Abstract
The ontogeny of two gonadotropin-releasing-hormone (GnRH) systems, salmon GnRH (sGnRH) and chicken GnRH-II (cGnRH-II), was investigated in zebrafish (Danio rerio). In situ hybridization (ISH) first detected sGnRH mRNA-expressing cells at 1 day post-fertilization (pf) anterior to the developing olfactory organs. Subsequently, cells were seen along the ventral olfactory organs and the olfactory bulbs, reaching the terminal nerve (TN) ganglion at 5–6 days pf. Some cells were detected passing posteriorly through the ventral telencephalon (10–25 days pf), and by 25–30 days pf, sGnRH cells were found in the hypothalamic/preoptic area. Continuous documentation in live zebrafish was achieved by a promoter-reporter expression system. The expression of enhanced green fluorescent protein (EGFP) driven by the sGnRH promoter allowed the earlier detection of cells and projections and the migration of sGnRH neurons. This expression system revealed that long leading processes, presumably axons, preceded the migration of the sGnRH neuron somata. cGnRH-II mRNA expressing cells were initially detected (1 day pf) by ISH analysis at lateral aspects of the midbrain and later on (starting at 5 days pf) at the midline of the midbrain tegmentum. Detection of red fluorescent protein (DsRed) driven by the cGnRH-II promoter confirmed the midbrain expression domain and identified specific hindbrain and forebrain cGnRH-II-cells that were not identified by ISH. The forebrain DsRed-expressing cells seemed to emerge from the same site as the sGnRH-EGFP-expressing cells, as revealed by co-injection of both constructs. These studies indicate that zebrafish TN and hypothalamic sGnRH cell populations share a common embryonic origin and migratory path, and that midbrain cGnRH-II cells originate within the midbrain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All vertebrate species studied to date possess two or three molecular forms of gonadotropin-releasing hormone (GnRH). These are mainly distributed in three distinct anatomical areas in the brain: (1) the midbrain tegmentum (MBT), (2) along the terminal nerve (TN), and (3) the septo-preoptic hypothalamic area (Fernald and White 1999; Dubois et al. 2002; Yamamoto 2003). The MBT population expresses the highly conserved chicken GnRH-II (cGnRH-II) form, present in all jawed vertebrates (Millar 2003). The hypothalamic population contains a GnRH form that exerts the hypophysiotropic action of gonadotoropin-releasing activity, and its peptide sequence varies among vertebrates (Somoza et al. 2002). In species that have two forms of GnRH, the TN population contains the same peptide that is expressed in the hypothalamus. In acanthopterygians (modern teleosts), the TN population expresses a third molecularly distinguished form, i.e., salmon GnRH (sGnRH; Yamamoto 2003).
Studies in diverse species have described the migratory route of the TN and hypothalamic GnRH-producing neurons. These cells are first detected in the nasal placode and follow the olfactory/vomeronasal or terminal tracts to the olfactory bulbs and then posteriorly to the TN ganglion (TNg). The cells of the hypothalamic GnRH population continue to migrate along the ventral telencephalon to their final and functional position in the hypothalamic/preoptic area (Wray 2002). Studies in mammals indicate that abnormal developmental migration and targeting of the hypothalamic GnRH-producing neurons results in hypogonadism and infertility (MacColl et al. 2002). Hence, an understanding of the mechanisms that regulate this migration is both fundamental and clinically relevant.
Zebrafish has become an important vertebrate model for studying the genetic bases of developmental processes. Among the advantages of this species is its rapid development and accessible transparent embryos. These traits allow investigations in live animals. Zebrafish has two GnRH genes: sGnRH and cGnRH-II (Torgersen et al. 2002; Steven et al. 2003). In adult fish, sGnRH is expressed in the olfactory bulbs, TNg region, ventral telencephalon and preoptic area, whereas cGnRH-II is expressed exclusively in the MBT (Steven et al. 2003). To begin to investigate the molecular mechanisms underlying GnRH neuronal migration, the present study characterizes the ontogeny of the GnRH systems in zebrafish by in situ hybridization (ISH) and the transient expression of GnRH promoter-reporter constructs in live intact animals.
Materials and methods
Animals
Zebrafish embryos (AB line) were generated by natural mating. Embryos were raised in egg water (60 ppm Sea Salts) containing methylene blue (0.3 ppm) at 28.5°C and under a 12 h light/12 h dark cycle. To prevent pigmentation, 0.2 mM phenylthiourea was added to the water on days 2–5 post-fertilization (pf). At 5 days pf, larvae were transferred to a biologically filtered recirculating water system and held under the conditions mentioned above. Larvae were fed twice daily with dried and fresh live food. For ISH analysis, zebrafish (n=40) at various stages of development were anesthetized (0.6 mM methanesulfonate salt) until movement had stopped, fixed overnight with 4% paraformaldehyde in phosphate-buffered saline (PBS), and stored in 100% methanol.
ISH analysis
Fragments of zebrafish sGnRH cDNA (nt 26–379, GenBank accession number AJ304429; Torgersen et al. 2002) and cGnRH-II cDNA (nt 165–629, GenBank accession number AF511531; Steven et al. 2003) were cloned into pGEM-T vector. These clones (pzfsGnRH and pzfcGnRH-II) were linearized with NcoI and NotI, respectively, and used as templates to synthesize digoxigenin (Dig)-labeled antisense RNA probes (Dig RNA labeling kit, Roche Diagnostics, Basel, Switzerland). Sense RNAs were synthesized and used as negative controls. Whole-mount ISH was carried out according to established protocol (Westerfield 1995) with slight modifications. Fixed and methanol-preserved animals were rinsed in cold acetone for 8 min, dehydrated through a graded series of methanol concentrations, rinsed twice for 5 min with PBS, and treated with proteinase K (10 μg/ml in PBS) for 5–60 min (depending on the developmental stage of the animal). Samples were then fixed for 20 min (4% paraformaldehyde in PBS), washed in PBTw (0.1% Tween-20 in PBS), and prehybridized for 3 h at 65°C in hybridization buffer composed of 50% formamide, 5× standard sodium citrate (SSC), 5 mM EDTA, 0.1% Tween-20, 0.1% CHAPS, 50 mg/ml heparin, and 1 mg/ml yeast RNA. Hybridization was carried out overnight (65°C, 2 μg/ml riboprobe in hybridization buffer), and subsequently, samples were rinsed in washing buffer 1 (2×30 min, 65°C, 50% formamide, 2×SSC, 0.15% CHAPS), washing buffer 2 (15 min, 2×SSC, 0.3% CHAPS), washing buffer 3 (2×30 min, 0.2% SSC, 0.3% CHAPS), and finally MAB buffer (0.1 M maleic acid, 150 mM NaCl, pH 7.5) at room temperature. Following pre-incubation in blocking solution (2% blocking reagent, 5% calf serum in MAB buffer) for 3 h, samples were incubated for 3 h with anti-DIG antibody conjugated to alkaline phosphatase (AP; 1:5000; Roche). The riboprobe-antibody complex was detected by the enzymatic reaction of AP with a chromogenic substrate (BM Purple AP substrate, Roche) yielding a purple precipitate. For image analysis, the heads of embryos/larvae were photographed from the dorsal aspect using an Olympus dissecting microscope (SZX12, Olympus, Japan) equipped with digital camera (DP70, Olympus). In some cases, the eyes were removed prior to photography for better detection and documentation.
Following whole-mount ISH, selected larvae were dehydrated in ethanol (50%, 70%, 95%), infiltrated with a plastic resin (JB4, Polysciences, Warrington, USA), and then embedded in fresh JB4. Blocks were sagittally sectioned at a thickness of 3 μm. Sections were counterstained with 0.1% aqueous toluidine blue O, dried, and coverslipped with DPX mountant (BDH Laboratory Supplies, Poole, England). Images were captured with an Optiphot microscope (Nikon, Japan) equipped with digital sight camera (DS-L1, Nikon).
Generation of promoter-reporter constructs and transient expression assays
For sGnRH-EGFP, a 2.4-kb genomic fragment containing 1291 bp of the sGnRH 5′-flanking region and the 5′-untranslated region (UTR), including 1140 bp of the intronic sequence (Fig. 1a), was amplified by the polymerase chain reaction (PCR) from zebrafish genomic DNA. The product (corresponding to nt 365–2797, GenBank accession number AF490354) was subcloned into pBlueScript vector. The resulting clone was then digested with Hind-III and BamH-I, and the insert was ligated into Hind-III/BamH-I-cut enhanced green fluorescent protein expression vector, pEGFP-1 (Clontech, Palo Alto, Calif. USA).
Representation of the promoter-reporter genes constructs (Ex exon). a sGnRH-EGFP: Zebrafish sGnRH fragment containing 1291 bp of the 5′-flanking region and 1140 bp of the 5′-UTR, including intron 1, fused to the EGFP gene. b cGnRH-II-DsRed: Zebrafish cGnRH-II fragment containing 2019 bp of 5′-flanking region and 1543 bp of 5′-UTR, including intron 1, fused to the DsRed gene
For cGnRH-II-DsRed, a zebrafish cGnRH-II fragment containing 2019 bp of the 5′-flanking region and 1543 bp of the 5′-UTR, including intron 1 (Fig. 1b), was PCR-amplified using a previously cloned zebrafish cGnRH-II gene as template (Steven et al. 2003). The product was subcloned into pGEM-T vector. This clone was then digested with SalI and SacII and the insert (corresponding to nt 1–3565, GenBank accession number AF511532) was ligated into SalI/SacII-cut red fluorescent protein expression vector, pDsRed-1 (Clontech).
Transient expression of the two DNA constructs was obtained by microinjection of zebrafish embryos as described (Gothilf et al. 2002). Approximately 2 nl were injected into the cytoplasm of one- or two-cell-stage wild-type zebrafish zygotes using a micromanipulator and PV830 Pneumatic Pico Pump (World Precision Instruments, Sarasota, Fl., USA). The constructs were injected separately (200 ng/μl) or together (150 ng/μl each) into more than 400 embryos. Injected embryos were kept under light/dark (12 h:12 h) cycles and repeatedly observed on days 1 to 40 pf. Green and red fluorescence in live embryos was detected using an Olympus dissecting microscope (SZX12) equipped with appropriate filter sets.
Real-time PCR quantification of sGnRH and cGnRH-II mRNAs
The forebrain and midbrain were dissected from 4-day old sGnRH-EGFP/cGnRH-II-DsRed-microinjected embryos under a fluorescent dissecting microscope. Tail tissue was used as a negative control. Total RNA was extracted using EZ-RNA isolation reagent (Biological Industries, Israel) according to manufacturer’s instructions. The mRNA (1 μg) was reverse-transcribed using Oligo(dT) primer and MMLV reverse transcriptase (Promega, Wis., USA). sGnRH and cGnRH-II mRNA levels were determined by real-time PCR using the ABI Prism 7000 thermocycler (Applied Biosystems, Calif., USA) following the manufacturer’s instructions. Triplicate first-strand cDNA aliquots (25 ng) from each sample served as templates in a PCR consisting of master mix, SYBR Green I fluorescent dye (Applied Biosystems), and 500 nM gene-specific primers [sGnRH primers: 5′-tagtttgtgtgttggaggtcagtctt-3′ and 5′-catcctgaatgttgcctccatt-3′; cGnRH-II primers: 5′-ctgagaccgcagggaagaaac-3′ and 5′-gagggcatccagcagtattgtc-3′]. The copy number in the samples was determined by comparing CT values with those of recombinant plasmid standards (0.5×10−6–5 pg) containing the cDNA inserts.
Results
ISH analysis of sGnRH mRNA
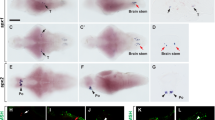
The development of the zebrafish sGnRH system as detected by whole-mount ISH followed by sagittal sectioning is illustrated in Figs. 2 and 3. The earliest appearance of sGnRH transcript was at 24 h pf in cell clusters anterior to the developing olfactory organs (Fig. 2a, arrows). During the second day of development, sGnRH-producing cells were seen along the ventral aspect of the olfactory organs (Fig. 3b,c, arrowheads). On the third day of development, sGnRH-producing cells were also seen outside of the olfactory organs, at the boundary between the olfactory organs and the olfactory bulbs (Figs. 2b, 3d,e). At this stage, the signal intensity increased, probably reflecting increased cell number. On days 5–6 pf, sGnRH neurons were detected in TNg at the olfactory bulb/telencephalon border (Figs. 2c, 3f,g). Two bilateral continua of sGnRH-producing neurons (from the TNg, along the lateral aspects of the ventral telencephalon to the hypothalamus) were observed at 10–25 days pf (Fig. 2d). The final positioning of the sGnRH-producing neurons at the preoptic area (POA) was observed in a number of larvae at 25–30 days pf (Fig. 2e,f). The partial detection of POA sGnRH neurons at this stage probably reflected the reduced penetration of the riboprobe into the developed brain. No signal was observed at any stage when the sense riboprobe was used (data not shown). At all ages, background reactivity was minimal, and all positive results were found consistently in multiple specimens.
Whole-mount ISH for sGnRH-producing neurons at various developmental stages of zebrafish larvae (MB midbrain). Dorsal view. sGnRH mRNA expressing neurons are visualized as dark purple signals. a At 1 day pf, sGnRH mRNA (arrows) is localized in the vicinity of the developing olfactory organ (Olf). b At 3 days pf, the expression of sGnRH (arrows) increases and the cells seem in transitional area between the olfactory organ (Olf) and olfactory bulb (OB). c At 5 days pf and later, the sGnRH population is seen in the terminal nerve ganglion (TNg; arrowheads) adjacent to the olfactory bulb/telencephalon (Tel) border (white dashed line). d At 20 days pf, a continuum of sGnRH neurons is seen from the olfactory area (small arrow) through the TNg (arrowheads), into the telencephalon (Tel) and then further on inside of the hypothalamus (arrows). e At 25 days pf, sGnRH mRNA is localized to the TNg (arrowheads) caudally to the olfactory bulb (OB; dotted line) and along the lateral aspects of the telencephalon (Tel; arrows), and pioneer cells are seen at the preoptic area (POA; asterisk). f At 30 days pf, sGnRH neurons are visualized migrating along the telencephalon (Tel; arrow) towards the POA (asterisk). The black dashed line in e, f depicts the location of the eyes before their removal. Bars 100 μm
Localization of sGnRH producing neurons in sagittal sections of whole-mount ISH specimens (MB midbrain, Tel telencephalon). a Representation of the sagittal section (dashed line). Larval head seen from above. Section 1 plane (S1) contains the sGnRH-producing neurons (purple circles) localized adjacent to the olfactory organ (Olf)/olfactory bulb (OB) boundary. b–g Sagittal sections showing sGnRH mRNA expressing neurons as dark purple signal (arrowheads) on a blue background. The boxed areas are shown at higher magnification right panels. b, c At 2 days pf, sGnRH mRNA is localized to the ventral part of the olfactory organ (Olf). d, e By 3 days pf, the cells expressing sGnRH can be visualized migrating in a medial-caudal direction outside of the olfactory organ (Olf) toward the olfactory bulb. f, g At 6 days pf, the signals are seen in the olfactory bulb (OB). Bars 50 μm (b, d, f), 20 μm (c, e, g)
sGnRH promoter-EGFP expression analysis
Continuous documentation in live intact animals was achieved using transient expression of a fluorescent marker (EGFP) of the sGnRH specific promoter. Microinjection of sGnRH-EGFP resulted in 25% EGFP-expressing embryos, almost all of which exhibited a similar spatio-temporal pattern. Repeated observations of sGnRH neurons in a representative live larva are shown in Fig. 4. EGFP-expressing cells were detected initially at 24 h pf in the vicinity of the developing olfactory organs (Fig. 4a). EGFP-positive projections were first detected after hatching (2 days pf) as bilateral projections from the EGFP-expressing somata extending toward the brain (Fig. 4b). Later on, these projections crossed the telencephalon, formed a chiasm, and then entered the hypothalamus (Fig. 4d,e). Cases of unilateral EGFP expression patterns clearly indicated that the projections targeted both contralateral and ipsilateral hypothalamic regions (data not shown). During their migration, sGnRH somata appeared to follow the path of their own projections. At 3 days pf, EGFP-positive cells were detected in a transitional area between the olfactory organs and the olfactory bulb (Fig. 4c) and at 6 days pf in the TNg (Fig. 4d). A few cells were detected as early as 10 days pf in the hypothalamus (Fig. 4e). The entire migration path of the EGFP-expressing cells from the olfactory organ through the TNg to the hypothalamus was seen at 15 days pf (Fig. 4f).
Repeated observations of sGnRH neurons in a representative live larva expressing the sGnRH-EGFP construct. Lateral (a, b) and dorsal views (c–f) of the bilateral migration path of EGFP-expressing cells. a Earliest detection of EGFP, at 1 day pf, in the prehatched embryo adjacent to the developing olfactory organ (arrow). b After hatching (2 days pf), neurons expressing EGFP (arrows) send projections toward the developing brain. c At 3 days pf, EGFP expression (arrows) appears in the transitional area between the olfactory organ and olfactory bulb (OB) and a few positive cells lie within the telencephalon (arrowhead). d By 6 days pf, clusters of cells migrate backward to the presumptive terminal nerve ganglion (TNg, arrowheads), and additional cells are seen in the telencephalon in association with the projection pathway. e Initial EGFP-positive cells in the presumptive hypothalamus (H, arrows) are observed at 10 days pf. Note the bilateral leading processes that cross the telencephalon, form a chiasm anteriorly to the hypothalamus, and target the hypothalamus. f The entire migration path of the EGFP-expressing cells from the olfactory organ (Olf) through the TNg to the hypothalamus (H) is seen at 15 days pf. Bars 100 μm
ISH analysis of cGnRH-II mRNA
The development of zebrafish cGnRH-II system as detected by whole-mount ISH followed by sagittal sectioning is illustrated in Figs. 5 and 6. cGnRH-II mRNA expression was first detected at 24 h pf and was localized at lateral aspects of the midbrain (Fig. 5a, arrows). By 3 days pf, cGnRH-II neurons were found closer to the midline (Figs. 5b, 6b,c), and at 5 days pf, cGnRH-II-producing cells were exclusively in the median MBT (Figs. 5c, 6d). The location of cGnRH-II mRNA expression remained the same at later stages (8, 14, 21, and 30 days pf; data not shown). ISH revealed that cGnRH-II was not expressed elsewhere at any stage. Background staining was minimal at all ages, and the same results were found consistently in multiple specimens. No signal was observed at any stage when the sense riboprobe was used (data not shown).
Whole-mount ISH of cGnRH-II-producing neurons in various developmental stages of zebrafish larvae (MB midbrain, Tel telencephalon). Dorsal view of head. a cGnRH-II mRNA, first detected at 24 h pf, is located in the lateral midbrain (arrows). b Starting on day 3, the bilateral cGnRH-II-positive populations are now detected closer to the midline (arrows). c From day 5 onward, the cGnRH-II-producing neurons are localized in the midline of the midbrain tegmentum (MBT). Bars 100 μm
Localization of cGnRH-II-producing neurons in sagittal sections from whole-mount ISH specimens (Olf olfactory organ, OB olfactory bulb). a Orientation of the sagittal sections planes (dashed lines). The lateral-medial detection of cells expressing cGnRH-II mRNA is indicated (purple circles). b–d cGnRH-II mRNA expressing neurons are visualized as dark purple signals (arrowheads) on a blue background. b, c At 3 days pf, cGnRH-II-mRNA-producing cells extend from the lateral aspects (S1 plane) to the midline (S2 plane) of the MBT. d By 5 days pf, cGnRH-II mRNA is localized only in the MBT (S2 plane). No signal was detected in other sections, including the S1 planes (data not shown). Bars 50 μm
cGnRH-II promoter-DsRed expression assay
Microinjection of cGnRH-II-DsRed resulted in 25% DsRed-positive embryos. Expression in the MBT was detected in 80% of the DsRed-positive larvae starting on day 3 pf (Fig. 7, asterisk). This expression site was consistent with that seen when using ISH for cGnRH-II mRNA. In contrast with the sGnRH neurons, neuronal projections were not detected in these MBT DsRed cells. Two additional DsRed-expression sites were consistently observed: around 30%–40% of the larvae exhibited temporary DsRed-positive expression in the hindbrain (see Fig 8b, arrowhead) and a specific fluorescent signal in the olfactory area was detected in 90% of the larvae at 3 days pf (Fig. 7a, arrows). Over the next few days, these rostral DsRed-positive cells were seen migrating posteriorly to the olfactory bulb/telencephalon boundary and then penetrated the telencephalon (Fig. 7b,c).
Repeated observations of cGnRH-II neurons in a representative live larva expressing the cGnRH-II-DsRed construct. DsRed expression (red fluorescent signals) in the head region of one representative live larva at 3 (a), 6 (b), and 9 (c) days pf. a–c A stable population of DsRed-expressing cells was detected in the MBT (stars) from 3 days pf onward. Other DsRed-expressing cells were detected in the olfactory area (arrows). At 6 days pf (b), the olfactory-derived DsRed-expressing cells were observed migrating to the olfactory bulb/telencephalon boundary and along the lateral aspects of the telencephalon (arrowheads). By 9 days pf (c), additional DsRed-expressing cells were found in the telencephalon, and the intensity of the fluorescent signals had increased. Bars 100 μm
Expression of co-injected cGnRH-II-DsRed and sGnRH-EGFP constructs. Ventral (a) and dorsal (b) view of the head region of a representative live larva. a Co-expression of both reporter genes is seen at 3 days pf between the olfactory organ and olfactory bulb (arrows). The plane of focus is on the DsRed expression right and on the EGFP expression left (yellow/orange overlap of expression of both reporter genes). The red appearance of the eyes represents the auto-fluorescence of the background. b By 14 days pf, the different distribution patterns of cells expressing cGnRH-II-DsRed and sGnRH-EGFP are apparent. Three cGnRH-II-DsRed populations are observed: in the forebrain (white arrows), MBT (white star), and HB (white arrowhead). The sGnRH-EGFP expressing cells were seen as a continuum from the olfactory bulb (large yellow arrow) to the TNg (yellow arrowhead), following their projections. A weak EGFP signal was found at the hypothalamus (small yellow arrow). Note that the EGFP-expressing fibers target a variety of areas in the brain. Bars 100 μm
Real-time PCR quantification of GnRH transcripts
To determine whether the conflicting results of cGnRH-II localization reflected a lower sensitivity of the ISH analysis or an artifact of the promoter expression assay, the expression of cGnRH-II and sGnRH mRNA in the olfactory/forebrain and midbrain was analyzed by a sensitive real-time PCR. Double-injected EGFP- and DsRed-positive embryos were used to dissect the olfactory/forebrain and midbrain. This analysis revealed that sGnRH was expressed only in the olfactory/forebrain (0.5 pg/μg RNA), whereas cGnRH-II was expressed in both the midbrain (0.2 pg/μg RNA) and, at lower levels, in the olfactory/forebrain region (0.03 pg/μg RNA).
Colocalization of cGnRH-II-DsRed and sGnRH-EGFP cells
The localization of DsRed-expressing cGnRH-II cells in the olfactory area (Fig. 7) resembled that of EGFP-positive cells at the same age (Fig. 4). Therefore, co-injection experiments (150 ng/μl each construct) were performed in order to determine whether these cells co-expressed both constructs. Approximately 25% of the injected embryos expressed the fluorescent proteins, of which two thirds exhibited both EGFP and DsRed expression. Initially, co-expression of the constructs was detected in the same area in the olfactory zone, as indicated by the appearance of an orange fluorescence (Fig. 8a). Later, although both DsRed- and EGFP-expressing neurons migrated to the telencephalon, the constructs were expressed in different cells, and the migration paths appeared to be separate (Fig. 8b).
Discussion
Studies in various classes of vertebrates indicate that the GnRH cell populations of the TN and hypothalamus are derived from the nasal placode and form a continuum during their developmental migration (Schwanzel-Fukuda et al. 1989; Wray et al. 1989; Murakami et al. 1992; Yamamoto et al. 1996; Parhar et al. 1995). In contrast, different embryonic origins for these two populations have been suggested by studies in teleosts that possess three forms of GnRH (Parhar et al. 1998; Pandolfi et al. 2002; Amano et al. 2004). In the current study, the monitoring of sGnRH neuronal ontogeny by ISH and EGFP expression in live individuals has revealed that sGnRH is expressed in cells that form a continuum extending from the nasal epithelium, along the TN and ventral telencephalon, to the hypothalamus. This distribution pattern is in agreement with previous ISH results in adult zebrafish brain (Steven et al. 2003) and with the well-accepted view of the forebrain GnRH migration route (for reviews, see Wray 2002; Schwanzel-Fukuda 1999). These results also support the hypothesis that the TN and hypothalamic GnRH populations contain the same cell type. We have also found that sGnRH projections reach both ipsilateral and contralateral hypothalamic regions in zebrafish, confirming the results of unilateral olfactory placode ablation studies in Xenopus (Setalo 1996).
A third distinct group of immunoreactive cells, not defined molecularly, has been detected in the zebrafish as early as 56 h after fertilization and is referred to as hypothalamic GnRH cells (Whitlock et al. 2003). Based on lineage tracing, these cells have been suggested to arise from the adenohypophyseal regions of the neural plate, whereas the TN sGnRH cells were shown to originate from the cranial neural crest and to be transiently associated with the olfactory organ (Whitlock et al. 2003). In the current study, however, this early hypothalamic population has not been detected, and the origin of the TN and hypothalamic GnRH populations was indistinguishable. The presence of a third form of GnRH in zebrafish cannot be completely ruled out, although our enduring efforts to find such a gene by thoroughly searching the zebrafish genome database have so far been fruitless. Additional supportive data have been obtained by identification of only two GnRH forms in zebrafish brain extracts as determined by their elution position in high pressure liquid chromatography (HPLC) followed by radioimmunoassay (Powell et al. 1996). A recent model for the evolution of the GnRH genes proposes that some fish, including the zebrafish, have only two GnRH molecular forms because of a loss of the mammalian GnRH ortholog gene (Kuo et al. 2005).
During their ontogeny, the GnRH-containing neurons migrate in a mode called “tangential migration” in which neurons progress in parallel to the brain surface (Murakami et al. 2002). The continuous detection of EGFP-labeled sGnRH neurons in live animals has revealed that long leading processes, presumably axons, precede the migration of the cell soma. Indeed, tangential neural migration is suggested as an elaboration of the normal process of axon elongation (Gilthorpe et al. 2002). Known examples of neurons that migrate tangentially by following the elongation of leading processes are the cerebellar rhombic lip derivatives (Gilthorpe et al. 2002; Koster and Fraser 2001), pontine neurons (Yee et al. 1999), and neuroblasts that form the nucleus magnocellularis (Book and Morest 1990). Further investigations, including cell labeling and time lapse confocal microscopy, is needed to determine whether GnRH neurons use axonal extensions as a guiding cue for the migration of the cell body.
The ISH method has revelaed clusters of cGnRH-II mRNA expressing neurons moving caudo-medially from the lateral aspects of the midbrain to its midline. At later developmental stages, cGnRH-II mRNA is exclusively expressed at this site, as has been previously shown in the adult zebrafish (Steven et al. 2003). Interestingly, in addition to the MBT, DsRed-expressing cGnRH-II cells have been detected at specific regions of the hindbrain and forebrain, the expression of cGnRH-II mRNA in the forebrain being confirmed by real-time PCR analysis. Wong and colleagues (2004) have also detected temporary cGnRH-II expression in the hindbrain in embryos of two other teleosts, namely, the gilthead seabream (Sparus aurata) and European sea bass (Dicentrarchus labrax), and immunoreactive cGnRH-II cells have been previously identified in the forebrain of the closely related goldfish (Lin and Peter 1997; Yu et al. 1998). The DsRed-expressing cells in the forebrain seem to emerge from the same site as the EGFP-labeled sGnRH cells, as revealed by co-injection of the two constructs. Both cell types migrate from the vicinity of the olfactory organs to the TNg and subsequently into the telencephalon; however, their migratory routes are not identical. The overlapping spatial expression reflects the evolutionary relationship of the two genes and possible promoter similarities. Further transient and stable expression analyses of different promoter constructs are required for identifying regulatory elements that determine the cell-type-specific expression of each gene.
The molecular mechanism of GnRH neuronal migration is presently ambiguous. The establishment of an in vivo experimental system for the study of the regulation of GnRH neuronal migration should facilitate the elucidation of its mechanism. The specific spatio-temporal expression of the reporter proteins driven by GnRH promoters shown herein indicates that the injected DNA constructs contain the relevant transcription regulatory elements. The generation of transgenic lines in which stable reporter proteins linked to GnRHs promoters are expressed can now be used to study the roles of various external and internal factors in the regulation of GnRH neuronal migration.
References
Amano M, Okubo K, Yamanome T, Oka Y, Kawaguchi N, Aida K, Yamamori K (2004) Ontogenic development of three GnRH systems in the brain of a pleuronectiform fish, barfin flounder. Zool Sci 21:311–317
Book KJ, Morest DK (1990) Migration of neuroblasts by perikaryal translocation: role of cellular elongation and axonal outgrowth in the acoustic nuclei of the chick embryo medulla. J Comp Neurol 297:55–76
Dubois EA, Zandbergen MA, Peute J, Goos HJ (2002) Evolutionary development of three gonadotropin-releasing hormone (GnRH) systems in vertebrates. Brain Res Bull 57:413–418
Fernald RD, White RB (1999) Gonadotropin-releasing hormone genes: phylogeny, structure, and functions. Front Neuroendocrinol 20:224–240
Gilthorpe JD, Papantoniou EK, Chedotal A, Lumsden A, Wingate RJ (2002) The migration of cerebellar rhombic lip derivatives. Development 129:4719–4728
Gothilf Y, Toyama R, Coon SL, Du SJ, Dawid IB, Klein DC (2002) Pineal-specific expression of green fluorescent protein under the control of the serotonin-N-acetyltransferase gene regulatory regions in transgenic zebrafish. Dev Dyn 225:241–249
Koster RW, Fraser SE (2001) Direct imaging of in vivo neuronal migration in the developing cerebellum. Curr Biol 11:1858–1863
Kuo MW, Lou SW, Postlethwait J, Chung BC (2005) Chromosomal organization, evolutionary relationship, and expression of zebrafish GnRH family members. J Biomed Sci 12:629–639
Lin XW, Peter RE (1997) Cloning and expression pattern of a second [His5Trp7Tyr8]gonadotropin-releasing hormone (chicken GnRH-H-II) mRNA in goldfish: evidence for two distinct genes. Gen Comp Endocrinol 107:262–272
MacColl G, Quinton R, Bouloux PM (2002) GnRH neuronal development: insights into hypogonadotrophic hypogonadism. Trends Endocrinol Metab 13:112–118
Millar RP (2003) GnRH II and type II GnRH receptors. Trends Endocrinol Metab 14:35–43
Murakami S, Kikuyama S, Arai Y (1992) The origin of the luteinizing hormone-releasing hormone (LHRH) neurons in newts (Cynops pyrrhogaster): the effect of olfactory placode ablation. Cell Tissue Res 269:21–27
Murakami S, Seki T, Arai Y (2002) Structural and chemical guidance cues for the migration of GnRH neurons in the chick embryo. Prog Brain Res 141:31–44
Pandolfi M, Parhar IS, Ravaglia MA, Meijide FJ, Maggese MC, Paz DA (2002) Ontogeny and distribution of gonadotropin-releasing hormone (GnRH) neuronal systems in the brain of the cichlid fish Cichlasoma dimerus. Anat Embryol (Berl) 205:271–281
Parhar IS, Iwata M, Pfaff DW, Schwanzel-Fukuda M (1995) Embryonic development of gonadotropin-releasing hormone neurons in the sockeye salmon. J Comp Neurol 362:256–270
Parhar IS, Soga T, Ishikawa Y, Nagahama Y, Sakuma Y (1998) Neurons synthesizing gonadotropin-releasing hormone mRNA subtypes have multiple developmental origins in the medaka. J Comp Neurol 401:217–226
Powell JF, Krueckl SL, Collins PM, Sherwood NM (1996) Molecular forms of GnRH in three model fishes: rockfish, medaka and zebrafish. J Endocrinol 150:17–23
Schwanzel-Fukuda M (1999) Origin and migration of luteinizing hormone-releasing hormone neurons in mammals. Microsc Res Tech 44:2–10
Schwanzel-Fukuda M, Bick D, Pfaff DW (1989) Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res 6:311–326
Setalo G (1996) Gonadotropin-releasing hormone neuroblasts from one olfactory placode can be present in both hemispheres in the clawed toad Xenopus laevis. Neuroendocrinology 63:408–414
Somoza GM, Miranda LA, Strobl-Mazzulla P, Guilgur LG (2002) Gonadotropin-releasing hormone (GnRH): from fish to mammalian brains. Cell Mol Neurobiol 22:589–609
Steven C, Lehnen N, Kight K, Ijiri S, Klenke U, Harris WA, Zohar Y (2003) Molecular characterization of the GnRH system in zebrafish (Danio rerio): cloning of chicken GnRH-II, adult brain expression patterns and pituitary content of salmon GnRH and chicken GnRH-II. Gen Comp Endocrinol 133:27–37
Torgersen J, Nourizadeh-Lillabadi R, Husebye H, Alestrom P (2002) In silico and in situ characterization of the zebrafish (Danio rerio) gnrh3 (sGnRH) gene. BMC Genomics 3:25
Westerfield M (1995) The zebrafish book. University of Oregon Press, Oregon
Whitlock KE, Wolf CD, Boyce ML (2003) Gonadotropin-releasing hormone (GnRH) cells arise from cranial neural crest and adenohypophyseal regions of the neural plate in the zebrafish, Danio rerio. Dev Biol 257:140–152
Wong TT, Gothilf Y, Zmora N, Kight KE, Meiri I, Elizur A, Zohar Y (2004) Developmental expression of three forms of gonadotropin-releasing hormone and ontogeny of the hypothalamic-pituitary-gonadal axis in gilthead seabream (Sparus aurata). Biol Reprod 71:1026–1035
Wray S (2002) Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol 23:292–316
Wray S, Grant P, Gainer H (1989) Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86:8132–8136
Yamamoto N (2003) Three gonadotropin-releasing hormone neuronal groups with special reference to teleosts. Anat Sci Int 78:139–155
Yamamoto N, Uchiyama H, Ohki-Hamazaki H, Tanaka H, Ito H (1996) Migration of GnRH-immunoreactive neurons from the olfactory placode to the brain: a study using avian embryonic chimeras. Brain Res Dev Brain Res 95:234–244
Yee KT, Simon HH, Tessier-Lavigne M, O’Leary DM (1999) Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron 24:607–622
Yu KL, He ML, Chik CC, Lin XW, Chang JP, Peter RE (1998) mRNA expression of gonadotropin-releasing hormones (GnRHs) and GnRH receptor in goldfish. Gen Comp Endocrinol 112:303–311
Acknowledgements
We thank Ms. Barbara Coloroni, National Center for Mariculture, Eilat, for her technical assistance, Dr. Helit Nabel-Rosen for sharing her excellent micro-dissection skills with us, and Professor (Emeritus) Zvi Yaron for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the US-Israel Bi-national Agricultural Research and Development (BARD) Foundation (grant 3428-03).
Rights and permissions
About this article
Cite this article
Palevitch, O., Kight, K., Abraham, E. et al. Ontogeny of the GnRH systems in zebrafish brain: in situ hybridization and promoter-reporter expression analyses in intact animals. Cell Tissue Res 327, 313–322 (2007). https://doi.org/10.1007/s00441-006-0279-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0279-0