Abstract
To probe pro-fibrotic mechanisms in dystrophic muscle, we isolated primary fibroblasts from Duchenne muscular dystrophy (DMD) and control muscle biopsies and induced transdifferentiation in myofibroblasts by transforming growth factor β1 (TGF-β1) treatment. We compared proliferating activity, soluble collagen production, and transcript and protein levels of decorin, myostatin, TGF-β1, matrix metalloproteinase-1 (MMP-1; interstitial collagenase), MMP-2 (gelatinase), MMP-3 (stromelysin), MMP-7 (matrilysin), and the tissue inhibitors of metalloproteinases inhibitors (TIMPs) 1–4, in fibroblasts and myofibroblasts. Principal differences included a significantly greater proliferation rate and soluble collagen production, a significant upregulation of decorin, myostatin and MMP-7 transcripts and proteins, and a significant downregulation of MMP-1 and TIMP-3 transcripts (with MMP-1 protein being reduced as shown by enzyme-linked immunosorbent assay and TIMP-3 protein apparently being reduced on Western blot), in untreated DMD fibroblasts compared with controls. TGF-β1 transdifferentiation significantly lowered decorin and myostatin and significantly increased TGF-β1 transcript and protein, significantly increased MMP-1 and TIMP-3, and significantly lowered MMP-7 transcript and protein in DMD cells compared with pretreatment controls. The differences between DMD and control fibroblasts showed that DMD fibroblasts had a profibrotic phenotype, accentuated by TGF-β1 treatment. Dystrophin absence itself could exert a direct influence on the homeostasis of the extracellular matrix (ECM) by allowing leakage of cellular components to the extracellular space or by abnormal cellular uptake of extracellular growth factors, cytokines, or enzymes influencing muscle fibroblasts either directly by altering adhesion properties or indirectly by interactions with molecules released into the ECM by muscle or inflammatory cells. The transdifferentiation of muscle fibroblasts might serve as a simplified model of fibrosis for further elucidation of the mechanisms of muscle fibrosis and for testing possible anti-fibrotic agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Duchenne muscular dystrophy (DMD), caused by mutations in the dystrophin gene on the X chromosome, is one of the most common inherited neuromuscular diseases, affecting 1 in 3500 male births. Dystrophin, the dystrophin-associated glycoprotein complex, and laminin α-2 form a link between the extracellular matrix (ECM) and the intracellular cytoskeleton; this link is crucial for maintaining the structural integrity of muscle fibers (Matsumura and Campbell 1994; Blake et al. 2002). The main consequences of dystrophin absence in skeletal muscle are sarcolemmal instability and increased fiber vulnerability to mechanical stress, resulting in fiber degeneration, followed to some extent by regeneration. However, complete regeneration is prevented by the proliferation of connective tissue (fibrosis), which progressively replaces muscle tissue.
Fibrosis is a complex incompletely understood process characterized by excessive accumulation of collagens and other ECM components. It occurs in conditions affecting skeletal muscle, liver (Kossakowska et al. 1998), kidney (Schnaper et al. 1996), myocardium (Li et al. 2000; Herpel et al. 2006), lung (Selman et al. 2001), and biliary tract (Milani et al. 1990).
The extensive structural disorganization and remodeling that characterize the fibrotic process involve an imbalance of matrix metalloproteinases (MMPs) and their specific tissue inhibitors (TIMPs), the release of fibrogenic cytokines including transforming growth factor β1 (TGF-β1; Mauviel 2005), and the alteration of proteoglycans such as decorin and biglycan (Westergren-Thorsson et al. 1993). Numerous functions of decorin have been documented, including protein-protein interactions, cell adhesion, signal transduction, and DNA repair (Vogel et al. 1984; De Luca et al. 1996; Iozzo 1999). Functions of likely importance in muscle diseases include binding to collagens, TGF-β1, and myostatin (Yamaguchi et al. 1990; Hildebrand et al. 1994; Miura et al. 2006). Myostatin regulates the growth of myocytes (McPherron and Lee 1997). Recent work has shown that myostatin also directly stimulates the proliferation of muscle fibroblasts and the production of ECM proteins in vitro and in vivo, and that muscle fibroblasts express myostatin and its putative receptor (Li et al. 2008).
TGF-β1 intervenes in the regulation of ECM composition through the stimulation of protein production, the inhibition of matrix degradation, and the control of adhesion proteins required for cell-matrix interactions (Montesano and Orci 1988; Kissin et al. 2002). Its aberrant expression has been implicated in fibrotic and inflammatory conditions in kidney, liver, and lung (Border and Ruoslahti 1992; Sime et al. 1997; Iredale 2007). In muscle tissue from dystrophin- and LAMA2-mutated muscular dystrophy patients, TGF-β1 transcript levels have been shown to be greatly increased (Bernasconi et al. 1995; Zanotti et al. 2005). However, the molecular mechanisms involved in the profibrotic role of TGF-β1 are not fully understood. The cytokine is a direct inducer of the myofibroblast phenotype (Desmoulière et al. 1993; Ronnov-Jessen and Petersen 1993). Myofibroblasts occur in wound healing and fibrosis (Sappino et al. 1990; Gabbiani 2003) and play a major role in these processes because of their ability to synthesize various ECM components such as fibronectin and collagen (Ignotz and Massagué 1986), growth factors, cytokines (Finlay et al. 2000), growth factor receptors (Thannickal et al. 1998), and integrins (Heino et al. 1989) involved in the repair and remodeling of connective tissue. Normally, when tissue repair is completed, myofibroblasts disappear, probably as a result of apoptosis, and normal tissue function is restored (Desmoulière et al. 1995). However, if the tissue repair program is not terminated appropriately, myofibroblasts persist in the lesion and may give rise to scarring and chronic fibrotic conditions (Lorena et al. 2002; Desmoulière et al. 2005).
In order to increase our understanding of pro-fibrotic mechanisms in dystrophic muscle, we have isolated primary human fibroblasts from DMD and control muscle biopsies and induced them to differentiate into myofibroblasts with TGF-β1. We have investigated, in both fibroblasts and myofibroblasts, not only proliferating activity and soluble collagen production, but also transcript and protein levels of decorin, myostatin, TGF-β1, MMP-1 (interstitial collagenase), MMP-2 (gelatinase), MMP-3 (stromelysin), MMP-7 (matrilysin), and the MMP inhibitors TIMP-1, TIMP-2, TIMP-3, and TIMP-4.
Materials and methods
Cell cultures
Quadriceps muscle biopsies were obtained after informed parental consent from four DMD patients (aged 1–7 years) and four controls (aged 1–10 years) suspected of neuromuscular disease, but who had normal muscle on biopsy. Investigations on human tissue were approved by our institutional review board. DMD was diagnosed by dystrophin testing and gene analysis.
Primary fibroblasts were derived from muscle biopsies by immunoselection from the myogenic lineage (Zanotti et al. 2007). After being cultured in M growth medium consisting of Dulbecco’s modified Eagle’s medium (DMEM; Cambrex Corporation, East Rutherford, N.J., USA), 20% heat-inactivated fetal bovine serum (FBS; Cambrex), 1% penicillin-streptomycin (Cambrex), 2 mM L-glutamine (Cambrex), 10 μg/ml insulin (Sigma Aldrich, St. Louis, Mo., USA), 2.5 ng/ml basic fibroblast growth factor (bFGF; Gibco Life Technologies, Carlsbad, Calif., USA), and 10 ng/ml epidermal growth factor (EGF; Gibco), myoblasts and fibroblasts were separated in a midiMACS cell separator (Milteny Biotec, Bergisch Gladbach, Germany) by using immunomagnetic beads to which the anti-human CD56 surface marker (Dickson et al. 1987) was attached. After allowing CD56-negative cells (fibroblasts) to pass through the column, CD56-positive cells (myoblasts) were flushed out by using the plunger. CD56-positive and CD56-negative cell fractions were subsequently seeded onto collagen-coated Petri dishes; fibroblasts were grown in DMEM containing 10% FBS, 1% penicillin-streptomycin, and L-glutamine (F growth medium), whereas myoblasts were grown in M growth medium and frozen for future analysis.
Myofibroblasts were obtained by treatment with TGF-β1 as follows: fibroblast growth was first arrested by serum withdrawal, rinses with PBS, and replacement with medium without serum; 48 h later the cells were stimulated by addition of 10 ng/ml h-recombinant TGF-β1 (Peprotech, London) for 48 h. The cellular response to TGF-β1 was evaluated by quantitating α-smooth muscle actin (α-SMA) transcripts by real time polymerase chain reaction (PCR), and α-SMA protein by Western blot and immunocytochemistry with a commercial monoclonal antibody (Sigma Aldrich).
Immunocytochemistry
Fibroblasts and myofibroblasts were fixed in 4% paraformaldehyde, permeabilized in PBS with 0.1% Triton X-100 for 30 min, and incubated overnight with one of the following mouse monoclonal antibodies: anti-prolyl-4-hydroxylase (anti-fibroblast), anti-desmin (both from DAKO, Copenhagen, Denmark; 1:500 and 1:200, respectively), anti-CD44, anti-CD31, anti-α-SMA (all from Sigma; all diluted 1:200), or anti-collagen I (polyclonal from Chemicon, diluted 1:250). The cells were then incubated with goat anti-mouse Alexa-488 (Invitrogen; 1:2000) for 2 h, followed by DAPI (4,6-diamidino-2-phenylindole; Sigma; 1:3000). As negative control, primary antibody was omitted.
Phalloidin staining to demonstrate F-actin was performed on paraformaldehyde-fixed cells by incubation in fluorescein isothiocyanate (FITC)-phalloidin (Sigma; 1:1000) for 40 min, in combination with either α-SMA or collagen I immunostaining. Cells were examined under Zeiss Axioplan fluorescence.
Cell proliferation assay
Cells were plated onto 96-well plates (104 cells/well) and incubated overnight with 100 μl F growth medium, which was then replaced with serum-free medium for 24 h in order to synchronize the cells. Cell proliferation was then determined by measuring the incorporation of 5-bromo-2′-deoxyuridine (BrdU), after a 4-h incubation, by means of an enzyme immunoassay kit (BrdU cell proliferation assay kit; Roche Diagnostic, Penzberg, Germany), according to the manufacturer’s instructions. The enzymatic reaction was stopped with 25 μl 2 N sulfuric acid, and absorbances were read at 450 nm on a Victor Wallac 1420 multi-label reader (Perkin-Elmer, Waltham, Mass., USA).
Collagen measurements
Total soluble (non-cross-linked) collagen was determined in culture supernatants by a quantitative dye-binding method with the Sircol collagen assay (Biocolor, Belfast, N. Ireland, UK) according to the manufacturer’s instructions.
cDNA synthesis
Total RNA was isolated from fibroblasts or myofibroblasts by using TRI Reagent (Ambion, Austin, Tex., USA) according to the manufacturer’s instructions and checked spectrophotometrically for quantity and purity. Aliquots of RNA (1 μg) were reverse-transcribed in the presence of 5× first strand buffer (Invitrogen), 1 mM each deoxynucleoside triphosphate, 8 pM random hexamers, 10 μM dithiothreitol, 1 IU/µl RNAse inhibitor (Roche Molecular Biochemicals, Basel, Switzerland), and 10 IU/µl M-MLV reverse transcriptase (Invitrogen) with incubation at 37°C for 1 h and at 95°C for 5 min. The reaction product was stored at −20°C pending use. cDNA integrity was assessed by PCR amplification of human D-glyceraldehyde-3-phosphate dehydrogenase (GenBank accession no. M33197) with specific primers (forward 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse 5′-GAAGATGGTGATGGGATTTC-3′). PCR conditions were: 94°C 1 min, 54°C 1 min, and 72°C 1 min, for 35 cycles.
Real time PCR
Target gene expression was analyzed by quantitative real time PCR. TaqMan Universal PCR MasterMix and Assays-on-Demand Gene Expression probes (Applied Biosystems, Calif,. USA; see Table 1) were used for the PCR step. Reactions were performed in 96-well plates with 25-μl volumes. All samples were analyzed in triplicate. Cycling parameters were: 2 min at 50°C, 95°C for 10 min, followed by 40 cycles of PCR (15 s at 95°C and 1 min at 60°C). Products were detected with the ABI Prism 7000 sequence detection system (Applied Biosystems). The expression of each target gene in control and DMD fibroblasts was normalized to the expression of β-actin and determined as the ratio of the target gene to β-actin gene calculated by \( {{\text{2}}^{ - \Delta {\text{Ct}}}} \), where \( \Delta {\text{Ct}} = {\text{C}}{{\text{t}}^{\text{Target}}} - {\text{C}}{{\text{t}}^{\beta - {\text{actin}}}} \). Differences in gene expression between basal and TGF-β1-treated control and DMD cells were calculated by using the mathematical model based on PCR efficiency (E), described by Pfaffl (2001). According to this model, differences in expression were determined by \( {{\text{E}}^{\Delta {\text{CTgene basal}} - \Delta {\text{CT gene TGF}} - \beta {\text{1 stimulated}}}} \).
Protein extraction
Cells were washed with PBS and lysed in extraction buffer (10 mM TRIS-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.5% Nonidet-P, 1% Triton X-100) containing a protease inhibitor cocktail (Pierce Biotechnology, Rockford, Ill., USA) for 30 min on ice. The lysate was centrifuged at 18,000g for 15 min at 4°C. The supernatant was collected, and the protein concentration was determined with the DC Protein Assay Reagent (Bio-Rad).
Zymography
MMP-1 and MMP-2 activities were detected in the medium after 5× concentration by using an Ultrafree-4 centrifugal filter unit (Millipore Corporation, Billerica, Mass., USA), and protein concentration was determined with the DC Protein Assay Reagent.
Samples (10 µg of the 5× concentrated preparations) were then diluted 1:1 in non-reducing buffer (0.5 M TRIS-HCl, pH 6.8, 10% SDS, 20% glycerol, 0.1% bromophenol blue) and electrophoresed on 7.5% SDS-polyacrylamide gels containing 1 mg/ml of either β-casein (for detection of MMP-1 activity) or gelatin type A from pig skin (for detection of MMP-2 activity; both from Sigma). The gels were washed twice for 15 min in 2.5% (v/v) Triton X-100, rinsed with distilled H2O at room temperature, and incubated overnight in developing buffer (50 mM TRIS-HCl, pH 7.5, 5 mM CaCl2, 200 mM NaCl) at 37°C. The gels were then stained with 0.5% Coomassie brilliant blue R-250 (in 30% methanol, 10% acetic acid) for 3 h and de-stained with aqueous methanol (30%) and acetic acid (10%). An area in which the gelatin had been degraded by proteolytic activity were seen as an absence of staining.
Western blot
For detection of α-SMA, 10 μg cell protein extracts were solubilized in 2× Laemmli buffer (0.5 M TRIS-HCl, pH 6.8, 20% glycerol, 2% SDS, 5% 2-mercaptoethanol, 1% bromophenol blue), boiled, separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, N.H., USA). Membranes were probed with anti-α-SMA (diluted 1:250) and anti-β-tubulin (internal standard; diluted 1:2500; both monoclonal antibodies from Sigma). Biotin-conjugated secondary antibody was then applied (1:2500; Jackson ImmunoResearch, Westgrove, Pa., USA), followed by peroxidase-conjugated streptavidin (1:3000; Jackson ImmunoResearch), and detection with the ECL chemiluminescence reagent (Amersham Biosciences, Buckinghamshire, UK).
MMP-2, MMP-3, TIMP-1, TIMP-2, and TIMP-3 (all secreted extracellularly) were detected in supernatants prepared and concentrated as described above. Samples (10 µg) of supernatant proteins were solubilized in 2× Laemmli buffer, boiled, separated by 12.5% SDS-PAGE, and transferred to nitrocellulose membranes. Membranes were probed with one of the following rabbit polyclonal antibodies, viz., anti-MMP-2, anti-TIMP-2 (both from Biomol), anti-MMP-3, anti-TIMP-1, or anti-TIMP-3 (Immunological Sciences, Rome, Italy; all diluted 1:250), and detected as described above.
For the detection of decorin, 20 μg cell protein extract or cell medium was digested with 25 mU chondroitinase ABC (Sigma) for 6 h at 37°C. Samples were then solubilized in 2× Laemmli buffer, boiled, electrophoresed on 12% SDS-PAGE, and transferred to nitrocellulose membranes. These were probed with goat polyclonal anti-decorin (Calbiochem, Merck Chemicals, Nottingham, UK; 1:100) and revealed as described above.
Enzyme-linked immunosorbent assay
MMP-1, MMP-7, TGF-β1, and myostatin in the extracellular medium were not detected by Western blot and were therefore evaluated by ELISA. MMP-1 was detected by the Calbiochem sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions, whereas MMP-7, TGF-β1, and myostatin were detected with direct ELISA assays. Briefly, plates were coated with 100 μl medium or protein standards (for the calibration curve) and incubated at 4°C overnight. Unbound antigen was removed from the plates by tapping off the liquid and several washes with PBS plus 0.1% Triton X-100. To block non-specific binding, 200 μl 1% bovine serum albumin/PBS was added for 2 h at room temperature, followed by several washes as above. Aliquots (100 μl) of polyclonal anti-MMP-7 (Biomol), monoclonal anti-TGF-β1 (BioSource International, Camarillo, Calif., USA), or polyclonal anti-myostatin (Chemicon), all diluted 1:100, was then added, and the wells were incubated overnight at 4°C. After several washes, the wells were incubated with the second-step alkaline-phosphatase-conjugated antibody for 2 h, followed by repeated washes, incubation with 100 μl substrate solution for 1 h, and finally, addition of stopping solution. The wells were then read on an ELISA Victor Wallac 1420 multi-label plate reader.
Statistical analysis
All experiments, except for Western blots, represent data from at least three patients and three controls, always performed at least nine times. For the Western blots, concentrated supernatants from three or four patients were pooled, as were those from at least three controls.
For reverse transcription with PCR (RT-PCR), because of inherent variability in data from different cell isolates, all quantitative data in basal conditions were expressed as “fold” changes relative to control levels expressed as 1. Quantitative RT-PCR data after TGF-β1 treatment were expressed as fold changes relative to basal values.
The results are expressed as means±SD. Differences between the two groups (DMD and control) were assessed by using the Wilcoxon non-parametric test and considered significant at P≤0.05.
Results
Cell characterization
All CD56-negative cells from both DMD and controls were immunopositive with anti-prolyl-4-hydroxylase (Fig. 1a, b) and were therefore fibroblasts. Furthermore, none of the cells were immunostained with anti-CD44 (marker of interstitial cells) or anti-CD31 (marker of endothelial cells), whereas less than 1% of the cells were immunopositive for desmin (marker of myogenic cells) both in DMD and in control populations (data not shown). We therefore concluded that our fibroblast populations were homogeneous and comparable.
a, b Immunostaining with anti-prolyl-4-hydroxylase (green) and DAPI (blue) shows that DMD and control (Ctrl) fibroblast cultures contain fibroblasts, exclusively. c–f Anti-α-SMA immunostaining shows considerably increased expression of α-SMA in the stress fibers of myofibroblasts both in control and in DMD cells (c, d before TGF-β1 treatment, e, f after TGF-β1 treatment). Bars 20 μm. g Real time PCR and Western blot demonstrate that TGF-β1-treated cells produce abundant α-SMA, which is present in low levels in untreated cells. h Cell proliferation assay as determined by 5-bromo-2′-deoxyuridine (BrdU) incorporation shows a significantly greater proliferation rate in DMD than in control (CTRL) fibroblasts and myofibroblasts and a significantly greater proliferation rate in TGF-β1-treated cells compared with untreated cells. i Total soluble collagen as measured by the colorimetric Sircol assay shows a significantly greater collagen concentration in media of DMD fibroblast and myofibroblast cultures than in media of respective control (CTRL) cultures (asterisks statistical significance)
After treatment with TGF-β1, FITC-phalloidin staining showed marked modification of the cytoskeletal protein F-actin both in DMD and in control fibroblasts, evident as the formation of new stress fibers (Fig. 2e, k); α-SMA immunostaining showed considerably increased expression of α-SMA in the stress fibers, this being more intense in DMD than in control cells (Fig.1c–f). Collagen I immunostaining showed greater positivity in DMD fibroblasts than in control fibroblasts, after TGF-β1 treatment (Fig. 2d, j).
α-SMA transcript levels in DMD fibroblasts did not differ significantly (0.51 ± 0.25, P = 0.10) from those in control fibroblasts (considered as 1). After TGF-β1 treatment, both DMD (7.78 ± 1.96, P = 0.02) and control (5.38 ± 1.80, P = 0.02) myofibroblasts expressed significantly increased α-SMA transcript levels compared with basal levels (Fig. 1g). The difference between DMD and control myofibroblasts was also significant (P = 0.03).
By Western blot, the band corresponding to α-SMA increased in intensity after TGF-β1 treatment, both in DMD and in control cells (Fig. 1g).
From these data, we conclude that, after TGF-β1 treatment, our cell populations show features characteristic of myofibroblasts. From now on, we refer to cells before TGF-β1 treatment as fibroblasts (although a few myofibroblasts were present in both cell populations) and to those after TGF-β1 treatment as myofibroblasts.
The cell proliferation assay showed that DMD fibroblasts and myofibroblasts incorporated significantly more BrdU than control cells. Absorbances were: 0.27 ± 0.04 vs. 0.24 ± 0.06 (P = 0.001) in fibroblasts and 0.34 ± 0.06 vs. 0.29 ± 0.04 (P = 0.001) in myofibroblasts; TGF-β1 treatment caused a further significant increase in BrdU incorporation compared with basal values both in DMD and control cells (P = 0.001; Fig. 1h; values were means of four independent experiments each including three cell lines seeded in 4–6 wells for a total of 16–24 determinations per condition).
Soluble collagen production in the culture medium was significantly greater in DMD fibroblasts than normal fibroblasts: 124.28 ± 56.16 μg/ml vs. 28.87 ± 13.45 μg/ml (P = 0.001). TGF-β1 treatment caused a further significant increase in total soluble collagen production in both cell populations: 268.05 ± 63.96 μg/ml in DMD vs. 175.47 ± 44.70 μg/ml in control myofibroblasts. Values differed significantly both between groups and before and after transdifferentiation to myofibroblasts (P = 0.01; Fig. 1i).
Real time PCR
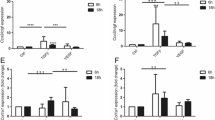
DMD fibroblasts expressed significantly more myostatin (4.86 ± 1.73, P = 0.02) and decorin (2.35 ± 0.54, P = 0.010) mRNA than controls, whereas TGF-β1 mRNA (0.79 ± 0.25, P = 0.06) did not differ significantly (Fig. 3a).
a Real time polymerase chain reaction for decorin, myostatin and TGF-β1 shows significantly higher levels of decorin and myostatin transcripts but not significantly different TGF-β1 transcript levels in DMD than in control (CTRL) fibroblasts. b TGF-β1 treatment significantly lowered decorin and myostatin and significantly increased TGF-β1 transcript levels in DMD compared with pretreatment cells; changes in controls were not significant
In DMD myofibroblasts, TGF-β1 treatment significantly lowered transcript levels (compared with basal) of decorin (0.14 ± 0.03, P = 0.02) and myostatin (0.14 ± 0.06, P = 0.02) and increased TGF-β1 (1.97 ± 0.55, P = 0.02). These differences were not significant from controls (decorin: 0.11 ± 0.08, P = 0.07; myostatin: 0.42 ± 0.32, P = 0.07; TGF-β1: 1.92 ± 0.69, P = 0.07; Fig. 3b).
DMD fibroblasts expressed significantly less MMP-1 (0.18 ± 0.10, P = 0.05; Fig. 4a) and significantly more MMP-7 (7.75 ± 1.83, P = 0.05; Fig. 4b) transcripts than controls. After TGF-β1 treatment, MMP-1 levels increased significantly (5.23 ± 2.23, P = 0.02; Fig. 4a), and MMP-7 levels decreased significantly, in DMD myofibroblasts (2.92 ± 1.13, P = 0.02; Fig. 4b), compared with basal values, whereas in controls, neither MMP transcripts changed significantly following treatment (MMP-1: 0.50 ± 0.36, P = 0.19; MMP-7: 1.91 ± 0.46, P = 0.07).
Real time PCR of MMP-1 (a) and MMP-7 (b) shows that DMD fibroblasts had significantly lower transcript levels of MMP-1 and significantly higher MMP-7 levels than control (CTRL)fibroblasts. TGF-β1 treatment significantly increased MMP-1 (a) transcripts and significantly lowered MMP-7 (b) transcripts in DMD compared with pretreatment; changes in controls were not significant
TIMP-3 transcript levels (0.55 ± 0.11, P = 0.02) were significantly lower in DMD fibroblasts than controls and increased significantly after TGF-β1 treatment, both in DMD (3.85 ± 1.13, P = 0.01) and in controls (6.81 ± 2.16, P = 0.02; Fig. 5a).
Real time PCR (a) and Western blot (b) of TIMP-3. a TIMP-3 transcript levels were significantly lower in DMD fibroblast cultures, and TGF-β1 treatment significantly increased transcript levels in both DMD and controls (CTRL). b The TIMP-3 protein band was more prominent in treated DMD and treated control (Ctrl) cells (Myofibroblasts) compared with untreated cells (Fibroblasts)
MMP-2, MMP-3, TIMP-1, TIMP-2, and TIMP-4 transcript levels did not differ significantly between DMD and control cells before TGF-β1 treatment and did not change significantly after treatment with TGF-β1 (data not shown).
Zymography
For MMP-1, only the medium of DMD fibroblasts after TGF-β1 treatment showed positivity, the presence of a single band at 48 kDa corresponding to the active form of MMP-1 (Fig. 6).
Top MMP-1 zymography shows a single band at 48 kDa corresponding to the active form of the MMP-1 in DMD myofibroblasts but not in fibroblasts. MMP-1 is absent from control (Ctrl) cells. Lanes 1, 2 Protein standard and positive control for MMP-1, MMP-2, and MMP-3. Bottom MMP-2 zymography shows a 66-kDa gelatinolytic band corresponding to the inactive form of MMP-2, which is more intense in DMD fibroblasts than in controls (Ctrl), while the 62-kDa band corresponding to the active form is absent or barely discernible either in DMD or in control cultures. TGF-β1 treatment reduced the intensity of the 66-kDa band, whereas the 62-kDa band was no longer visible
MMP-2 zymography showed the presence of a 66-kDa gelatinolytic band (inactive form of MMP-2) in medium from fibroblasts; this was more intense in DMD than in controls. The 62-kDa band (active form) was absent or barely discernible in DMD and control culture media. TGF-β1 treatment reduced the intensity of the 66-kDa band, whereas the 62-kDa band was no longer discernible (Fig. 6).
Western blot
Visual inspection of Western blots from cell media (control and DMD samples loaded with equal quantities of total protein) suggested that the intensities of the MMP-2, MMP-3, TIMP-1, and TIMP-2 bands were similar in controls and DMD, both before and after TGF-β1 treatment. The TIMP-3 band was more intense in controls than DMD both before and after TGF-β1 treatment (Fig. 5b).
The intensity of the decorin core protein band, evaluated in cell protein extracts, was greater in DMD than in control fibroblasts. After TGF-β1 treatment, band intensity reduced markedly in DMD myofibroblasts and was no longer discernible in control myofibroblasts (Fig. 7a).
a Western blot of decorin from DMD and control fibroblast and myofibroblast cell extracts showing that TGF-β1 treatment reduced the intensity of the decorin core protein band in DMD, whereas it was no longer visible in controls (Ctrl). Lane 1 Undigested bovine decorin standard (Chondroitinase −), lane 2 digested bovine decorin standard (Chondroitinase +), lanes 3–6 fibroblast and myofibroblast cell extracts. b Western blot of decorin from DMD and control fibroblast and myofibroblast cell media before (−) and after (+) chondroitinase ABC digestion, showing bands of greater intensity in DMD than in controls before TGF-β1 treatment and of similar intensity in both cell types after TGF-β1 treatment
In undigested cell media, decorin appeared as a smear at 95–130 kDa; intensity was greater in DMD than controls before TGF-β1 treatment and similar in both after TGF-β1 treatment. After chondroitinase treatment (the decorin core protein remains) of fibroblast media, the decorin band at approximately 45 kDa was more intense in media from DMD fibroblasts than controls; the core protein bands were of similar intensity after TGF-β1 treatment (Fig. 7b).
Enzyme-linked immunosorbent assay
MMP-1 protein levels were significantly lower (0.037 ± 0.004 ng/ml vs. 0.096 ± 0.018 ng/ml, P = 0.01; Fig. 8a), and MMP-7 protein levels were significant higher (2.040 ± 0.439 ng/ml vs. 0.993 ± 0.135 ng/ml, P = 0.001; Fig. 8b), in DMD fibroblast medium than in control fibroblast medium. TGF-β1 protein levels (0.374 ± 0.144 ng/ml vs. 0.059 ± 0.019 ng/ml, P = 0.001; Fig. 8c) and myostatin protein levels (2.368 ± 0.349 ng/mlvs. 1.818 ± 0.20 ng/ml, P = 0.03) were also significantly higher in DMD fibroblast medium than control medium (Fig. 8d).
Enzyme-linked immunosorbent assay of MMP-1 (a), MMP-7 (b), TGF-β1 (c), and myostatin (d), all from media. MMP-1 was significantly lower, whereas MMP-7, TGF-β1, and myostatin were significantly higher, in DMD fibroblast media than incontrol media. TGF-β1 treatment increased MMP-1 significantly in DMD, but not in controls, and significantly decreased MMP-7, TGF-β1, and myostatin in DMD, but not in controls
After TGF-β1 treatment, a significant increase, compared to basal, was seen in MMP-1 secretion both in DMD (0.60 ± 0.07 ng/ml, P = 0.0003) and control (0.20 ± 0.01 ng/ml, P = 0.019) media (Fig. 8a). TGF-β1 treatment also caused: a significant decrease in MMP-7 protein levels in DMD cells (1.20 ± 0.30 ng/ml, P = 0.007), but not in controls (1.19 ± 0.25 ng/ml, P = 0.13; Fig. 8b); a significant decrease in TGF-β1 protein levels in (0.09 ± 0.01 ng/ml, P = 0.001) and a significant increase in controls (0.11 ± 0.03 ng/ml, P = 0.007; Fig. 8c); a significant decrease in myostatin protein levels in both populations (DMD: 0.78 ± 0.14 ng/ml, P = 0.006; controls: 1.13 ± 0.20 ng/ml, P = 0.001; Fig. 8d).
Discussion
Fibroblasts play a central role in maintaining the integrity and composition of the ECM by synthesizing and degrading ECM components. The various distinct functional subtypes of fibroblasts are distinguished by their anatomical origin and gene expression profile (retained in culture; Chang et al. 2002).
Our study shows that primary fibroblasts derived from DMD muscle biopsies differ from control fibroblasts in a number of respects. First, DMD fibroblasts proliferate at a faster rate than control fibroblasts. An increased proliferation rate has been observed in fibroblasts isolated from fibrotic lung compared with control lung (Jordana et al. 1988; Raghu et al. 1988) and in fibroblasts isolated from hypertrophic scar tissue compared with those from normal skin (Zhang et al. 2007).
The second difference between DMD and control fibroblasts is that the production of soluble collagens by DMD fibroblasts is significantly higher than that in controls. Similar increases in collagen production have been found in several fibrotic conditions including idiopathic pulmonary fibrosis (Ramos et al. 2001) and cultured fibroblasts from scleroderma (Pannu et al., 2006).
Third, we have found a significant increase in decorin transcripts in DMD fibroblasts and have also observed an apparent increase in decorin protein both in cell extracts and in media. After production within the cell, the decorin core protein is rapidly glycosylated and secreted. We have revealed that both the digested core protein and the glycosylated form are of higher intensity in DMD fibroblast media than in control media. Fadic et al. (2006) have also reported increased decorin (and also biglycan synthesis) in muscle-derived fibroblasts from a DMD patient compared with controls. Kuroda and Shinkai (1997) have detected higher levels of decorin transcripts in systemic sclerosis fibroblasts, and Westergren-Thorsson et al. (2004) have reported increased levels of decorin secreted by primary fibroblasts derived from a fibrotic lung. In our previous study on the ECM of primary DMD myotube cultures (practically devoid of fibroblasts), we have shown that decorin transcript levels are lower than those in controls (Zanotti et al. 2007), in accord with our findings in DMD patient muscle (Zanotti et al. 2005). These data are in marked contrast to our present finding that decorin transcripts are increased in DMD fibroblasts and could reflect inherent differences in decorin production between the cell types or differences between fibrotic and non-fibrotic muscle areas. Moreover, the inflammation present in DMD muscle in vivo might affect the situation. Further investigation, possibly by laser micro-dissection of DMD muscle samples, might clarify this aspect.
Fourth, DMD fibroblasts also produce significantly more myostatin transcripts and protein. The protein is not detected by Western blot, probably because of its low concentration relative to total protein, but has been detected by ELISA. Myostatin expression in fibroblasts from injured skeletal muscle was first detected by Yamanouchi et al. (2000) indicating that fibroblasts could be a source of myostatin. More recently, Zhu et al. (2007) have shown that myostatin stimulates fibroblast proliferation and induces α-SMA expression, as does TGF-β1, whereas Li et al. (2008) have found that biologically active myostatin is expressed and secreted by muscle-derived fibroblasts. We have detected up-regulated myostatin mRNA and protein in myotube cultures obtained from the same DMD patients as those evaluated in the present study (Zanotti et al. 2007) but have found reduced myostatin mRNA in their muscle biopsies (in preparation). These differences could reflect differences between in vitro and in vivo conditions; the in vitro cell population consists mainly of myoblasts and myotubes, whereas the inflammatory component is present in vivo but not in vitro. Interactions of myostatin with decorin, TGF-β1, or other regulatory molecules might give rise to a persistent positive autocrine feedback loop that results in the over-production of matrix proteins and subsequent fibrosis (Schmid et al. 1998).
Although TGF-β1 transcript levels are unchanged, protein levels are significantly increased in DMD fibroblasts as shown by ELISA. TGF-β1 is finely regulated by interactions with various cellular and extracellular players and by positive autocrine feedback (Schmid et al. 1998). Furthermore, increased levels could in part be attributable to release of the cytokine from decorin, as it is degraded by (increased activity of) MMP-2.
Another finding of the present study is that MMP-7 transcript and protein levels are elevated in DMD fibroblasts. The increase in transcript expression is marked, whereas the increase in protein expression is less striking but still significant. Like several other secreted proteins, MMP-7 has not been detected by Western blot. As detected by the more sensitive ELISA method, its expression is significantly greater in DMD than in control fibroblasts. Increased levels of MMP-7 have been found in experimental and pathological fibrotic conditions, such as idiopathic pulmonary fibrosis (Zuo et al. 2002), liver fibrosis (Huang et al. 2005), and tubulointerstitial fibrosis (Surendran et al. 2004). Zuo et al. (2002) have demonstrated that MMP-7 −/− knockout mice are protected from bleomycin-induced fibrosis, showing that this metalloproteinase plays an important role in the development of pulmonary fibrosis. Huang et al. (2005) have shown that MMP-7 expression directly correlates with the progression of liver fibrosis. The MMP-7 produced by DMD fibroblasts is therefore probably also involved in skeletal muscle fibrosis, as is also suggested by our finding that MMP-7 appears to be increased (as shown by immunohistochemistry) in DMD muscle biopsies (in preparation).
We have also found that MMP-1 mRNA and protein levels are significantly lower in DMD fibroblasts than in controls. MMP-1 plays an important role in limiting fibrosis by degrading type I and III collagen fibrils (Brinckerhoff et al. 1987). In the early stages of fibrosis, a slight increase in collagen III has been reported, whereas collagen I is highly increased and remains the major collagen type later on (Eckes et al. 2000). Takeda et al. (1994) have shown that MMP-1 is downregulated and its activity reduced in scleroderma. Furthermore, mice expressing an α1 chain of collagen I with a defective MMP-1 cleavage site develop fibrotic skin alterations similar to those in scleroderma (Liu et al. 1995). Our data, showing excessive soluble collagen production, increased collagen I expression, and a concomitant reduction in MMP-1 expression in DMD fibroblasts, suggest a scenario similar to that observed in systemic scleroderma fibroblasts. The recent work of Kaar et al. (2008) has demonstrated that active MMP-1 can effectively reduce muscle scarring because of its ability to digest collagen; MMP-1 therefore emerges as a possible treatment of fibrosis.
The ultimate finding of the present study is that TIMP-3 transcript levels in DMD fibroblasts are significantly reduced. In addition to its role in counteracting MMPs, TIMP-3 is able to induce apoptosis in various cell types (Ahonen et al. 1998; Baker et al. 1998). This characteristic has been attributed to the tight binding of TIMP-3 to the ECM (Yu et al. 2000), an event that confers the unique ability of inhibiting members of the ADAM (a disintegrin and metalloprotease domain) family of enzymes such as tumor necrosis factor-α-converting enzyme (Amour et al. 1998). Thus, DMD fibroblasts might be more resistant to apoptosis than normal fibroblasts.
The detailed significance of all the differences that we have found between normal and DMD fibroblasts is not clear at present. Nevertheless, DMD fibroblasts are clearly characterized by a pro-fibrotic phenotype. This is further confirmed when fibroblasts transdifferentiate into myofibroblasts following TGF-β1 treatment; both control and DMD transdifferentiated cells express significantly more α-SMA and apparently more collagen I and produced more soluble collagens than fibroblasts, in addition to assuming myofibroblast morphology. However, the proliferation rate and collagen production of DMD myofibroblasts remains significantly higher than those of control myofibroblasts. These findings are in agreement with observations in fibrotic conditions including lung disease and liver fibrosis (Gharaee-Kermani et al. 2009; Le Bousse-Kerdilès et al. 2008).
DMD fibroblasts are probably predisposed to a profibrotic phenotype as a consequence of their primary genetic defect. In our previous study of DMD myotubes (Zanotti et al. 2007), we have shown that mechanisms regulating ECM homeostasis in muscle tissue are altered and have suggested that dystrophin absence itself could exert a direct influence on ECM homeostasis by allowing leakage of cellular components to the extracellular space or abnormal cellular uptake of extracellular growth factors, cytokines, or enzymes. Based on the findings of the present study, we speculate that the absence of dystrophin influences the muscle fibroblast subtype either directly by altering its adhesion properties or indirectly by interactions with molecules released into the ECM by muscle or inflammatory cells.
Myofibroblast transdifferentiation by TGF-β1 induces lower levels of decorin and myostatin both in DMD and in control cells. These changes are probably related to the existence of co-regulatory interrelationships between TGF-β1, myostatin, and decorin in the fibrotic process (Miura et al. 2006; Li et al. 2008; Zhu et al. 2007).
In vitro, TGF-β1 induces variation in the gene expression of MMPs and TIMPs (Roberts and Sporn 1996; Wells 2000). Differing effects of TGF-β1 on different MMP isoforms have also been reported (Uria et al. 1998). We have found a reduction in MMP-7 transcript and protein levels in DMD cells after TGF-β1 treatment, but an increase in MMP-1 transcripts and protein. As noted, MMP-1 is the only enzyme able to initiate the breakdown of the interstitial collagens type I, II, and III. We hypothesize that the increase in MMP-1 in DMD cells might be a compensatory effect of the increase in collagens.
Finally, the significant increase in TIMP-3 transcript and apparent increase in protein levels after TGF-β1 treatment, both in DMD and in controls, is likely to be related to a generic up-regulation of TIMPs by this cytokine (Wells 2000).
This study has thus revealed several differences between fibroblasts from DMD muscle and those from normal muscle, particularly as regards factors likely to affect ECM turnover; these differences are accentuated by TGF-β1 treatment. Our findings confirm that fibroblasts and myofibroblasts play a major role in muscle fibrosis, as also reported for other tissues, and indicate that the quantity and localization of both cell populations should be evaluated in in vivo studies. We propose that the transdifferentiation of muscle fibroblasts can be employed as a simplified model of fibrosis and may be useful for elucidating muscle fibrosis mechanisms occurring at the various steps of ECM deposition and for testing possible anti-fibrotic agents.
Abbreviations
- ECM:
-
Extracellular matrix
- DMD:
-
Duchenne muscular dystrophy
- α-SMA:
-
α-Smooth muscle actin
- MMPs:
-
Matrix metalloproteinases
- TIMPs:
-
Tissue inhibitors of metalloproteinases
- TGF-β1:
-
Transforming growth factor β1
References
Ahonen M, Baker AH, Kähäri VM (1998) Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res 58:2310–2315
Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knäuper V, Docherty AJ, Murphy G (1998) TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett 435:39–44
Baker AH, Zaltsman AB, George SJ, Newby AC (1998) Divergent effects of tissue inhibitor of metalloproteinase-1, −2, or −3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest 101:1478–1487
Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R (1995) Expression of transforming growth-factor beta 1 in dystrophic patient muscles correlates with fibrosis. J Clin Invest 96:1137–1144
Blake DJ, Weir A, Newey SE, Davies KE (2002) Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82:291–329
Border WA, Ruoslahti E (1992) Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest 90:1–7
Brinckerhoff CE, Ruby PL, Austin SD, Fini ME, White HD (1987) Molecular cloning of human synovial cell collagenase and selection of a single gene from genomic DNA. J Clin Invest 79:542–546
Chang HY, Chi JT, Dudoit S, Bondre C, M van de Rijn, Botstein D, Brown PO (2002) Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA 99:12877–12882
De Luca A, Santra M, Baldi A, Giordano A, Iozzo RV (1996) Decorin-induced growth suppression is associated with up-regulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem 271:18961–18965
Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122:103–111
Desmoulière A, Redard M, Darby I, Gabbiani G (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146:56–66
Desmoulière A, Chaponnier C, Gabbiani G (2005) Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 13:7–12
Dickson G, Gower HJ, Barton CH, Prentice HM, Elsom VL, Moore SE, Cox RD, Quinn C, Putt W, Walsh FS (1987) Human muscle neural cell adhesion molecule (N-CAM): identification of a muscle-specific sequence in the extracellular domain. Cell 50:1119–1130
Eckes B, Zigrino P, Kessler D, Holtkötter O, Shephard P, Mauch C, Krieg T (2000) Fibroblast-matrix interactions in wound healing and fibrosis. Matrix Biol 19:325–332
Fadic R, Mezzano V, Alvarez K, Cabrera D, Holmgren J, Brandan E (2006) Increase in decorin and biglycan in Duchenne muscular dystrophy: role of fibroblasts as cell source of these proteoglycans in the disease. J Cell Mol Med 10:758–769
Finlay GA, Thannickal VJ, Fanburg BL, Paulson KE (2000) Transforming growth factor-beta 1-induced activation of the ERK pathway/activator protein-1 in human lung fibroblasts requires the autocrine induction of basic fibroblast growth factor. J Biol Chem 275:27650–27656
Gabbiani G (2003) The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200:500–503
Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR (2009) Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast. Curr Med Chem 16:1400–1417
Heino J, Ignotz RA, Hemler ME, Crouse C, Massagué J (1989) Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem 264:380–388
Herpel E, Pritsch M, Koch A, Dengler TJ, Schirmacher P, Schnabel PA (2006) Interstitial fibrosis in the heart: differences in extracellular matrix proteins and matrix metalloproteinases in end-stage dilated, ischaemic and valvular cardiomyopathy. Histopathology 48:736–747
Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E (1994) Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 302:527–534
Huang CC, Chuang JH, Chou MH, Wu CL, Chen CM, Wang CC, Chen YS, Chen CL, Tai MH (2005) Matrilysin (MMP-7) is a major matrix metalloproteinase upregulated in biliary atresia-associated liver fibrosis. Mod Pathol 18:941–950
Ignotz RA, Massagué J (1986) Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 261:4337–4345
Iozzo RV (1999) The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem 274:18843–18846
Iredale JP (2007) Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest 117:539–548
Jordana M, Schulman J, McSharry C, Irving LB, Newhouse MT, Jordana G, Gauldie J (1988) Heterogeneous proliferative characteristics of human adult lung fibroblast lines and clonally derived fibroblasts from control and fibrotic tissue. Am Rev Respir Dis 137:579–584
Kaar JL, Li Y, Blair HC, Asche G, Koepsel RR, Huard J, Russell AJ (2008) Matrix metalloproteinase-1 treatment of muscle fibrosis. Acta Biomater 4:1411–1420
Kissin EY, Lemairem R, Kornm JH, Lafyatis R (2002) Transforming growth factor beta induces fibroblast fibrillin-1 matrix formation. Arthritis Rheum 46:3000–3009
Kossakowska AE, Edwards DR, Lee SS, Urbanski LS, Stabbler AL, Zhang CL, Phillips BW, Zhang Y, Urbanski SJ (1998) Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol 153:1895–1902
Kuroda K, Shinkai H (1997) Gene expression of types I and III collagen, decorin, matrix metalloproteinases and tissue inhibitors of metalloproteinases in skin fibroblasts from patients with systemic sclerosis. Arch Dermatol Res 289:567–572
Le Bousse-Kerdilès MC, Martyré MC, Samson M (2008) Cellular and molecular mechanisms underlying bone marrow and liver fibrosis: a review. Eur Cytokine Netw 19:69–80
Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, Feldman AM (2000) Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci USA 97:12746–12751
Li ZB, Kollias HD, Wagner KR (2008) Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem 283:19371–19378
Liu X, Wu H, Byrne M, Jeffrey J, Krane S, Jaenisch R (1995) A targeted mutation at the known collagenase cleavage site in mouse type I collagen impairs tissue remodeling. J Cell Biol 130:227–237
Lorena D, Uchio K, Costa AM, Desmoulière A (2002) Normal scarring: importance of myofibroblasts. Wound Repair Regen 10:86–92
Matsumura K, Campbell KP (1994) Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve 17:2–15
Mauviel A (2005) Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med 117:69–80
McPherron AC, Lee SJ (1997) Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94:12457–12461
Milani S, Herbst H, Schuppan D, Kim KY, Riecken EO, Stein H (1990) Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology 98:175–184
Miura T, Kishioka Y, Wakamatsu J, Hattori A, Hennebry A, Berry CJ, Sharma M, Kambadur R, Nishimura T (2006) Decorin binds myostatin and modulates its activity to muscle cells. Biochem Biophys Res Commun 340:675–680
Montesano R, Orci L (1988) Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA 85:4894–4897
Pannu J, Gardner H, Shearstone JR, Smith E, Trojanowska M (2006) Increased levels of transforming growth factor beta receptor type I and up-regulation of matrix gene program: a model of scleroderma. Arthritis Rheum 54:3011–3021
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR.Nucleic Acids Res 29:e45
Raghu G, Chen YY, Rusch V, Rabinovitch PS (1988) Differential proliferation of fibroblasts cultured from normal and fibrotic human lungs. Am Rev Respir Dis 138:703–708
Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A (2001) Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 24:591–598
Roberts AB, Sporn MB (1996) Transforming growth factor-β. In: Clark RAF (ed) The molecular and cellular biology of wound repair. Plenum, New York, pp 275–308
Ronnov-Jessen L, Petersen OW (1993) Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest 68:696–707
Sappino AP, Masouyé I, Saurat JH, Gabbiani G (1990) Smooth muscle differentiation in scleroderma fibroblastic cells. Am J Pathol 137:585–591
Schmid P, Itin P, Cherry G, Bi C, Cox DA (1998) Enhanced expression of transforming growth factor-beta type I and type II receptors in wound granulation tissue and hypertrophic scar. Am J Pathol 152:485–493
Schnaper HW, Kopp JB, Poncelet AC, Hubchak SC, Stetler-Stevenson WG, Klotman PE, Kleinman HK (1996) Increased expression of extracellular matrix proteins and decreased expression of matrix proteases after serial passage of glomerular mesangial cells. J Cell Sci 109:2521–2528
Selman M, King TE, Pardo A (2001) Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 134:136–151
Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J (1997) Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100:768–776
Surendran K, Simon TC, Liapis H, McGuire JK (2004) Matrilysin (MMP-7) expression in renal tubular damage: association with Wnt4. Kidney Int 65:2212–2222
Takeda K, Hatamochi A, Ueki H, Nakata M, Oishi Y (1994) Decreased collagenase expression in cultured systemic sclerosis fibroblasts. J Invest Dermatol 103:359–363
Thannickal VJ, Aldweib KD, Rajan T, Fanburg BL (1998) Upregulated expression of fibroblast growth factor (FGF) receptors by transforming growth factor-beta1 (TGF-beta1) mediates enhanced mitogenic responses to FGFs in cultured human lung fibroblasts. Biochem Biophys Res Commun 251:437–441
Uria JA, Jimenez MG, Balbin M, Freije JMP, Lopez-Otin C (1998) Differential effects of transforming growth factor-beta on the expression of collagenase-1 and collagenase-3 in human fibroblasts. J Biol Chem 273:9769–9777
Vogel KG, Paulsson M, Heinegard D (1984) Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J 223:587–597
Wells RG (2000) Fibrogenesis V. TGF-β signaling pathways. Am J Physiol Gastrointest Liver Physiol 279:G845–G850
Westergren-Thorsson G, Hernnas J, Sarnstrand B, Oldberg A, Heinegard D, Malmstrom A (1993) Altered expression of small proteoglycans, collagen, and transforming growth factor-beta 1 in developing bleomycin-induced pulmonary fibrosis in rats. J Clin Invest 92:632–637
Westergren-Thorsson G, Sime P, Jordana M, Gauldie J, Sarnstrand B, Malmstrom A (2004) Lung fibroblast clones from normal and fibrotic subjects differ in hyaluronan and decorin production and rate of proliferation. Int J Biochem Cell Biol 36:1573–1584
Yamaguchi Y, Mann DM, Rouslahti E (1990) Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 19:281–284
Yamanouchi K, Soeta C, Naito K, Tojo H (2000) Expression of myostatin gene in regenerating skeletal muscle of the rat and its localization. Biochem Biophys Res Commun 270:510–516
Yu WH, Yu S, Meng Q, Brew K, Woessner JF Jr (2000) TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem 275:31226–31232
Zanotti S, Negri T, Cappelletti C, Bernasconi P, Canioni E, Di Blasi C, Pegoraro E, Angelini C, Ciscato P, Prelle A, Mantegazza R, Morandi L, Mora M (2005) Decorin and biglycan expression is differentially altered in several muscular dystrophies. Brain 128:2546–2555
Zanotti S, Saredi S, Ruggieri A, Fabbri M, Blasevich F, Romaggi S, Morandi L, Mora M (2007) Altered extracellular matrix transcript expression and protein modulation in primary Duchenne muscular dystrophy myotubes. Matrix Biol 26:615–624
Zhang Z, Li XJ, Liu Y, Zhang X, Li YY, Xu WS (2007) Recombinant human decorin inhibits cell proliferation and downregulates TGF-β1 production in hypertrophic scar fibroblasts. Burns 33:634–641
Zhu J, Li Y, Shen W, Qiao C, Ambrosio F, Lavasani M, Nozaki M, Branca MF, Huard J (2007) Relationships between transforming growth factor-beta1, myostatin, and decorin: implication for skeletal muscle fibrosis. J Biol Chem 282:25852–25863
Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Pollini L, Morris D, Kim Y, DeLustro B, Sheppard D, Pardo A, Selman M, Heller RA (2002) Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 99:6292–6297
Acknowledgments
The authors are grateful to Professor Renato Iozzo of the Thomas Jefferson University of Philadelphia for helpful suggestions on the detection of the glycosylated form of decorin by Western blot and to Don Ward for help with the English.The EuroBioBank and Telethon Network of Genetic Biobanks (GTB07001F) are also gratefully acknowledged for providing biological samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
The financial support of the Italian Ministry of Health is gratefully acknowledged.
Rights and permissions
About this article
Cite this article
Zanotti, S., Gibertini, S. & Mora, M. Altered production of extra-cellular matrix components by muscle-derived Duchenne muscular dystrophy fibroblasts before and after TGF-β1 treatment. Cell Tissue Res 339, 397–410 (2010). https://doi.org/10.1007/s00441-009-0889-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0889-4