Abstract
The hypophysial pars tuberalis (PT) acts as an important interface between neuroendocrine brain centers (hypothalamus, pineal organ) and the pars distalis (PD) of the hypophysis. Recently, we have identified an endocannabinoid system in the PT of hamsters and provided evidence that 2-arachidonoylglycerol is a messenger molecule that appears to play an essential role in seasonal reproduction and prolactin release by acting on the cannabinoid receptors in the PD. We now demonstrate the enzymes involved in endocannabinoid synthesis and degradation, namely sn-1-selective diacylglycerol lipase α, N-acylphosphatidylethanolamine-specific phospholipase D, and monoacylglycerol lipase, in the PT of man by means of immunohistochemistry. High-performance liquid chromatography coupled with tandem mass spectrometry revealed 2-arachidonoylglycerol and other endocannabinoids in the human PT. Furthermore, we detected the expression of the cannabinoid receptor 1 (CB1), a primary receptor for endocannabinoids, in the PD. Double-immunofluorescence staining for CB1 and various hypophysial hormones or S-100, a marker for folliculostellate (FS) cells, revealed that CB1 immunoreactivity was mainly localized to corticotrophs and FS-cells. A limited number of lactotrophs and somatotrophs also showed CB1 immunoreactivity, which was however absent from gonadotrophs and thyrotrophs. Our data thus indicate that the human PT comprises an endocannabinoid system, and that corticotrophs and FS-cells are the main target cells for endocannabinoids. The functional significance of this newly discovered pathway remains to be elucidated in man; it might be related to the control of stress responses and/or reflect a remnant seasonal control of hypophysial hormonal secretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pars tuberalis (PT), which is located between the median eminence, the portal vessels of the hypophysis, and the pars distalis (PD), plays an important role within neuroendocrine circuits of the hypothalamo-hypophysial system. Studies with hamster and sheep have unraveled an important function of the PT as an interface between the photoperiodic melatonin signal and the seasonal control of reproduction. Melatonin is secreted from the pineal gland at night and encodes the length of the night (Arendt 1995; Reiter 1980). The PT contains a high density of MT1 melatonin receptors (von Gall et al. 2002b), which mediate photoperiodic responses in mammals (Yasuo et al. 2009) and control the temporal expression of clock genes in the PT (Hazlerigg et al. 2001; Lincoln et al. 2003; von Gall et al. 2002a, 2005). Recent studies have identified a “retrograde” pathway from the PT to the hypothalamus, a pathway that is crucial for the photoperiodic response of the gonads in Japanese quail (Nakao et al. 2008), sheep (Hanon et al. 2008), melatonin-proficient mouse strains (Ono et al. 2008; Unfried et al. 2009, 2010), and hamster (Yasuo et al. 2010a). In the PT, this pathway involves the thyrotropin beta-subunit (TSHB), which acts upon the ependymal cell layer of the infundibular recess and activates deiodinase type 2, an enzyme that converts thyroxin into triiodothyronin and thereby controls the local concentration of the thyroid hormones in the third ventricle, which in turn control the release of gonadotropin-releasing hormone. In mammals, this pathway is influenced by the melatonin signal. On the other hand, melatonin signals also impinge upon an “anterograde” pathway from the PT to the PD, a pathway that controls the secretion of gonadotropic hormones such as prolactin (Morgan 2000). The “anterograde” messenger molecules from the PT have been called “tuberalins” and are supposed to be secreted from the PT in a photoperiod-dependent manner (Morgan 2000). The tuberalins have been suspected to belong to the class of neuropeptides, but our recent study with the golden hamster provides good evidence for the hypothesis that the anterograde pathway from the PT to the PD employs endocannabinoids as messenger molecules (Yasuo et al. 2010b).
Endocannabinoids are endogenous ligands for G-protein-coupled cannabinoid receptors 1 (CB1) and 2 (CB2), which are mainly localized in brain and immune cells (Matsuda et al. 1990; Munro et al. 1993). They are involved in many biological functions such as food intake, thermoregulation, nociceptive processing, synaptic plasticity, and immunomodulation (Di Marzo et al. 2004; Piomelli 2003; Kunos 2007). Two major endocannabinoids, viz., N-arachidonoyl-ethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG), have been identified and are also involved in the control of neuroendocrine functions such as the regulation of the gonadotropic and lactotropic axes (Wang et al. 2006; Murphy et al. 1998; Olah et al. 2008). AEA and 2-AG are lipophilic molecules that are synthesized on demand from their membrane lipid precursors, viz., N-arachidonoylphosphatidylethanolamine and diacylglycerol. The synthesis of AEA is mediated by N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD), and the synthesis of 2-AG is mediated by two sn-1-selective diacylglycerol lipases (DAGLs: DAGLα and DAGLβ; Okamoto et al. 2004; Bisogno et al. 2003). AEA is inactivated by fatty acid amide hydrolase (FAAH), which catalyzes the hydrolysis of AEA to arachidonic acid and ethanolamine, whereas 2-AG is metabolized by both FAAH and monoacylglycerol lipase (MAGL; Cravatt et al. 1996; Karlsson et al. 1997).
A recent study with golden hamsters has revealed the expression of NAPE-PLD, DAGLs, FAAH, and MAGL in the PT and of CB1 in the PD by means of in situ hybridization and immunocytochemistry (Yasuo et al. 2010b). The expression of DAGLβ and immunosignals for DAGLα, enzymes involved in 2-AG synthesis, and 2-AG levels in the PT are higher in animals kept under long-day conditions than those kept under short-day conditions. These data suggest that endocannabinoids are signaling molecules produced in the PT and targeted to the PD, and that 2-AG acts as a tuberalin in rodents (Yasuo et al. 2010b). The purpose of this study has been to extend these studies into humans: we have therefore investigated the localization of immunosignals of the enzymes involved in endocannabinoid synthesis and degradation (DAGLα, NAPE-PLD, and MAGL) in the human PT and identified the cell types of the PD expressing the CB1 receptor by using the double-immunofluorescence method.
Materials and methods
Human tissues
This study was performed with samples from four females and six males (for age and sex of the individuals, see Table 1). Hypothalami and hypophyses (PD and PT) were obtained during autopsy for forensic reasons; investigations were approved by the Ethics Committee of the Medical Faculty of the Goethe University, Frankfurt am Main (139/10). For immunohistochemistry, tissue from four females and three males was fixed with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) for 24-72 h, cryoprotected by increasing concentrations of sucrose, and cut into sagittal frozen sections (20 μm thick). Endocannabinoid levels were determined in the PT and the PD, which were dissected from three males and snap-frozen on dry ice immediately after dissection performed 8–27 h after death. Endocannabinoid levels were also determined in the hypophysis of six male C3H mice killed between 09.00 and 10.00. The PT and PD were dissected from three mice immediately after death and from three mice that had been kept at 4ºC for 27 h after death in order to show chemical stability of endocannabinoids in postmortem tissue. Animal experiments were approved by the local Ethics Committee and were performed in accordance with Federal guidelines and the European Council Directive.
Immunohistochemistry
The primary antibodies used in this study are listed in Table 2. After the blocking of endogenous peroxidase with 1% hydrogen peroxide, sections were washed in PBS supplemented with 0.3% Triton X-100 (PBST) and incubated in 10% normal goat serum for 30 min, followed by incubation with the primary antibody plus 1% bovine serum albumin overnight at room temperature. Subsequently, the sections were washed with PBST and incubated with biotinylated goat anti-rabbit IgG (Sigma, Deisenhofen, Germany; 1:100; 1 h) and ExtrAvidin (Sigma; 1:100, 1 h) at room temperature. Visualization of the immunocomplexes was performed by use of 0.05% 3,3’-diaminobenzidine and 0.015% hydrogen peroxide. The sections were observed with a Zeiss Axioplan microscope connected to a digital camera. To test the specificity of immunoreactivity, each antibody was preabsorbed with an excess amount of the respective antigen peptide and applied to the sections, which were then processed as described above.
Double-immunofluorescence
Sections of the hypophysis were prepared as described above. They were incubated in 10% normal donkey serum for 30 min and then with rabbit polyclonal anti-CB1 and with one of the antibodies recognizing either hypophysial hormones or S-100 protein, a marker for FS-cells (for antibody information, see Table 2) overnight at room temperature. Immunosignals for CB1 were detected by Alexa Fluor 488 donkey anti-rabbit IgG (Invitrogen, 1:200), whereas those for hypophysial hormones and S-100 protein were detected by Cy3-conjugated donkey anti-goat IgG, anti-sheep IgG, or anti-mouse IgG (Jackson Immunoresearch, 1:400). Slides were observed with a confocal laser scanning microscope (LSM510, Zeiss).
Determination of endocannabinoids by high-performance liquid chromatography coupled with tandem mass spectrometry
For the quantification of endocannabinoids, the PT and the PD were dissected out from three males and snap-frozen on dry ice. The tissue samples were homogenized and extracted by liquid-liquid extraction as described in detail previously (Yasuo et al. 2010b). The extracts were evaporated under nitrogen, and the reconstituted samples were analyzed for AEA, N-palmitoylethanolamide (PEA), N-oleoylethanolamide (OEA), 2-AG, and 1-AG. The respective deuterated substances AEA-d8, PEA-d4, 1-AG- d5, 2-AG-d5, and OEA-d2 were used as internal standards. High-performance liquid chromatography (HPLC) analysis was performed under gradient conditions through a Luna HST C18(2) column (100 mm length x 2 mm internal diameter, 2.5 μm particle size; Phenomenex) as described in detail previously (Yasuo et al. 2010b). Mass spectrometry (MS) and tandem MS (MS/MS) analyses were performed on an API 5000 triple quadrupole mass spectrometer with a Turbo V source (Applied Biosystems) in the negative ion mode. Precursor-to-product ion transitions of m/z 346→259 for AEA, m/z 354→86 for AEA-d8, m/z 298→268 for PEA, m/z 302→272 for PEA-d4, m/z 377→303 for 2-AG and 1-AG, m/z 382→303 for 2-AG-d5 and 1-AG- d5, m/z 324→86 for OEA, and m/z 326→86 for OEA-d2 were used for multiple reaction monitoring (MRM) with a dwell time of 70 ms. Concentrations of the calibration standards, quality controls, and unknowns were evaluated by Analyst software (version 1.4; Applied Biosystems).
To estimate the postmortem degradation, endocannabinoid levels were also determined in hypohyses dissected from male C3H mice either immediately or after a postmortem period of 27 h during which the cadavers were kept at 4°C.
Results
Localization of DAGLα, NAPE-PLD, and MAGL in human PT
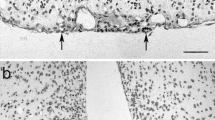
The location of the human PT is shown in a sagittal section through the whole hypophysial area (Fig. 1a). Most cells of the PT were immunoreactive for the common glycoprotein alpha-subunit (CGA; Fig. 1b, c) and TSHB (Fig. 1d). The TSHB-immunreactive PT cells were smaller and displayed a weaker TSHB immunoreaction than the thyrotropes in the PD (Fig. 1e). In all specimens investigated, numerous cells of the PT displayed immunosignals for DAGLα, NAPE-PLD, and MAGL (Fig. 2a-f). NAPE-PLD (Fig. 2a, d) was concentrated in the perinuclear region of virtually all PT cells, whereas DAGLα (Fig. 2b, e) and MAGL (Fig. 2c, f) were localized in the cytoplasm. All immunosignals were totally blocked when the respective primary antibody had been preabsorbed with antigen peptides.
a Sagittal section of the human hypophysis showing the pars tuberalis (PT), pars distalis (PD), pars intermedia (PI), and pars nervosa (PN). The boxed areas indicate the relevant regions examined by immunohistochemistry. b–e Immunohistochemical demonstration of the common glycoprotein alpha-subunit in the pars tuberalis (b, c) and of the thyrotropin beta-subunit in the pars tuberalis (d) and pars distalis (e) of man. Bars 1 mm (a), 100 μm (b, d), 20 μm (c, e)
Immunohistochemical demonstration of N-acylphosphatidylethanolamine-specific phospholipase D (a, d), diacylglycerol lipase-alpha (b, e), and monoacylglycerol lipase (c, f) in the pars tuberalis (PT) of man; low (a-c) and high (d-f) magnification images. The extent of the pars tuberalis layer is shown by lines in a-c. Bars 100 μm (a-c), 20 μm (d-f)
Determination of endocannabinoid levels in human PT
As revealed by HPLC-MS/MS, the concentration of 2-AG in the human PT ranged between 0.309 to 0.104 ng/mg tissue and exceeded that of all other endocannabinoids determined (AEA: 0.006 to 0.003 ng/mg tissue; PEA: 0.012 to 0.008 ng/mg tissue; OEA: 0.013 to 0.011 ng/mg tissue; see Table 3). Investigations of hypophyses of mice also showed higher concentrations of 2-AG as compared with AEA, OEA, and PEA. Importantly, the ratio between the levels of 2-AG, AEA, OEA, and PEA remained constant, irrespective of whether the tissue was dissected and snap frozen either immediately or 27 h after the death of the animals.
Localization of CB1 in human PD
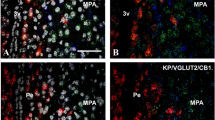
Immunosignals for CB1 were found in the cytoplasm of many PD cells. The distribution of CB1-immunoreactive cells within the PD was not homogeneous: some regions contained densely packed CB1-immunoreactive cells (Fig. 3a), whereas such cells were only scattered or even absent in other regions (Fig. 3b). Moreover, the distribution of the CB1 immunoreactivity within a single cell was not homogeneous; some cells contained either coarse or delicate granular-like CB1-immunoreactive areas in their cytoplasm (large and small arrows in Fig. 3c, respectively), whereas others displayed small dot-like immunosignals located in the periphery of the cytoplasm (large arrowheads in Fig. 3c) or at junctions connecting the cells and/or forming the follicles (small arrowheads in Fig. 3c).
Immunohistochemical demonstration of cannabinoid receptor 1 (CB1) in the pars distalis of man at low (a, b) and high (c) magnification. Immunosignals for CB1 are concentrated (c, large arrows) or scattered (c, small arrows) in the cytoplasm. CB1-immunoreactive dots are often located in the periphery of the cytoplasm (c, large arrowheads) or at junctions connecting the cells and/or forming the follicles (c, small arrowheads). Follicles are indicated by asterisks. Bars 100 μm (a, b), 20 μm (c)
Identification of cell types containing CB1 immunoreactivity in human PD
To identify the CB1-immunoreactive cells in the PD, we performed double-immunofluorescence staining with antibodies against CB1 and hypophysial hormones, viz., prolactin (PRL), adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), and growth hormone (GH), or against S-100 protein, a marker of folliculo-stellate (FS)-cells. Most of the cells displaying CB1 immunoreactivity were also immunoreactive for either ACTH or S-100-protein (Fig. 4b, h, n, f, l, r). Interestingly, the CB1 immunoreaction was mainly cytoplasmic in ACTH-immunoreactive cells (Fig. 4b, h, n) and appeared as dot-like structures in the periphery of the S-100-protein-immunoreactive cells (Fig. 4f, l, r). CB1 immunosignals were also detected in a limited number of PRL- and GH-immunoreactive cells (1%-2% or less), but no overlapping was found between CB1 and FSH- or TSH- immunosignals (Fig. 4).
Double-immunolabeling of cannabinoid receptor 1 (CB1) and hypophysial hormones viz., prolactin (PRL), adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), and growth hormone (GH), or S-100 protein (S-100), a marker for folliculostellate cells, in the pars distalis of man. Immunosignals for CB1 (a-f), PRL (g), ACTH (h), FSH (i), TSH (j), GH (k), and S-100 (l) and merged images for CB1 and each hormone/protein (m-r) are presented in each row (arrowheads overlapping signals, asterisks follicles). Bar 20 μm
Discussion
The PT is located between the median eminence, the portal vasculature, and the hypophysial PD and forms an important interface within the hypothalamo-hypophysial system. Studies with photoperiodic animals have pinpointed an important role of the PT for the control of seasonal reproduction. In photoperiodic mammals, the functional state of the PT strongly depends on the melatonin signal from the pineal organ (see Introduction), and TSHB has been identified as a melatonin-controlled messenger that transmits signals from the PT back into the hypothalamus (Nakao et al. 2008; Hanon et al. 2008; Ono et al. 2008; Unfried et al. 2009, 2010; Yasuo et al. 2010a). Moreover, our own recent studies with hamsters have identified an endocannabinoid system in the PT and provided strong evidence that 2-AG acts as important messenger from the PT (Yasuo et al. 2010b).
The aim of the present study has been to gain insight into the messenger molecules of the PT in man. First of all, we have been able to demonstrate that numerous PT-specific cells in man display immunosignals for TSHB and CGA. To our knowledge, the only study describing TSH-immunoreactive cells in the human PT was published by Gross (1984) who found TSH-like immunoreactivity only in a few large cells. Our results show TSHB and CGA immunoreactivity in numerous PT cells whose somata are smaller than those of the thyrotropes in the PD. A similar distribution of TSHB immunoreactivity has been observed in hamster (Yasuo et al. 2010a), mice (Unfried et al. 2009), and sheep (Böckers et al. 1996; Bockmann et al. 1997). These data support the hypothesis that TSHB is a conserved molecular messenger in the PT, and they suggest a functional role for TSHB also in man.
Moreover, the present study of the human PT has revealed immunoreactivities for DAGLα, NAPE-PLD, and MAGL, enzymes involved in synthesis and degradation of 2-AG and AEA, respectively. Accordingly, the human PT has been found to contain 2-AG and lower levels of AEA, PEA, and OEA. A similar ratio between 2-AG, AEA, PEA, and OEA has been described in hamsters (Yasuo et al. 2010b). These data suggest that 2-AG and other endocannabinoids are produced and metabolized in the human PT, and that the intrinsic endocannabinoid system that has been recently discovered in the hamster PT (Yasuo et al. 2010b) is conserved in man. An important question relates to the functional significance of the endocannabinoid system in the PT of man. As is well known, endocannabinoids participate in the neuroendocrine control of gonadotropins, prolactin, stress hormones, growth hormones, and thyroid hormones (Wang et al. 2006; Murphy et al. 1998; Olah et al. 2008), and the expression or protein levels of DAGLs (synthesizing enzyme for 2-AG) and 2-AG levels are upregulated in the PT of hamsters kept under long-day conditions as compared with those kept under short-day conditions (Yasuo et al. 2010b). Experimental evidence with hamsters suggests that the endocannabinoids produced in the PT exert their actions upon specific cells in the PD. To search for putative targets of the endocannabinoids produced in human PT, we have performed immunocytochemical analyses of CB1, a major endocannabinoid receptor and, indeed, have found CB1 immunosignals in the human PD. Thus, our results are consistent with previous reports demonstrating CB1 mRNA and protein in the PD of rats (Gonzalez et al. 1999; Wenger et al. 1999), hamsters (Yasuo et al. 2010b), and humans (Pagotto et al. 2001). Moreover, we have succeeded in identifying the major cell types containing CB1 receptors as being ACTH-producing cells and FS-cells by means of double-immunofluorescence for CB1 with ACTH or S-100 protein. These data suggest that endocannabinoids produced in the PT act on corticotrophs and FS-cells for the regulation of hormonal secretion. Endocannabinoids are known to regulate neuroendocrine functions mainly by hypothalamic control through hypothalamic peptide or monoamine signals that then control hormonal secretion from the hypophysial PD (Wang et al. 2006; Murphy et al. 1998; Pagotto et al. 2006; Olah et al. 2008). However, other data indicate the direct action of endocannabinoids on the PD. For example, the CB1 agonist Δ9-tetrahydrocannabinol has been shown to induce prolactin release and to increase cAMP levels in primary cell cultures of the rat PD (Rodriguez de Fonseca et al. 1999). Similarly, AEA has been demonstrated to inhibit or activate LH, PRL, ACTH, and GH in dispersed PD cell cultures of the rat (Wenger et al. 2000). In the present study, most cells expressing the CB1 receptor in the human PD have been found to be either corticotrophs or FS-cells. Thus, endocannabinoids might control the endocrine activity of the PD primarily via direct action on corticotrophs or indirect action on FS-cells, which produce various tropic factors to modulate hormonal secretions in neighboring endocrine cells (Devnath and Inoue 2008).
The seasonal changes of the photoperiod affect various physiological processes and behaviors, including stress response and mood. The demonstration of an endocannabinoid system in the PT, a neuroendocrine center for the photoperiodic control of biological physiology, and the existence of CB1 receptors in ACTH-producing cells suggest the involvement of an endocannabinoid system in the control of stress responses in man. Clearly, this assumption needs further experimental verification but, if confirmed, will open new avenues to decipher further the functional significance of the PT, which so far has been mainly related to the control of seasonal reproduction. Intriguingly, in human corticotropinoma cells, CB1 agonists do not modify the basal levels of ACTH secretion but potentiate the inductive action of corticotropin-releasing factors (CRF) on ACTH secretion (Pagotto et al. 2001). These actions might be driven by endocannabinoids produced seasonally in the PT and underlie the mechanism of seasonal affective disorder (SAD). SAD is a condition of regularly occurring depressions in winter with a remission the following spring or summer; it affects 1%-3% of adults in temperate climates (Rosenthal et al. 1988; Magnusson and Boivin 2003) and is related to abnormal pituitary-adrenal responses to CRF (Joseph-Vanderpool et al. 1991).
In conclusion, we have identified an endocannabinoid system in the human PT and have localized the majority of CB1 receptors within the PD to corticotrophs and FS-cells. These data suggest that endocannabinoids are signaling molecules that, also in man, are produced in the PT and conveyed to the PD to regulate hypophysial hormone secretion by either direct action on corticotrophs or indirect action on FS-cells. In view of the prominent role of the PT for seasonal physiology in photoperiodic mammals, it remains to be elucidated whether the endocannabinoid system within the human PT-PD plays a role in the development of SADs.
References
Arendt J (1995) Melatonin and the mammalian pineal gland. Chapman & Hall, London
Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P (2003) Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163:463–468
Böckers TM, Bockmann J, Fauteck JD, Wittkowski W, Sabel BA, Kreutz MR (1996) Evidence for gene transcription of adenohypophyseal hormones in the ovine pars tuberalis. Neuroendocrinology 63:16–27
Bockmann J, Böckers TM, Winter C, Wittkowski W, Winterhoff H, Deufel T, Kreutz MR (1997) Thyrotropin expression in hypophyseal pars tuberalis-specific cells is 3, 5, 3’-triiodothyronine, thyrotropin-releasing hormone, and pit-1 independent. Endocrinology 138:1019–1028
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lemer RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87
Devnath S, Inoue K (2008) An insight to pituitary folliculo-stellate cells. J Neuroendocrinol 20:687–691
Di Marzo V, Bifulco M, De Petrocellis L (2004) The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 3:771–784
Gall C von, Garabette ML, Kell CA, Frenzel S, Dehghnai F, Schumm-Draeger PM, Weaver DR, Korf HW, Hastings MH, Stehle JH (2002a) Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci 5:234–238
Gall C von, Stehle JH, Weaver DR (2002b) Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res 309:151–162
Gall C von, Weaver DR, Moek J, Jilg A, Stehle JH, Korf HW (2005) Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann NY Acad Sci 1040:508–511
Gonzalez S, Manzanares J, Berrendero F, Wenger T, Corchero J, Bisogno T, Romero J, Fuentes JA, Di Marzo V, Ramos JA, Fernandez-Ruiz J (1999) Identification of endocannabinoids and cannabinoid CB(1) receptor mRNA in the pituitary gland. Neuroendocrinology 70:137–145
Gross DS (1984) The mammalian hypophysial pars tuberalis: a comparative immunocytochemical study. Gen Comp Endocrinol 56:283–298
Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pevet M, Morgan PJ, Hazlerigg DG (2008) Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol 18:1147–1152
Hazlerigg DG, Morgan PJ, Messager S (2001) Decoding photoperiodic time and melatonin in mammals: what can we learn from the pars tuberalis? J Biol Rhythms 16:326–335
Joseph-Vanderpool JR, Rosenthal NE, Chrousos GP, Wehr TA, Skwerer R, Kasper S, Gold PW (1991) Abnormal pituitary-adrenal responses to corticotropin-releasing hormone in patients with seasonal affective disorder: clinical and pathophysiological implications. J Clin Endocrinol Metab 72:1382–1387
Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C (1997) cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. J Biol Chem 272:27218–27223
Kunos G (2007) Understanding metabolic homeostasis and imbalance: what is the role of the endocannabinoid system? Am J Med 120:S18–S24
Lincoln GA, Andersson H, Loudon A (2003) Clock genes in calendar cells as the basis of annual timekeeping in mammals—a unifying hypothesis. J Endocrinol 179:1–13
Magnusson A, Boivin D (2003) Seasonal affective disorder: an overview. Chronobiol Int 20:189–207
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564
Morgan PJ (2000) The pars tuberalis: the missing link in the photoperiodic regulation of prolactin secretion? J Neuroendocrinol 12:287–295
Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65
Murphy LL, Munoz RM, Adrian BA, Villanua MA (1998) Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis 5:432–446
Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, Kasukawa T, Iigo M, Sharp PJ, Iwasawa A, Suzuki Y, Sugano S, Niimi T, Mizutani M, Namikawa T, Ebihara S, Ueda HR, Yoshimura T (2008) Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 452:317–322
Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N (2004) Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem 279:5298–5305
Olah M, Milloh H, Wenger T (2008) The role of endocannabinoids in the regulation of luteinizing hormone and prolactin release. Differences between the effects of AEA and 2AG. Mol Cell Endocrinol 286:S36–S40
Ono H, Hoshino Y, Yasuo S, Watanabe M, Nakane Y, Murai A, Ebihara S, Korf HW, Yoshimura T (2008) Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc Natl Acad Sci USA 105:18238–18242
Pagotto U, Marsicano G, Fezza F, Theodoropoulou M, Grubler Y, Stalla J, Arzberger T, Milone A, Losa M, Di Marzo V, Lutz B, Stalla GK (2001) Normal human pituitary gland and pituitary adenomas express cannabinoid receptor type 1 and synthesize endogenous cannabinoids: first evidence for a direct role of cannabinoids on hormone modulation at the human pituitary level. J Clin Endocrinol Metab 86:2687–2696
Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R (2006) The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 27:73–100
Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884
Reiter RJ (1980) The pineal and its hormones in the control of reproduction in mammals. Endocr Rev 1:109–131
Rodriguez de Fonseca F, Wenger T, Navarro M, Murphy LL (1999) Effects of delta9-THC on VIP-induced prolactin secretion in anterior pituitary cultures: evidence for the presence of functional cannabinoid CB1 receptors in pituitary cells. Brain Res 841:114–122
Rosenthal NE, Sack DA, Skwerer RG, Jacobsen FJ, Wehr TA (1988) Phototherapy for seasonal affective disorder. J Biol Rhythms 3:101–120
Unfried C, Ansari N, Yasuo S, Korf HW, Gall C von (2009) Impact of melatonin and molecular clockwork components on the expression of thyrotropin β-chain (Tshb) and the Tsh receptor in the mouse pars tuberalis. Endocrinology 150:4653–4662
Unfried C, Burbach G, Korf HW, Gall C von (2010) Melatonin receptor 1-dependent gene expression in the mouse pars tuberalis as revealed by cDNA microarray analysis and in situ hybridization. J Pineal Res 48:148–156
Wang H, Dey SK, Maccarrone M (2006) Jekyll and Hyde: two faces of cannabinoid signaling in male and female fertility. Endocr Rev 27:427–448
Wenger T, Fernandez-Ruiz JJ, Ramos JA (1999) Immunocytochemical demonstration of CB1 cannabinoid receptors in the anterior lobe of the pituitary gland. J Neuroendocrinol 11:873–878
Wenger T, Jamali KA, Juaneda C, Bacsy E, Tramu G (2000) The endogenous cannabinoid, anandamide regulates anterior pituitary secretion in vitro. Addict Biol 5:59–64
Yasuo S, Yoshimura T, Ebihara S, Korf HW (2009) Melatonin transmits photoperiodic signals through the MT1 melatonin receptor. J Neurosci 29:2885–2889
Yasuo S, Yoshimura T, Ebihara S, Korf HW (2010a) Photoperiodic control of TSH-β expression in the mammalian pars tuberalis has different impacts on the induction and suppression of the hypothalamo-hypophysial gonadal axis. J Neuroendocrinol 22:43–50
Yasuo S, Koch M, Schmidt H, Ziebell S, Bojunga J, Geisslinger G, Korf HW (2010b) An endocannabinoid system is localized to the hypophysial pars tuberalis of Syrian hamsters and responds to photoperiodic changes. Cell Tissue Res 340:127–140
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the Alfons und Gertrud Kassel-Stiftung, Frankfurt am Main, Germany, and the LOEWE Lipid Signaling Forschungszentrum Frankfurt (LiFF).
Rights and permissions
About this article
Cite this article
Yasuo, S., Unfried, C., Kettner, M. et al. Localization of an endocannabinoid system in the hypophysial pars tuberalis and pars distalis of man. Cell Tissue Res 342, 273–281 (2010). https://doi.org/10.1007/s00441-010-1066-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-010-1066-5