid uptake in skeletal muscle of type 2 diabetic subjects. Am J Physiol Endocrinol Metab 285:E354–E362
Yamamoto DL, Csikasz RI, Li Y, Sharma G, Hjort K, Karlsson R, Bengtsson T (2008) Myotube formation on micro-patterned glass: intracellular organization and protein distribution in C2C12 skeletal muscle cells. J Histochem Cytochem 56:881–892
Yasin R, Van Beers G, Nurse KC, Al-Ani S, Landon DN, Thompson EJ (1977) A quantitative technique for growing human adult skeletal muscle in culture starting from mononucleated cells. J Neurol Sci 32:347–360
Zammit PS, Partridge TA, Yablonka-Reuveni Z (2006) The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 54:1177–1191
Zierath JR, Hawley JA (2004) Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol 2:e348
Zuurveld JG, Oosterhof A, Veerkamp JH, Moerkerk HT van (1985) Oxidative metabolism of cultured human skeletal muscle cells in comparison with biopsy material. Biochim Biophys Acta 844:1–8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aas, V., Bakke, S.S., Feng, Y.Z. et al. Are cultured human myotubes far from home?. Cell Tissue Res 354, 671–682 (2013). https://doi.org/10.1007/s00441-013-1655-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1655-1