Abstract
Whereas animal models of depression are associated with decreased adult hippocampal neurogenesis, antidepressant treatments, including pharmacotherapy but also electroconvulsive therapy, have the opposite action, as they stimulate cell proliferation and the survival and maturation of newborn dentate gyrus neurons. Although the lack of these new cells is not causally involved in depression, as their absence does not trigger a depressive-episode per se, their loss has been shown to be causally involved in the ability of chronic monoaminergic antidepressants to achieve remission. However, the process by which the stimulation of hippocampal neurogenesis can elicit recovery after a depressive-like episode is poorly understood. The accepted view is that hippocampal newborn neurons integrate into the hippocampal network and thus participate in hippocampal cognitive functions crucial for remission. The hippocampus is associated with a wide range of such functions, including spatial navigation, pattern separation, encoding of new contextual information, emotional behavior and control over the hypothalamic-pituitary-adrenal axis. The present review aims at discussing each of these functions and tries to identify the process by which newborn cells participate in remission after successful therapy. Finally, future directions are proposed for a better understanding of these mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the discovery that chronic treatment with antidepressant drugs elicits an increase in cell proliferation in the hippocampus (Malberg et al. 2000), a growing body of research and enthusiasm has been generated regarding the contribution of adult-generated new hippocampal neurons in major depression and in the mechanisms involved in remission after successful therapy. Interest in this field became even higher with the discovery, in 2003, that these new neurons are crucial for the action of the antidepressants, as ablation of these cells via focal irradiation of the dentate gyrus of the hippocampus suppresses the ability of chronic monoaminergic antidepressants to induce recovery in mice (Santarelli et al. 2003). Indeed, this showed that the link between hippocampal neurogenesis and the therapeutic action of the antidepressant is not solely correlative but rather causal. Later on, additional data supported this hypothesis. For example, the pro-neurogenic action of treatments endowed with beneficial effects in the treatment of depressive-like states has been demonstrated not to be restricted to the classical monoaminergic-acting antidepressants, as similar findings have been observed with other drugs eliciting antidepressant-like effects in animal models, including tianeptine (Czéh et al. 2001; McEwen et al. 2002), CRF1 or vasopressin V1b receptor antagonists (Alonso et al. 2004), glutamatergic agents (Yoshimizu and Chaki 2004), endocannabinoid ligands (Jiang et al. 2005), or a melanin-concentrating hormone antagonist (David et al. 2007). Furthermore, the mood stabilizers lithium and valproate also increase both proliferation and survival of newborn hippocampal neurons (Chen et al. 2000; Hanson et al. 2011a; Hao et al. 2004; Silva et al. 2008). Finally, non-pharmacological therapies of major depression, such as electroconvulsive therapy (Malberg et al. 2000) or vagal nerve stimulation, also share the ability to increase hippocampal neurogenesis (Revesz et al. 2008). All this evidence seems convergent on the general idea that an increase in the number of newborn neurons of the hippocampus during adulthood is a property shared by all treatments enabling remission to be achieved, even if some data suggest that the picture is not fully homogeneous, results that have somewhat reduced initial enthusiasm in this field. For example, repetitive transcranial magnetic stimulation, which is also effective in achieving recovery, only partly abolishes the stress-induced decrease of cell proliferation, whereas it suppresses the survival rate of proliferating cells (Czéh et al. 2002). Further, the dual orexin receptor antagonist almorexant restores behavioral alterations that occur after chronic stress and normalizes the hypothalamis-pituitary-adrenal (HPA) axis function but, at the same time, decreases cell proliferation and neurogenesis within the ventral hippocampus (Nollet et al. 2012). The clinical relevance of this body of evidence obtained from preclinical data has also been debated (Gass and Henn 2009). Indeed, research with human subjects is difficult to undertake, as no tools that enable the in vivo imaging of new hippocampal neurons in patients are available and so their characterization entails post-mortem immunohistochemistry. This is extremely difficult to carry out in human subjects, as it requires not only access to hippocampal samples of humans that have newly died but also the phenotypic characterization of these patients (e.g., whether they used antidepressant medication, or if they were depressed). Therefore, only a few studies have been undertaken. Interestingly, some of these studies have confirmed the preclinical data, as pro-neurogenic effects of antidepressants have been found in humans (Boldrini et al. 2012; Boldrini et al. 2009), whereas others have not (Reif et al. 2006).
The objective of this paper is not to review the literature on this subject extensively, as such reviews have recently been published (Bambico and Belzung 2012; David et al. 2010; Hanson et al. 2011b; Petrik et al. 2012; Tanti and Belzung 2010a) but rather to try to provide a frame enabling us to understand the way that these new hippocampal neurons can contribute crucially to the etiopathogeny of major depression or to the ability of drugs to achieve remission. Indeed, major depression is a complex disorder related to a complex set of symptoms such as sadness, anhedonia, motivational decline, apathy and appetite changes that are not usually related to a defect of hippocampal functions. Further, this disease also elicits alterations of the morphology and the function of a set of brain areas, including of course the hippocampus but also other areas such as the amygdala, the lateral habenula, the nucleus accumbens, the cingulate cortex and several parts of the prefrontal cortex (for reviews, see Tanti and Belzung 2010b; Willner et al. 2012) together with alterations in the regulation of the HPA axis (Belzung and Billette de Villemeur 2010) and in several neurotransmitter systems (serotonin, noradrenalin, dopamine, glutamate, gamma amino butyric acid [GABA] and several neuropeptides). So, how can new neurons that are restricted to the dentate gyrus of the hippocampus participate in the recovery of a set of symptoms and of dysfunctions related to such a global brain network? Does the restoration of a normal level of hippocampal neurogenesis restore a crucial process that is dysfunctional in depression? What function that is so important for remission is achieved by the newborn hippocampal neurons? This review will try to answer these questions. However, before we can understand which of the functions related to hippocampal neurogenesis can explain its critical importance for recovery, we have to analyze, in a more detailed way, the findings regarding the impact of the new hippocampal cells in affective disorders and therapeutic effects. Indeed, do the current findings point to an involvement of neurogenesis (1) in the precipitation of a depressive episode, (2) in the vulnerability of subjects to depression, or (3) in the recovery after therapy? Before answering this question, we have to understand more precisely the way in which antidepressants act on newborn neurons and, particularly, whether they target a specific stage of the maturation of these cells. We should therefore first detail the process leading to the generation of newborn neurons.
Generation of newborn hippocampal neurons
New hippocampal neurons do not arise spontaneously. They are the result of a long maturation process starting with the proliferation of neural progenitors termed Type-1 progenitors (sometimes also defined as neural stem cells), which are located in the most inner part of the granule cell layer of the dentate gyrus of the hippocampus (for a review, see Duan et al. 2008). These cells express glial fibrillary acidic protein (GFAP), as do astrocytes but they can be distinguished from the latter by their lack of expression of S100β and by their co-expression of brain lipid-binding protein, MUSASHI-1 (an RNA-binding protein), Nestin and Sry-related HMG box transcription factor Sox2. These cells are capable of self-renewal but can also generate Type-2 progenitors (also sometimes referred to as intermediate progenitors) that no longer express GFAP and possess the ability to generate Type 3 neural progenitors (neuroblasts) that are still mitotic cells. This linear model is however sometimes questioned, as it appears that Type 1 progenitors can also be generated from Type 2 progenitors (Suh et al. 2007). All three types of progenitors are capable of self-renewal. Fate determination occurs after the Type 2 stage, as in Type 3 progenitors the aptitude to differentiate into glial cells is lost. The next stage corresponds to immature neurons. At this stage, cells express the microtubule-associated protein doublecortin (DCX), the polysialated form of the neural cell adhesion molecule (PSA-NCAM) and Prox 1. Immature neurons are post-mitotic cells that start tangential migration in the granule cell layer; during this process, they develop dendritic arborization, contact afferents from the entorhinal cortex and output mostly on pyramidal cells in the CA3 region of the hippocampus. By 8 weeks after cell birth, the process has enabled the generation of mature neurons. Immature neurons show some unique properties, probably related to the function of these cells (see below). For example, in these cells, the neurotransmitter GABA induces depolarization instead of hyperpolarization that is seen in adult neurons; this is related to a specific pattern of expression of some ionic co-transporters. Further, these immature neurons show enhanced excitability and a low long-term potentiation (LTP) induction threshold indicating that they possess specific properties associated with plasticity. At the same time, they undergo glutamatergic-related competitive survival; if these cells are not able to integrate into a functional network, they die. Later on (when 4–6 weeks old), these neurons display a larger LTP amplitude (Schmidt-Hieber et al. 2004). At approximately 7–8 weeks after the first division, the newborn cells become functionally indistinguishable from mature granule cells (Mongiat and Schinder 2011; Zhao et al. 2008).
Various methodologies enable us to assess each stage of this maturation process. A first approach consists in labeling the cells with bromodeoxyuridine (BrdU). Depending upon the time between the BrdU injection and the killing of the animals, one can assess proliferation (if BrdU has been administered 24 h before death) or the survival of the newborn cells (for example, if the injection occurred 4 weeks before death). However, this does not reveal the phenotype (glial or neural) of these cells, the demonstration of which requires double-labeling with a neuronal marker (for example NeuN) or the use of different combinations of endogenous markers. Interpretation of the data is sometimes difficult. For example, an increase in the number of immature neurons can be masked by an acceleration of the maturation of the neurons, the first resulting in an increase in the number of DCX-positive (DCX+) cells and the second having the opposite action. In this case, alternative methods consist in studying the proportion of DCX+ cells exhibiting tertiary/quaternary dendrites, which is an index of maturation. Assessment of the proportion of new neurons expressing immediate early genes after a given stimulation is also possible; as only the more mature cells will express the immediate early gene, this proportion indicates the maturation speed (Snyder et al. 2009).
Do animal models of depression or antidepressant therapies alter a specific stage of the process leading to the generation of new neurons in the dentate gyrus?
Two recent reviews have carefully analyzed the literature (David et al. 2010; Hanson et al. 2011b). Indeed, various protocols considered as models of depression, including bulbectomy, chronic stress, or chronic corticosterone administration, have been applied and the consequences on cell proliferation or survival have been assessed. On the forty-eight studies that have been considered by Hanson et al. (2011b), half determined a decrease in cell proliferation and an equivalent number did not describe any modification. One study even attempted a more precise characterization and showed that the effects of the selective serotonin reuptake inhibitor fluoxetine were restricted to Type 2 and not Type 1 progenitor cells (Encinas et al. 2006). The same picture was found with regard to the effects of chronic treatments with antidepressant drugs or electroconvulsive therapy ; half of the studies showed an increase in cell proliferation, whereas the others found no effect. For survival, the view is again balanced when considering the effects of models of depression, as more than half of the models of stress induced a decrease of survival, whereas a large majority of studies investigating the effects of treatment found the opposite, i.e., an increase. These variations are probably related to many factors, including the age of the animals (for example, chronic fluoxetine does not modify cell proliferation in middle-aged rodents; Couillard-Despres et al. 2009), the strain of the mice, the experimental models used to induce depressive-like behavior and the antidepressant drug used. Other studies have investigated the number of immature neurons by using DCX. Interestingly, although chronic fluoxetine treatment increased cell proliferation and cells expressing mature markers, it did not alter the number of DCX+ cells suggesting that it accelerated the maturation of progenitors into neurons (Wang et al. 2008). Similar findings were described by David et al. (2010) who used a Sholl analysis that enabled them to distinguish cells according to their dendritic morphology as DCX+ cells without tertiary dendrites and DCX+ cells with tertiary dendrites. They found that chronic treatment with fluoxetine increased the proportion of DCX+ cells exhibiting tertiary dendrites indicating that the antidepressant treatment accelerated their maturation. This means that probably more cells enter the DCX+ stage but that, as the maturation rate is increased by the treatment, they will remain for a shorter time in this stage, resulting in an apparent absence of effect on the total number of DCX+ cells. A possible interpretation is that the antidepressants have facilitated a function related to the new hippocampal cells and that this improves their incorporation into the corresponding functional network, thereby increasing their maturation and survival. If this is true, the ablation of hippocampal neurogenesis should deteriorate the corresponding function, thus inducing a behavioral or a cognitive phenotype. In this case, loss of these cells could, for example, modify the behavior of subjects when faced with particular conditions in which this function is required. Here, we aim to explore whether these newborn neurons are involved in functions related to depressive symptomatology. However, does ablation of newborn neurons alter depression-related behaviors?
Can the absence of new hippocampal neurons trigger depressive-like behaviour?
Nineteen experiments investigated the effects of the loss of new hippocampal neurons on depressive-like behavior of rodents (Table 1) that were not subjected to manipulations usually triggering depressive-like states. They thus assessed the intrinsic effects of the ablation of the newborn neurons. Suppression of neurogenesis was carried out by various strategies, including focal irradiation of the hippocampus (which only destroys newborn neurons of the hippocampus), peripheral injection of the anti-mitotic agent methylazoxymethanol (MAM), or genetic strategies (hGFAPtk or nestin-Bax mice; the latter two strategies suppress adult neurogenesis in the whole brain). The behavioral outcome was then assessed by using the forced swim test (eight studies), coat state deterioration (two studies), splash test (one study), sucrose consumption or preference (five studies), tail suspension (one study), cookie test (one study), or social interactions (one study). Only four experiments (21%) revealed behavioral alteration after neurogenesis ablation, whereas the large majority showed no effect (79%). Notably, the common point of these four studies was that they were all undertaken in rodents that had been subjected to ablation not only of hippocampal neurogenesis but also of olfactory bulb neurogenesis (hGFAPtk mice in two experiments and MAM-treated mice in two other experiments). Thus, relating the effects that were observed to a specific impact of the loss of the hippocampal new neurons is difficult, as these rodents also lacked new born neurons of the olfactory bulbs, a deficit that might play an important role in some behaviors that involve olfaction, such as feeding or drinking. Further, olfactory bulbectomy has also been used as an animal model of depression (Song and Leonard 2005), which might complicate the interpretation of these four studies. Taken together, most of these studies point to a consensus toward a lack of impact of neurogenesis ablation per se on depression-like behavior. However, the loss of newborn neurons might not trigger a depressive-like episode but rather alter vulnerability to the effects of factors involved in the etiology of depression. In this case, a double-hit (ablation of neurogenesis + triggering factor) would be necessary to observe a phenotype.
Does ablation of hippocampal new neurons affect vulnerability to depression?
The effects of the ablation of newborn hippocampal neurons on vulnerability to depression can be investigated by using two different experimental strategies. (1) We can study the effects of ablation of hippocampal neurogenesis on behaviors or factors associated with the onset of depression, such as increased anxiety (see Table 1) or a defect in the regulation of the HPA axis, which regulates the release of stress hormones. Indeed, heightened anxiety has been shown to precipitate depressive episodes and anxiety disorders are highly comorbid with major depression. Further, a defect in the regulation of the HPA axis, such as in Cushing disease, increases the rate of major depression episodes. (2) We can associate the ablation of hippocampal neurogenesis with other factors involved in the onset of depression, such as chronic stress. Here the idea is to study the addition of two vulnerability factors (a double-hit combining the loss of newborn neurons and stress) at the onset of a depressive-like episode with the view that the second hit will precipitate the onset of the symptoms or potentiate pre-existing infra-clinic symptoms. An analysis of the studies based on this second approach is presented in Table 2.
Studies investigating the basal corticosterone levels after neurogenesis ablation did not show any effect in basal (non-stressful) situations (Santarelli et al. 2003; Schloesser et al. 2009; Snyder et al. 2011; Surget et al. 2011) indicating that newborn neurons do not impact on the HPA functions in cases in which a subject has not been challenged by a stressful situation. However, if the animal is subjected to a challenge, such as a new situation or an acute restraint, differences appear both at the neuroendocrine and at the behavioral levels. Indeed, if mice are introduced into a brightly lighted new arena or are restrained, animals with a defect in neurogenesis show increased corticosterone levels (Schloesser et al. 2009; Snyder et al. 2011) and this parallels behavioral results. Moreover, anxiety-like behavior (Table 1) has been investigated in a range of test situations based on forced confrontation of rodents with novelty (novelty-induced suppression of feeding; 11 experiments), elevated plus maze (five experiments), light/dark boxes (five experiments), open field (4 experiments), O-maze (three experiments), novelty-induced hypophagia (two experiments), marble burying (one experiment) and predator avoidance (one experiment)). Interestingly, seven of these 32 experiments (again 21%) revealed an anxiogenic-like effect. This percentage remains low.
The second approach consists in combining a deficit in adult neurogenesis with factors conferring a vulnerability to depression, such as chronic stress or chronic corticosterone. The results are summarized in Table 2 and show a clear picture. Indeed, if we focus on studies that associate suppression of neurogenesis, vulnerability factors and assessment of depressive-related behaviors or on studies that combined the suppression of neurogenesis, stress and anxiety behavior, we find a total of 23 experiments (15 on depression-related behaviors and eight on anxiety-relate behaviors). Interestingly, none of the 15 experiments focusing on depression-related behavior have found a behavioral alteration, while two of the eight experiments on anxiety behavior revealed an effect of the ablation of neurogenesis, both using non-specific suppression of newborn cells (in the hippocampus and in the olfactory bulbs). This indicates that the idea that the ablation of hippocampal neurogenesis might sensitize the subjects to subsequent stressors does not receive strong experimental support as (1) the percentage of experiments revealing an effect of the ablation of newborn neurons on anxiety-behavior is quasi the same when mice are placed in basal conditions and when they have been challenged by experimental manipulations mimicking vulnerability factors; (2) a complete loss of brain neurogenesis, including not only hippocampal neurogenesis but also neurogenesis in the olfactory bulbs seems necessary to trigger such effects. The ablation of olfactory bulbs has also sometimes been considered as an animal model of depression. Even if chronic stress associated to the loss of neurogenesis does not induce convincing and reproducible behavioral modifications, chronic stress can indeed elicit an alteration of the HPA axis responsiveness at higher amplitude in animals showing a defect of neurogenesis (Snyder et al. 2011) supporting the idea that a combination of various susceptibility factors induces modifications relevant for the depressive-like phenotype.
Taken together, these studies suggest a small and not convincing trend toward an involvement of the newborn dentate gyrus neuron in vulnerability/resilience to depression, rather than in triggering depressive episodes per se. The other side of the coin concerns remission after effective therapy.
Is hippocampal neurogenesis crucial for recovery?
Strong evidence has been obtained regarding the impact of adult newborn neurons on the ability of chronic monoaminergic antidepressant drugs to achieve recovery. These studies were undertaken either on naive rodents that had not been subjected to experimental manipulations to induce a depressive-like state (Table 3) or on animals in which a depressive-like state had been induced (Table 4). With regard to the first category, all experiments were performed after focal irradiation and did not provide strong evidence that the loss of hippocampal newborn neurons suppressed the ability of monoaminergic drugs to achieve remission; indeed, two experiments showed that the effects of fluoxetine and imipramine were suppressed in the novelty-suppression feeding test and one showed the opposite. The picture is also balanced concerning the forced swimming test. However, the relevance of these studies is unclear, since monoaminergic antidepressant drugs do not elicit recovery in clinical situations in non-depressed subjects. If one explores the effect of these compounds in animals that have been stressed, strong evidence emerges indicating that the ability to achieve remission is suppressed after the focal suppression of hippocampal newborn neurons; indeed, antidepressant-like effects are suppressed in seven studies out of eight. The sole exception concerns an experiment involving the forced swim test, which is probably not relevant for depression but is instead a bio-assay. Anxiolytic effects of monoaminergtic compounds were also suppressed in three studies out of four after the suppression of neurogenesis induced either by irradiation or by anti-mitotic drugs. Hence, here, the picture seems monolithic, with however one exception: the antidepressant-like effects of monoaminergic antidepressants were not suppressed after antimitotic-induced suppression of neurogenesis, probably because the used protocol suppressed only extremely young cells (see below).
An important point to be addressed here concerns the developmental stage at which new neurons contribute to the therapeutic effects of monoaminergic antidepressants. Indeed, as can be seen from above, the process leading to the generation of adult-generated newborn neurons is long, lasting 6-8 weeks. Neural progenitors are unlikely to contribute to the recovery from depression; this might rather be a property of immature neurons or of neurons that have reached maturity recently. However, is there any experimental evidence for this? The first studies investigating the contribution of new neurons to the effects of fluoxetine used focal irradiation of the hippocampus. As this methodology can also elicit neuro-inflammation, the experiments usually tested the animals several weeks after the irradiation. For example, in the study of Surget et al. (2011), the behavioral experiments were undertaken 12 weeks after the focal irradiation of the hippocampus, so that most dentate gyrus neurons aged 0-12 weeks at the time of testing were missing. This does not provide a tight temporal window. However, other experiments have provided more precise information. For example, some studies used an immediate early gene (e.g., fos) to reveal the activity of precise populations of new neurons. This was performed by Surget et al. (2011) who found that fluoxetine increased the recruitment of 4-week-old cells to restore the normal function of the HPA axis (which is dysfunctional in depressive subjects). Two other studies have provided interesting results in this regard. First, Bessa et al. (2009) used MAM to suppress adult-generated neurons of the hippocampus and assessed the effects of chronic fluoxetine and imipramine at 2 weeks after the MAM injection. In this case, they found that the behavioral effects of the two monoaminergic drugs were not suppressed in the forced swimming test and in the sucrose preference test, suggesting a lack of impact of 2-week-old cells. These cells were probably too young to have any impact on the recovery after treatment with monoaminergic antidepressants. However, if the impact of MAM administration was studied on long-term recovery (for example, if the behavioral tests are applied 1 month after its administration), the effects of fluoxetine on the chronic stress-induced defects in anxiety-like behavior and in working memory were found to be absent after MAM injection (Mateus-Pinheiro et al. 2013). This indicates that newborn cells have to reach the age of 4-6 weeks to impact recovery after this pharmacological treatment. Remarkably the same profile can be found regarding the impact of new hippocampal neurons in depressive-like behavior; an effect in the forced swimming test is only found if 4-week-old cells are suppressed. These findings are also interesting with regard to the observation that antidepressants stimulate the maturation of new hippocampal cells. These drugs might therefore facilitate the recruitment of these neurons for precise functions, an event that might accelerate their maturation and facilitate their functional integration into the network and thus decrease their apoptosis.
If 4– to 6-week-old newborn hippocampal neurons contribute to the ability of monoaminergic-acting (and particularly serotoninergic) antidepressant drugs to achieve remission, little data has been found with respect to putative drugs acting via non-monoaminergic mechanisms (Tables 3, 4). Indeed, regarding non-aminergic-drugs endowed with putative antidepressant-like effects, the sole causal evidence has been obtained with the CB1 (cannabinoid receptor 1) agonist HU210, as this compound elicits increased neurogenesis together with antidepressant-like effects that are abolished after hippocampal irradiation (Jiang et al 2005). However, a similar picture is not found regarding the involvement of hippocampal neurogenesis in the effects of CRH-1 (corticotropin-releasing hormone receptor type 1), V1b (vasopressin type 1b receptor; Surget et al. 2008), or MCHR1 (melanocortin hormone receptor 1) ligands (David et al. 2007). Indeed, six studies have investigated the causal involvement of dentate gyrus neurogenesis in the antidepressant-like effects of these molecules and five have not detected its contribution, as the suppression of these cells does not abolish the effects of these molecules. Of course, one can argue that these drugs are in fact not antidepressant drugs, as despite a number of preclinical studies suggesting an antidepressant-like profile of these compounds, little clinical evidence has been found in Phase II trials with, for example, CRH1 antagonists or V1b antagonists (Griebel et al. 2012; Griebel and Holsboer 2012). The anxiolytic effects of these treatments provide more evidence in this regard, as out of four such studies, three have shown that the anxiolytic-like effects of the compounds are abolished after the suppression of the new neurons.
Finally, hippocampal neurogenesis interestingly also causally contributes to the antidepressant-like effects of non-pharmacological treatments of depressive-like phenotypes, such as environmental enrichment, adrenalectomy, or hypoxia. Indeed, if we consider only the studies that have explored this aspect of animal models of depression, we can observe that eight studies have been undertaken, all leading to positive effects as the antidepressant-like or anxiolytic-like effects of these different manipulations are all prevented after ablation of new neurons. A common denominator of these and pharmacological treatments could be the activation of the transcription factor, cAMP response element-binding protein and the growth factor, brain-derived neurotropic factor (Gass and Riva 2007).
Which function?
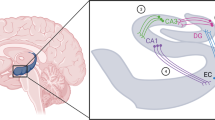
If we consider that neurogenesis in the hippocampus, even if not causally involved in triggering depressive episodes or in participating in the vulnerability to depression, is nonetheless crucial for the ability of monoaminergic antidepressants to achieve remission, we have now to determine the process by which this action might occur. Little data is however available on this subject, which also questions, in a more general way, the function of dentate gyrus neurogenesis. Indeed, to date, only a limited understanding of its function is available, as reflected by a list of factors, including executive functions, pattern separation, spatial/contextual memory, regulation of the HPA axis and behavioral coping with stressful situations (see Fig. 1). The view is that newborn hippocampal neurons enable the generation of a neural reserve that might be used in the performance of any of these functions in case the network underlying has been disturbed.
Potential involvement of hippocampal neurogenesis in the behavioral effects of antidepressants. By promoting the functional integration of newborn neurons into the hippocampal network, antidepressants might strengthen various hippocampus-related functions in which neurogenesis has been shown to participate. This could in return facilitate antidepressant-induced remission. According to the neurogenic reserve hypothesis, by increasing the pool of newborn hippocampal neurons, antidepressants might allow the (re)generation of a neural reserve that could be used to strengthen any of these functions and to enable better coping and resilience to stress when confronted with a challenging environment, such as during severe stress exposure and depression (+, – indicate, respectively, the strengthening and weakening of the hippocampus contribution to the listed functions
Neurogenic reserve
Kempermann (2008) has proposed the “neurogenic reserve hypothesis”. This theory derives from the “neural reserve theory” promoted by R. Katzman and P. Satz (Katzman 1993; Satz 1993; Stern 2003), which posits that, when faced with challenging situations that can disrupt normal cognitive function, the organism can adapt by recruiting specific neurons or networks that can thus be considered a neural reserve, enabling plasticity and adaptation. According to Kemperman (2008), the newborn hippocampal neurons (even when immature, i.e., during their critical time window) can be recruited to allow adaptation of the hippocampal network to situations that are experienced for the first time at moments in the life of the subject in which plasticity is lower, such as in older age. A prediction related to this theoretical framework is that the ablation of neurogenesis might have no direct consequences in normal situations, whereas it will have a huge impact in cases in which the subject has been placed in challenging situations such as chronic stress. One can thus hypothesize that, in such situations, increased hippocampal neurogenesis would also better enable the subject to face changes occurring within their surroundings, thus increasing adaptability to a challenging environment. Within this framework, newborn neurons generated throughout the life of the subject would allow improved coping with stressful situations occurring at older ages and better resilience during confrontations with environmental stress. According to this view, antidepressants would promote the generation of new neurons to compensate a loss of the neural reserve.
Executive functions
According to theoretical frameworks in the field of cognitive psychology, executive functions include three fully dissociable aspects: inhibition, task shifting and updating (Miyake et al. 2000). As is well established both from clinical and from preclinical research, the processing of executive functions is related to the prefrontal cortex (Kesner and Churchwell 2011). However, the hippocampus is also involved in this function to some extent and this has relevance to the field of depression research as, in depressed patients, the executive dysfunction correlates with the hippocampal volume (Frodl et al. 2006). Interestingly, the prefrontal cortex and the hippocampus are highly connected through reciprocal direct or indirect projections (Godsil et al. 2013; Laroche et al. 2000) and one can thus propose that the involvement of the hippocampus in this function partly relies on its connections with the prefrontal areas. However, little data is available on the involvement of dentate gyrus neurogenesis in this process, partly also because of the lack of relevant rodent tests of executive functions. Indeed, few tasks specific to the inhibition or to flexibility have been developed in rodents. One can however mention one study that involved a variant of the active place avoidance task in which an aversive zone was switched from one place to another (mice have thus to inhibit a learned response and to shift to a new task); this study showed that ablation of the newborn hippocampal neurons decreased performance (Burghardt et al. 2012). One can thus hypothesize that hippocampal neurogenesis deficit induces decreased executive performance, which causes non-response to antidepressant therapy. Indeed, in patients, decreased executive functions have been related to non-response to fluoxetine (Dunkin et al. 2000). However, these results have to receive further experimental and theoretical support.
Pattern separation
The idea that the main function of hippocampal neurogenesis is to enable pattern separation (the ability to discriminate among similar experiences, thus transforming identical memories into non-overlapping representations) has gained much popularity in last few years, first because this process is unique in having been related specifically to the dentate gyrus (Leutgeb et al. 2007) and second because it has received strong experimental support from studies assessing the effects of decreased or increased neurogenesis, which respectively have found a deterioration or improvement of pattern separation (Clelland et al. 2009; Nakashiba et al. 2012; Sahay et al. 2011; Tronel et al. 2012). However, the relationship between pattern separation and depression or the effects of an antidepressant remains unclear. First, no clinical or preclinical evidence reporting that antidepressant therapy modifies performance in tasks related to pattern separation in depression has been published as yet. The same applies to major depression or depression models in rodents, as no study has shown a deficit in this process under these conditions. Second, as noticed above, pattern separation enables two resembling contexts to be distinguished. Major depression is characterized by a focus on negative stimuli and by poor processing of hedonic information. A deficit in pattern separation should elicit an over-generalization for negative and for positive information; if the first of these two phenomenon can precipitate depressive-like behaviors, because it can induce a cognitive bias for negative events, the second should induce the opposite effect and one can hardly imagine a way in which this can participate in the onset of depression or in the ability of treatments to achieve remission. Future experiments should thus investigate the effects of stress, of depression and of antidepressants on pattern separation concerning emotionally relevant positive and negative information to further determine the way that increased pattern separation might contribute to recovery.
Processing of contextual information
The involvement of the hippocampus in the processing of contextual (particularly spatial) information and in declarative memory is well established. A logical proposal is thus that dentate gyrus neurogenesis might be involved in one or another of these processes. Interestingly, the suppression of hippocampal neurogenesis has been shown to worsen this process, whereas new hippocampal neurons are preferentially activated during spatial or contextual tasks. For example, use of the Morris water maze, the Barnes maze, or associative learning, such as contextual fear conditioning, has shown that the ablation of newborn hippocampal neurons induces a decline of performance (Deng et al. 2009; Dupret et al. 2008; Farioli-Vecchioli et al. 2008; Garthe et al. 2009; Jessberger et al. 2009; Shors et al. 2001), even if failure to see such effects has also been observed in some studies (for a review, see Marín-Burgin and Schinder 2012). Further, by using immediate early gene immunohistochemistry, increased recruitment of these new cells when compared to more mature granule cells of the dentate gyrus has also been described in relation to learning (Kee et al. 2007; Ramirez-Amaya et al. 2006; Sandoval et al. 2011; Trouche et al. 2009). These findings seem relevant to the topic of depression, as models of depression such as chronic stress have been found to impair performance in several hippocampus-dependent learning tasks or to induce a shift from spatial-based strategies to cued-based strategies (for a review, see Conrad 2010). Similar findings have been established by clinical studies assessing hippocampus-related learning in patients with major depression (Nissen et al. 2010). Finally, the cognitive decline induced by chronic stress can be prevented by antidepressant treatment (Elizalde et al. 2008). However, the mechanisms by which an increase of the processing of contextual information or of encoding of declarative memories participates in remission remains to be discovered. The increased number of new neurons might enable a shift from habit-based strategies associated with chronic stress and/or major depression to more flexible and adaptive strategies and this might, at the same time but independently, increase performance in declarative memory and elicit recovery from depressive-like behaviors.
HPA axis
As is well established, the hippocampus, together with other brain areas such as the prefrontal cortex, participates in negative feedback over the HPA axis (for a review, see Belzung and Billette de Villemeur 2010). However, under basal conditions, the involvement of hippocampal neurogenesis in this function is probably not prominent, as shown by the absence of HPA alterations after the removal of newborn neurons (Santarelli et al. 2003; Schloesser et al. 2009; Snyder et al. 2011; Surget et al. 2011), probably because the other brain areas involved in HPA regulation compensate the neurogenesis-related loss of function. A different picture is found after mild stress as, in this case, ablation of neurogenesis can compromise normal HPA function. Indeed, in this situation, corticosterone levels have been demonstrated to require more time to return to pre-stress levels and HPA regulation is blunted (Snyder et al. 2011). Notably, in this case, the function of other areas negatively regulating the HPA axis, such as the prefrontal cortex, is also compromised, whereas the function of areas positively regulating the HPA, such as the amygdala, is increased (McEwen 2002). In this instance, all the systems enabling the compensation for the loss of the hippocampal system are defective. Therefore, the recruitment of newborn neurons can be facilitated by antidepressant drugs (Surget et al. 2011), which might enable the loss of function related to the alteration in the other brain areas participating in this regulation to be compensated. This is certainly sufficient to explain the way that these cells can enable antidepressants drugs to achieve recovery. Newborn neurons are well established as synapsing on CA3 pyramidal cells and do not directly project to the paraventricular nucleus of the hypothalamus, the nucleus in which the endocrine stress axis initiates the process leading to the release of glucocorticoids. This action occurs via multisynaptic projections on relay areas such as the lateral septum, the bed nucleus of the stria terminalis and several hypothalamic nuclei (Surget et al. 2011).
Behavioural coping with stressful situations; anxiety behavior
Heightened anxiety might contribute to the onset or to the maintenance of depressive symptomatology and suppression of anxiety behavior by antidepressants might thus participate in recovery. The effect of neurogenesis loss on anxiety behavior occurs in part through connections arising from the basolateral amygdala. Indeed, activity within the basolateral nucleus of the amygdala has been shown to regulate the activity pattern of hippocampal newborn neurons, as lesions of this brain structure block the recruitment of new hippocampal neurons in a contextual fear conditioning task (Kirby et al. 2012). As high anxiety behavior is reversed by chronic antidepressant drugs (Guilloux et al. 2011), the treatment, by preventing the anxiety associated with the depressive symptomatology, might participate in remission. Interestingly, the presence of comorbid anxiety disorders in depressed patients predicts poor treatment outcome (Souery et al. 2007).
We have just reviewed the various functions that have been proposed to rely on hippocampal neurogenesis. Interestingly, recent evidence suggests that the hippocampus, even if long considered as being rather functionally homogeneous, displays functional dissociation along its septo-temporal axis, some hippocampal-dependant functions relying on the septal sub-regions and others on the more temporal sub-regions. For example, use of the Morris water maze, which enables the measurement of spatial learning, has shown that the amplitude of the decrease in performance in this test parallels the magnitude of dorsal (septal) hippocampal lesions (Moser et al. 1993, 1995). Similarly, lesions restricted to the dorsal hippocampal have been demonstrated to interfere with the acquisition of the tone-shock association in contextual fear conditioning, which is another hippocampus-dependant learning task (Kim and Fanselow 1992; Yoon and Otto 2007). Such effects have not been observed after a lesion restricted to the more ventral part of the hippocampus, a result that has led to the idea that only the dorsal hippocampus is involved in spatial or contextual learning. However, this does not mean that the ventral part has no functional contribution. Indeed, specific effects after an ablation restricted to the ventral hippocampus have also been observed, revealing that this subpart is instead associated with emotional behaviour. Moreover, in cases of a specific lesion of the ventral sub-region, the lesion elicits modified anxiety-like behaviour in various situations such as novelty suppression of feeding (Bannerman et al. 2002; Burns et al. 1996; McHugh et al. 2004), exposure to predator-related stimuli (Blanchard et al. 2005; Pentkowski et al. 2006), elevated plus maze (Degroot and Treit 2004; Kjelstrup et al. 2002; Trivedi and Coover 2004) and tone fear conditioning (Hunsaker and Kesner 2008; Maren and Holt 2004). Interestingly, lesions of the dorsal hippocampus do not induce such effects, indicating a double dissociation. These differences between the two sub-regions are probably related to a different pattern of inputs/ouputs of the dorsal and ventral sub-regions.
Therefore, a possible approach for clarifying the functions of the hippocampal newborn neurons relating to depression or to recovery might involve an exploration of whether animal models of depression or treatment with antidepressant compounds impact specifically on septal/dorsal or on temporal/ventral neurogenesis.
Is there a regional specificity of the impact of depression models and/or antidepressant treatment on hippocampal neurogenesis?
Some studies have investigated whether stress or more generally animal models of depression can impact newborn neurons specifically in a subpart of the hippocampus. This has been recently reviewed (A. Tanti and C. Belzung, submitted). Interestingly, most studies have shown that the effects are restricted to the more ventral part of the hippocampus. One can hypothesize that this might alter functions related to the ventral part of the hippocampus, as the newborn neurons located in this sub-region might have a pattern of input/outputs enabling them to interfere with the activity of the ventral hippocampus. However, strangely enough, a mirror picture is not obtained after pharmacotherapy with monoaminergic antidepressants. Indeed, chronic treatment with these molecules produces an increase in the number of newborn neurons not only in the ventral sub-region but also in the dorsal sub-region, whatever the state of the animals (irrespective of whether they have been subjected to experimental manipulations inducing a depressive-like state before application of the treatment). However, when focusing on the effects of putative non-monoaminergic antidepressants, the picture becomes more precise. For example, agomelatine induces an increase in neurogenesis that is restricted to the ventral sub-region, indicating that an action on neurogenesis in this sub-region is sufficient to induce recovery.
Concluding remarks and future directions
This review clearly underlines the idea that hippocampal neurogenesis, while not causally involved in the onset of depression-like symptomatology, is related to the ability of chronic monoaminergic antidepressants to achieve recovery. However, the cognitive or the biological mechanism explaining this phenomenon is still poorly understood and requires further investigations. Newborn neurons functionally integrate into the dentate gyrus network and thus participate in hippocampal functions crucial for remission. Several functions have been related to neurogenesis, including spatial navigation, pattern separation, processing of contextual information, neurogenic reserve, executive functions, anxiety behavior and control over the HPA axis. Even if experimental data strongly support some of these hypotheses (for example, the HPA axis), convincing evidence regarding the other processes is still missing. This action might in some cases not occur directly as a result of the deficit of the function of the dentate gyrus but rather indirectly, through connections of the hippocampus with other brain areas, such as the amygdala or areas of the HPA axis (Eisch and Petrik 2012). As the dorsal and the ventral sub-regions of the hippocampus have highly distinct patterns of connections, the impact of models of depression and antidepressants on dorsal or ventral sub-regions of the hippocampus might provide some interesting information in this regard.
Decisive progress in this field is unfortunately hampered by the lack of specific methodology. First, clinical investigations are hindered by the lack of tools enabling in vivo neuroimaging of neurogenesis. Therefore, the few studies that have explored neurogenesis changes in relation to depression or antidepressants have involved post-mortem immunohistochemistry, which does not permit the establishment of a comprehensive symptomatic phenotyping of the patients. Future research should develop radiopharmaceutical molecules tagged with positron-emitting isotopes that could specifically label proteins expressed in newborn neurons from various maturation stages. Some proposals have been made (Couillard-Despres and Aigner 2011) in this regard. Second, preclinical research lacks behavioral test situations allowing the easy targeting of some cognitive processes, such as executive functions. Some experimental protocols have been designed, such as the go/no go task to assess inhibition in mice (Gomez et al. 2007) or the attentional-set-shifting task to measure aspects of flexibility (Colacicco et al. 2002; McAlonan and Brown 2003) but all require long training procedures, so that an assessment of the contribution of newborn neurons of a precise maturation stage is not possible. Third, in the clinic, several subtypes of depression have been described, such as melancholic and atypical depression. These entities are related to different (sometimes opposite) cognitive and biological features, including differences in HPA axis abnormalities, coping styles, sleep pattern and memory. The availability of animal models of all the sub-types would certainly help to unravel the contribution of the new hippocampal cells in these different phenotypes. Finally, a more precise characterization of the involvement of dorsal or ventral neurogenesis would undoubtedly improve our understanding of the function of neurogenesis with regard to depression and recovery, possibly pin-pointing specific patterns of projections of these sub-regions.
References
Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K (2007) High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317:819–823
Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrié P (2004) Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatr 9:278–286
Bambico FR, Belzung C (2012) Novel insights into depression and antidepressants: a synergy between synaptogenesis and neurogenesis? Curr Top Behav Neurosci (in press)
Bannerman DM, Deacon RMJ, Offen S, Friswell J, Grubb M, Rawlins JNP (2002) Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci 116:884–901
Belzung C, Billette de Villemeur E (2010) The design of new antidepressants: can formal models help? A first attempt using a model of the hippocampal control over the HPA-axis based on a review from the literature. Behav Pharmacol(in press)
Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N (2009) The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatr 14:764-773
Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ (2005) Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a cat, cat odor, and nonpredator threat. Neurosci Biobehav Rev 29:1243–1253
Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V (2009) Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34:2376–2389
Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V (2012) Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry 72:562–571
Burghardt NS, Park EH, Hen R, Fenton AA (2012) Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus 22:1795–1808
Burns LH, Annett L, Kelley AE, Everitt BJ, Robbins TW (1996) Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: implication for limbic-striatal interactions. Behav Neurosci 110:60–73
Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK (2000) Enhancement of hippocampal neurogenesis by lithium. J Neurochem 75:1729–1734
Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325:210–213
Colacicco G, Welzl H, Lipp HP, Wurbel H (2002) Attentional set-shifting in mice: modification of a rat paradigm, and evidence for strain-dependent variation. Behav Brain Res 132:95–102
Conrad CD (2010) A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Biol Psychiatry 34:742–755
Couillard-Despres S, Aigner L (2011) In vivo imaging of adult neurogenesis. Eur J Neurosci 33:1037–1044
Couillard-Despres S, Wuertinger C, Kandasamy M, Caioni M, Stadler K, Aigner R, Bogdahn U, Aigner L (2009) Ageing abolishes the effects of fluoxetine on neurogenesis. Mol Psychiatry 14:856–864
Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E (2001) Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA 98:12796–12801
Czéh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Müller MB, Toschi N, Fuchs E, Keck ME (2002) Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry 52:1057–1065
David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, Craig DA, Zhong H, Swanson CJ, Hegde LG, Ping XI, Dong D, Marzabadi MR, Gerald CP, Hen R (2007) Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphenyl]-2-methylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J Pharmacol Exp Ther 321:237–248
David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux J-P, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493
David DJ, Wang J, Samuels BA, Rainer Q, David I, Gardier AM, Hen R (2010) Implications of the functional integration of adult-born hippocampal neurons in anxiety-depression disorders. Neuroscientist 16:578–591
Degroot A, Treit D (2004) Anxiety is functionally segregated within the septo-hippocampal system. Brain Res 1001:60–71
Deng W, Saxe MD, Gallina IS, Gage FH (2009) Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci 29:13532–13542
Duan X, Kang E, Liu CY, Ming G-L, Song H (2008) Development of neural stem cell in the adult brain. Curr Opin Neurobiol 18:108–115
Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S (2000) Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord 60:13–23
Dupret D, Revest J-M, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV (2008) Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 3:e1959
Eisch AJ, Petrik D (2012) Depression and hippocampal neurogenesis: a road to remission? Science 338:72–75
Elizalde N, Gil-Bea FJ, Ramirez MJ, Aisa B, Lasheras B, Del Rio J, Tordera RM (2008) Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology (Berl) 199:1–14
Encinas JM, Vaahtokari A, Enikolopov G (2006) Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA 103:8233–8238
Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cina I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F (2008) The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol 6:e246
Frodl T, Schaub A, Banac S, Charypar M, Jäger M, Kümmler P, Bottlender R, Zetzsche T, Born C, Leinsinger G, Reiser M, Möller H-J, Meisenzahl EM (2006) Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci 31:316–323
Fuss J, Ben Abdallah NM, Hensley FW, Weber K-J, Hellweg R, Gass P (2010) Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS One 5:e12769
Garthe A, Behr J, Kempermann G (2009) Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One 4:e5464
Gass P, Henn FA (2009) Is there a role for neurogenesis in depression? Biol Psychiatry 66:3–4
Gass P, Riva MA (2007) CREB, neurogenesis and depression. Bioessays 29:957–961
Godsil BP, Kiss JP, Spedding M, Jay TM (2013) The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol (in press)
Gomez P, Ratcliff R, Perea M (2007) A model of the go/no-go task. J Exp Psychol Gen 136:389–413
Griebel G, Holsboer F (2012) Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov 11:462–478
Griebel G, Beeske S, Stahl SM (2012) The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: results from 4 randomized, double-blind, placebo-controlled studies. J Clin Psychiatry 73:1403–1411
Guilloux J-P, David DJP, Xia L, Nguyen HT, Rainer Q, Guiard BP, Repérant C, Deltheil T, Toth M, Hen R, Gardier AM (2011) Characterization of 5-HT(1A/1B)-/- mice: an animal model sensitive to anxiolytic treatments. Neuropharmacology 61:478–488
Hanson ND, Nemeroff CB, Owens MJ (2011a) Lithium, but not fluoxetine or the corticotropin-releasing factor receptor 1 receptor antagonist R121919, increases cell proliferation in the adult dentate gyrus. J Pharmacol Exp Ther 337:180–186
Hanson ND, Owens MJ, Nemeroff CB (2011b) Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 36:2589–2602
Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould TD, Manji HK, Chen G (2004) Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci 24:6590–6599
Holick KA, Lee DC, Hen R, Dulawa SC (2008) Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology 33:406–417
Hunsaker MR, Kesner RP (2008) Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem 89:61–69
Jayatissa MN, Henningsen K, West MJ, Wiborg O (2009) Decreased cell proliferation in the dentate gyrus does not associate with development of anhedonic-like symptoms in rats. Brain Res 1290:133–141
Jessberger S, Clark RE, Broadbent NJ, Clemenson GD Jr, Consiglio A, Lie DC, Squire LR, Gage FH (2009) Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem 16:147–154
Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji S-P, Bai G, Zhang X (2005) Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest 115:3104–3116
Katzman R (1993) Education and the prevalence of dementia and Alzheimer’s disease. Neurology 43:13–20
Kee N, Teixeira CM, Wang AH, Frankland PW (2007) Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 10:355–362
Kempermann G (2008) The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci 31:163–169
Kesner RP, Churchwell JC (2011) An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem 96:417–431
Kim JJ, Fanselow MS (1992) Modality-specific retrograde amnesia of fear. Science 256:675–677
Kirby ED, Friedman AR, Covarrubias D, Ying C, Sun WG, Goosens KA, Sapolsky RM, Kaufer D (2012) Basolateral amygdala regulation of adult hippocampal neurogenesis and fear-related activation of newborn neurons. Mol Psychiatry 17:527–536
Kjelstrup KG, Tuvnes FA, Steffenach H-A, Murison R, Moser EI, Moser M-B (2002) Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA 99:10825–10830
Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ (2010) Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci USA 107:4436–4441
Laroche S, Davis S, Jay TM (2000) Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus 10:438–446
Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M (2013) Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J Neurosci 33:2961–2972
Leutgeb JK, Leutgeb S, Moser MB, Moser EI (2007) Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315:961–966
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110
Maren S, Holt WG (2004) Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci 118:97–110
Marín-Burgin A, Schinder AF (2012) Requirement of adult-born neurons for hippocampus-dependent learning. Behav Brain Res 227:391–399
Mateus-Pinheiro A, Pinto L, Bessa JM, Morais M, Alves ND, Monteiro S, Patricio P, Almeida OF, Sousa N (2013) Sustained remission from depressive-like behavior depends on hippocampal neurogenesis. Transl Psychiatry 3:e210
McAlonan K, Brown VJ (2003) Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res 146:97–103
McEwen BS (2002) Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging 23:921–939
McEwen BS, Magarinos AM, Reagan LP (2002) Structural plasticity and tianeptine: cellular and molecular targets. Eur Psychiatry 17 (Suppl 3):318–330
McHugh SB, Deacon RMJ, Rawlins JNP, Bannerman DM (2004) Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci 118:63–78
Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R (2006) Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci 9:729–731
Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000) The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol 41:49–100
Mongiat LA, Schinder AF (2011) Adult neurogenesis and the plasticity of the dentate gyrus network. Eur J Neurosci 33:1055–1061
Moser E, Moser MB, Andersen P (1993) Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci 13:3916–3925
Moser MB, Moser EI, Forrest E, Andersen P, Morris RG (1995) Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA 92:9697–9701
Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S (2012) Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149:188–201
Nissen C, Holz J, Blechert J, Feige B, Riemann D, Voderholzer U, Normann C (2010) Learning as a model for neural plasticity in major depression. Biol Psychiatry 68:544–552
Nollet M, Gaillard P, Tanti A, Girault V, Belzung C, Leman S (2012) Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology 37:2210–2221
Onksen JL, Brown EJ, Blendy JA (2011) Selective deletion of a cell cycle checkpoint kinase (ATR) reduces neurogenesis and alters responses in rodent models of behavioral affect. Neuropsychopharmacology 36:960–969
Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ (2006) Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci 23:2185–2196
Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, Lange C, Higley JD, Rosoklija G, Hen R, Sackeim HA, Coplan JD (2011) Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One 6:e17600
Petrik D, Lagace DC, Eisch AJ (2012) The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology 62:21–34
Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA (2006) Integration of new neurons into functional neural networks. J Neurosci 26:12237–12241
Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP (2006) Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry 11:514–522
Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN (2009) Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry 14:959–967
Revesz D, Tjernstrom M, Ben-Menachem E, Thorlin T (2008) Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol 214:259–265
Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R (2011) Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472:466–470
Sandoval CJ, Martinez-Claros M, Bello-Medina PC, Perez O, Ramirez-Amaya V (2011) When are new hippocampal neurons, born in the adult brain, integrated into the network that processes spatial information? PLoS One 6:e17689
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809
Satz P (1993) Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology 7:273–295
Saxe MD, Battaglia F, Wang J-W, Malleret G, David DJ, Monckton JE, Garcia ADR, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR (2006) Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA 103:17501–17506
Schloesser RJ, Manji HK, Martinowich K (2009) Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport 20:553–557
Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M (2010) Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry 15:1152–1163
Schmidt-Hieber C, Jonas P, Bischofberger J (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429:184–187
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410:372–376
Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E (2002) Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12:578–584
Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leão P, Almeida OFX, Sousa N (2008) Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience 152:656–669
Singer BH, Jutkiewicz EM, Fuller CL, Lichtenwalner RJ, Zhang H, Velander AJ, Li X, Gnegy ME, Burant CF, Parent JM (2009) Conditional ablation and recovery of forebrain neurogenesis in the mouse. J Comp Neurol 514:567–582
Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA (2009) Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci 29:14484–14495
Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476:458–461
Song C, Leonard BE (2005) The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev 29:627–647
Souery D, Oswald P, Massat I, Bailer U, Bollen J, Demyttenaere K, Kasper S, Lecrubier Y, Montgomery S, Serretti A, Zohar J, Mendlewicz J, Group for the Study of Resistant D (2007) Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J Clin Psychiatry 68:1062–1070
Stern Y (2003) The concept of cognitive reserve: a catalyst for research. J Clin Exp Neuropsychol 25:589–593
Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH (2007) In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1:515–528
Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C (2008) Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry 64:293–301
Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C (2011) Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 16:1177–1188
Tanti A, Belzung C (2010a) Neurogenic basis of antidepressant action: recent advances. In: Cryan JF, Leonard BE (eds) Modern trends in pharmacopsychiatry, vol 27. Karger, Basel, pp 224–242
Tanti A, Belzung C (2010b) Open questions in current models of antidepressant action. Br J Pharmacol 159:1187–1200
Trivedi MA, Coover GD (2004) Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem 81:172–184
Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN (2012) Adult-born neurons are necessary for extended contextual discrimination. Hippocampus 22:292–298
Trouche S, Bontempi B, Roullet P, Rampon C (2009) Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci USA 106:5919–5924
Wang J-W, David DJ, Monckton JE, Battaglia F, Hen R (2008) Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci 28:1374–1384
Willner P, Scheel-Krüger J, Belzung C (2012) The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev (in press)
Yoon T, Otto T (2007) Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiol Learn Mem 87:464–475
Yoshimizu T, Chaki S (2004) Increased cell proliferation in the adult mouse hippocampus following chronic administration of group II metabotropic glutamate receptor antagonist, MGS0039. Biochem Biophys Res Commun 315:493–496
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660
Zhu X-H, Yan H-C, Zhang J, Qu H-D, Qiu X-S, Chen L, Li S-J, Cao X, Bean JC, Chen L-H, Qin X-H, Liu J-H, Bai X-C, Mei L, Gao T-M (2010) Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci 30:12653–12663
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanti, A., Belzung, C. Hippocampal neurogenesis: a biomarker for depression or antidepressant effects? Methodological considerations and perspectives for future research. Cell Tissue Res 354, 203–219 (2013). https://doi.org/10.1007/s00441-013-1612-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1612-z