Abstract
Holothurians (sea cucumbers) have been known from ancient times to have the capacity to regenerate their internal organs. In the species Holothuria glaberrima, intestinal regeneration involves the formation of thickenings along the free mesentery edge; these thickenings will later give rise to the regenerated organ. We have previously documented that a remodeling of the extracellular matrix and changes in the muscle layer occur during the formation of the intestinal primordium. In order to analyze these changes in depth, we have now used immunocytochemical techniques and transmission electron microscopy. Our results show a striking disorganization of the muscle layer together with myocyte dedifferentiation. This dedifferentiation involves nucleic activation, disruptions of intercellular junctions, and the disappearance of cell projections, but more prominently, the loss of the contractile apparatus by the formation and elimination of spindle-like structures. Muscle dedifferentiation can be seen as early as 2 days following evisceration and continues during the next 2 weeks of the regeneration process. Dedifferentiation of myocytes might result in cells that proliferate and give rise to new myocytes. Alternatively, dedifferentiating myocytes could give rise to cells with high nuclear-to-cytoplasmic ratios, with some being eliminated by apoptosis. Our results, together with those in other regeneration models, show that myocyte dedifferentiation is a common event in regeneration processes and that the dedifferentiated cells might play an important role in the formation of the new tissues or organs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The regeneration of organs and tissues in both vertebrates and invertebrates is a complex phenomenon that has been studied since the nineteenth century. In the case of echinoderms, the regeneration of external and internal organs occurs after natural or induced autotomy or evisceration (ejection of the internal organs; Hyman 1955; Emson and Wilkie 1980; Byrne 1985, 2001; Dolmatov et al. 1996; Candia-Carnevali and Bonasoro 2001; García-Arrarás et al. 1998, 1999; García-Arrarás and Greenberg 2001). Our laboratory has investigated the intestinal regeneration process in the sea cucumber Holothuria glaberrima as an excellent model for the study of organogenesis in an adult organism (García-Arrarás et al. 1998, 1999; García-Arrarás and Greenberg 2001; Quiñones et al. 2002).
The regeneration process in holothurians (sea cucumbers) involves the formation of new internal organs including the digestive tube, the respiratory trees, and the hemal system, following tissue and organ rupture and evisceration (Hyman 1955; Emson and Wilkie 1980; Byrne 1985, 2001; Leibson 1992; Dolmatov et al. 1996; García-Arrarás et al. 1998, 1999; García-Arrarás and Greenberg 2001). The holothurian digestive system is anchored to the body wall by a series of mesenteries (designated as lateral, dorsal, and ventral) that play a crucial role in the regeneration process (see Quiñones et al. 2002). These mesenteries are composed of (from the outer to inner layers) a coelomic epithelium layer that lies over a muscle layer. These two layers are collectively known as the mesothelium and are separated from an inner connective tissue layer by a basal lamina (Smiley 1994). Previous studies have shown that regeneration of the intestinal system begins from a series of thickenings that are formed at the remaining free edge of the lateral and ventral mesenteries after evisceration and proceeds until it is complete in 3–4 weeks (García-Arrarás et al. 1998, 1999). The thickenings eventually give rise to a blastema-like structure, and various processes are associated with its formation, including cell division, migration of various cell populations from the esophagus, cloaca, and mesenteries, and changes in the extracellular matrix components of the connective tissue (García-Arrarás et al. 1998, 1999; García-Arrarás and Greenberg 2001; Quiñones et al. 2002; Murray and García-Arrarás 2004).
Changes that occur within the mesenterial tissues appear to be pivotal to the regeneration process. These include changes in extracellular matrix (ECM) components (Quiñones et al. 2002). Previous studies have shown that, during the first week of regeneration, both fibrous collagen labeling in the mesentery connective tissue and laminin labeling in the mesentery muscle cells disappear. This coincides with a disorganization of the muscle layers of the mesentery (Quiñones et al. 2002). In addition, selected markers for myocytes, such as a muscle-specific monoclonal antibody and phalloidin labeling (a toxin that labels the actin filaments present within the muscle cell contractile apparatus), are not found within the regenerating structure during the first days of regeneration and eventually appear as the new intestine is formed (Garcia-Arraras and Greenberg 2001; Murray and García-Arrarás 2004). These results suggest that the myocytes close to and within the regenerating structure undergo dramatic changes during the first few weeks of regeneration.
In previous work, we have suggested that intestinal regeneration in H. glaberrima occurs via a combination of morphallaxis and epimorphosis (García-Arrarás et al. 1998). The former implies the production of a blastema with dividing nondifferentiated cells. The cell division that mainly occurs during the second and third week of regeneration is characteristic of an epimorphic mechanism. On the other hand, morphallactic mechanisms are characterized by the formation of new body parts by the remodeling of existing cells, and the changes that occur during the first week of regeneration (Quiñones et al. 2002) are typical of this mechanism. However, the precise steps that take place in the mesentery remain unclear at the morphological level. Therefore, in the present study, we focus on the morphological changes that occur in the muscle component of the ventral mesentery during early intestinal regeneration in order to understand the processes that are involved in the initial formation of the regenerating blastema-like structure. To achieve our goal, we have used immunocytochemical techniques with HgM1, a monoclonal antibody against H. glaberrima muscular components (García-Arrarás et al. 1998; Murray and García-Arrarás 2004), and more importantly, transmission electron microscopy to analyze in detail the changes in the muscle cell layers. Our results provide interesting insights into the role of the mesentery and its cellular components during the holothurian intestinal regeneration process.
Materials and methods
Animals
Holothuria glaberrima specimens were collected on the northeast coast of Puerto Rico and maintained in the laboratory in recirculating seawater aquaria with constant oxygenation. Evisceration was induced by injecting 0.35 M KCl (3–5 ml) into the coelomic cavity. Animals were anesthetized by immersion in 6% MgCl2 for 1 h before dissection on various days after evisceration. Details of animal maintenance and dissection procedures were as described previously (García-Arrarás et al. 1998, 1999).
Immunocytochemistry
The immunocytochemical procedures used were as described elsewhere (García-Arrarás et al. 1998, 1999; Quiñones et al. 2002; Murray and García-Arrarás 2004). Briefly, normal noneviscerated and regenerating mesenteries were fixed in either picric acid/ formaldehyde (Zamboni) or 4% paraformaldehyde at 4°C for 24 h, processed, and maintained in 30% sucrose in phosphate-buffered saline (PBS) until use. Tissues were embedded in OCT embedding medium (Tissue Tek OCT; Miles), frozen at −20°C, and sectioned (10–20 μm) in a cryostat (Leica CM 1900). Sections were recovered on poly-lysine-treated slides and treated overnight with a culture supernatant of monoclonal antibody HgM1. This antibody is specific for H. glaberrima muscle components (García-Arrarás et al. 1998; Murray and García-Arrarás 2004). The following day sections were rinsed in PBS and treated with a 1:50 dilution of the secondary antibody, fluorescein isothiocyanate (FITC)-labeled goat-anti-mouse (BioSource Int.) for 1 h. Sections were rinsed again in PBS and mounted in buffered glycerol. Double-labeling with Hoechst nuclear dye (Sigma) was performed by immersing slides in 1 μM Hoechst for 5 min following the rinsing off of the monoclonal antibody (García-Arrarás et al. 1998; Murray and García-Arrarás 2004). Sections were examined and photographed by using a Nikon E600 microscope equipped with a I2/3 filter. Images were recorded by using MetaVue software (version 6.0; Universal Imaging) and processed with Adobe Photoshop software (version 7.0).
Western blot
Homogenates were prepared from the large intestine (or posterior intestine according to Feral and Massin 1982) of H. glaberrima specimens by using Triton X-100 (1% solution) and Laemmli sample buffer (2.5% SDS; Murray and García-Arrarás 2004). Some samples were treated with mercaptoethanol (20 μl/ml) and urea (0.3 g/ml). Protein concentrations were measured by Bio-Rad protein assay (Bio-Rad). From each sample, a volume equivalent to 40 μg soluble protein fraction was separated by 13% SDS-polyacrylamide gel electrophoresis. Biotinylated broad-range standards with avidin-horseradish peroxidase were used for molecular weight determination (Bio-Rad). Gels were equilibrated in Towbin transfer buffer with 15% methanol and transferred to polyvinylidene fluoride membranes (Amersham Pharmacia) in an electrophoresis transfer cell at 50 V for 2.5 h. After transfer, membranes were blocked with 5% nonfat milk, rinsed in PBS with 0.1% Tween 20 (pH 7.4), and incubated with the antibody HgM1 at a 1:50 dilution of the clone supernatant. The secondary antibody (sheep anti-mouse Ig coupled to horseradish peroxidase; Amersham Pharmacia) was used at a 1:5,000 dilution. Enhanced chemiluminescent development was performed following the manufacturer's instructions (Amersham Pharmacia).
Transmission electron microscopy
Noneviscerated and regenerating animals (at day−2, day−3, day−7, and day−14 after evisceration) were fixed by injecting 3% glutaraldehyde in 0.1 M PIPES buffer (2 ml, pH 7.4) into the body cavity of anesthetized animals. Posterior ventral mesenteries were dissected and placed in fresh fixative for 15 min at room temperature. Tissues were then transferred to fresh fixative (2.5% glutaraldehyde in 0.1 M PIPES buffer pH 7.4, containing 1% tannic acid) for 2 h at 4°C, rinsed in PIPES buffer, and post-fixed in 1% osmium tetroxide in distilled water for 1 h at 4°C. After being washed briefly with distilled water, tissues were stained en bloc with 1% aqueous uranyl acetate, dehydrated in graded series of ethanol, and embedded in Epon 812 resin (Electron Microscopy Sciences). Ultrathin sections (approximately 60 μm thick) were obtained with a LKB ultramicrotome by using a diamond knife, collected on copper grids, stained with 1% uranyl acetate and lead citrate, and examined with a Zeiss EM10 transmission electron microscope or a Philips 201 transmission electron microscope. Images were processed by using Adobe Photoshop software (version 7.0). Measurements of muscle cell area were performed by measuring the length and multiplying this by the width with the MetaVue program (version 6.0; Universal Imaging) and analyzed with Excel (Microsoft).
Results
Immunocytochemical analysis
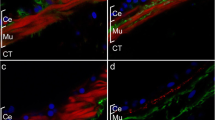
Previous experiments have shown that the HgM1 antibody specifically recognizes a plasma membrane epitope on the muscle cells of both noneviscerated and regenerating intestine and mesentery (García-Arrarás et al. 1998; Murray and García-Arrarás 2004). The use of this antibody together with various immunocytochemical techniques has given us an initial insight into the changes that occur in the muscle layers of the mesentery during the process of intestinal regeneration. At the light-microscopy level, the mesothelium from the noneviscerated mesentery is composed of well-differentiated cell layers that include a coelomic epithelial cell layer and a muscle layer, which in turn is innervated by nerve fibers from the visceral nerve plexus (García-Arrarás et al. 2001). Whereas the area of the regenerating mesentery that is close to the body wall shows the same muscle layer organization that is found in noneviscerated animals (Fig. 1a,b), dramatic changes have been observed to occur at the free end of the mesentery. During the first week of regeneration, myocytes within this area contract and round up, and there is a gradual disorganization of the muscle layer (Fig. 1c,d). Moreover, in the area of mesentery adjacent to the blastema-like structure, some of the myocytes appear to detach from their normal position just beneath the coelomic layer (Fig. 1c,d). In addition, projections originating from these cells can be observed throughout the connective tissue (Fig. 1e,f). Cells labeled with the muscle-specific antibody are found within the connective tissue layer, suggesting that the myocytes have left their usual location and have crossed the basal lamina. Disorganization of the muscle layer continues through the first 2 weeks of regeneration, resulting in a noticeable decrease in the number of immunolabeled myocytes; this is visually evident as regeneration progresses. The loss in cell numbers and muscle organization occurs gradually at both temporal and spatial levels. Disorganization is higher at the free end of the regenerative mesentery and becomes less disorganized toward the body wall. Similarly, the extent of mesentery where muscle is disorganized increases during the first week of regeneration.
HgM1 antibody labeling of the mesentery muscle cells in 5-day regenerating mesentery (a,c,e). The same tissue section areas are shown in phase contrast (b, d, f). Compared with the mesentery muscle layer near the body wall (a,b), the muscle layer near the regenerating pseudoblastema shows a disorganized morphology (CE coelomic epithelium, CT connective tissue). Myocytes (arrowheads) begin to round up presenting a smaller and irregular morphology and are observed at the connective tissue layer of the mesentery (c,d). Projections (arrowheads) from disorganized cells are present in the connective tissue (e,f), suggesting that they cross the basal lamina into the connective tissue. Bar 60 μm
The loss of the muscle cell markers was confirmed by Western blots as a reduction in the labeling of the HgM1 antigen (Fig. 2). The main band of 8 kDa (Murray and García-Arrarás 2004) disappeared during the first week of regeneration and reappeared in the subsequent weeks. Similarly, the weakly labeled 53-kDa band disappeared and reappeared following the same time course. In contrast, lesser bands present in normal control tissue (40 kDa, 31 kDa, and 24 kDa) did not reappear in the regenerating animals during the period of study and may have been breakdown products.
The Western blot and immunocytochemical data suggested that a loss of the mesentery muscle occurred during the early stages of intestine regeneration. Therefore, for an in-depth analysis of the ongoing changes in the myocytes, we examined the changes in the tissue at the ultrastructural level.
Ultrastructure observations
Initial observations of mesentery tissue from normal noneviscerated animals to determine the ultrastructural organization of the tissue show that the mesentery border is a pseudostratified myoepithelium according to the description of Rieger and Lombardi (1987). It consists of an apical layer of coelomic epithelial cells (or peritoneocytes) with mostly heterochromatic nuclei, cilia, and many microvilli and a more basal muscle layer formed by myocytes. This pseudostratified myoepithelium is separated from the connective tissue layer by a basal lamina. Throughout the mesentery border, the peritoneocytes extend fine lateral projections that form intercellular junctions or desmosomes connecting with each other (Fig. 3). The muscle layer is composed of several cells (up to five bundles in a single cross-sectional area). Because of the myocyte morphology, the cell nuclei are not always present in all cross sections, but when observed they are generally located in the apical part of the cell and mostly irregularly shaped and euchromic. Cells have a contractile system formed by densely packed myofilaments but show no discernible Z structure. Many mitochondria are usually present in the periphery. These cells are also characterized by long sarcoplasmic projections that extend toward the nerve plexus (whose fibers contain vesicles with various electron densities). Projections from the myocytes also extend toward the mesothelial basal lamina and, at their ends, contain hemidesmosome-like junctions. Desmosomes can be observed connecting the myocytes to each other.
Ultra-thin section of the noneviscerated mesentery showing that the mesentery mesothelium is typical of a pseudostratified epithelium and consists of an apical coelomic epithelial layer of peritoneocytes, with a collar of microvilli, and a basal muscle layer made of myocytes (BL basal lamina, CE coelomic epithelium, CT connective tissue, H hemidesmosome, IJ intercellular junction, M muscle layer, My myocyte). Both layers are separated from the connective tissue (CT) by a basal lamina (BL). Myocytes are characterized by sarcoplasmic projections (arrows) and desmosomal and hemidesmosomal junctions (arrowhead). Bar 4 μm
The first structural evidence indicating that a process of dedifferentiation is occurring in some of the myocytes in the mesentery can be seen between the second and third day of regeneration, when the contractile apparatus of some cells becomes disrupted and decreases in size. Muscle cells, as viewed by their contractile structure, can be observed to round up. These myocytes also lose their sarcoplasmic projections that previously connected them to the adjacent nerve plexus and basal lamina, and their junctional complexes (both desmosomes and hemidesmosomes). However, the major finding observed during muscle layer disorganization is the assemblage of myofilaments into spindle-like structures (SLS; Fig. 4a).
Cross-sectional anatomy of the mesothelium in the third-day regenerating mesentery showing myocyte dedifferentiation; transmission electron microscopy (PA phagocytic amoebocyte). a Muscle cells are present at various stages of dedifferentiation, showing a decrease in muscle size together with a rounder morphology. At this stage, cells lose certain cell structures including sarcoplasmic projections and cell junctions. Some muscle cells contain disorganized myofilaments that begin to arrange into spindle-like structures (SLS; arrows). SLS are also found within the cytoplasm of a coelomic epithelial (CE) cell (arrowhead). A phagocytic amoebocyte (PA) can be observed in the underlying connective tissue below the basal lamina (BL). b An extruded SLS is observed free in the body cavity. The presence of intact intercellular junctions (arrowheads) suggest that SLSs are exocytosed by the coelomic epithelium into the cavity. Bars 4 μm (a), 2 μm (b)
During this first stage of regeneration, SLSs are also found in the cytoplasm of peritoneocytes (Fig. 4a) and, in some cases, free in the coelomic cavity (Fig. 4b). This suggests that SLSs are further acquired by peritoneocytes and liberated into the adjacent body cavity space. Another finding that characterizes myocyte dedifferentiation is the appearance of highly developed nucleoli in their irregular euchromic nuclei (Fig. 5a–c). Areas in which the muscle layer has become completely disorganized have an increased number of peritoneocytes with euchromatic nuclei and well-developed nucleoli (as in the case of dedifferentiated myocytes), well-developed Golgi complexes, and an increased number of lysosomes and phagosomes containing residues of the contractile apparatus from muscle cells (Fig. 5c). Meanwhile, myocytes that are undergoing dedifferentiation acquire a rounded shape, with plasmalemmal invaginations that appear to separate the nucleus from the contractile apparatus (Fig. 5d,f). These cells separate from the apical coelomic peritoneocytes and have a high nucleus-to-cytoplasm ratio (Fig. 5d,e).
Cross sections of regenerating mesentery showing changes in myocyte nuclei during de-differentiation; transmission electron microsocpy (BL basal lamina, CE coelomic epithelium, IJ intercellular junction, IS intercellular space, M myoepithelial cell, SLS spindle-like structure). a A myocyte in the mesothelium of the second-day regenerating mesentery shows a typical, irregularly shaped, euchromic nucleus (arrow). b, c Changes in nuclei are observed at stages of active de-differentiation. b Nuclei with less-condensed chromatin are observed in the seventh-day mesentery. An SLS is present in the apical coelomic epithelial cytoplasm. c Similarly, a dedifferentiated myocyte showing residues of muscle cell components in its cytoplasm (arrow) is present within an area in the third-day mesothelium in which the muscle layer is completely disorganized. This area exhibits an increase in the number of coelomic epithelial cells, also with euchromatic nuclei and in some cases nucleoli and well-developed Golgi complexes. Lysosomes and phagosomes are also present in their cytoplasm containing dedifferentiated muscle cell components (arrowhead). d A cell that appears to be a dedifferentiating myocyte shows a round morphology, oval nucleus, and plasmalemmal invaginations (arrowhead). These invaginations separate the nucleus from the dedifferentiated residue of the contractile apparatus (arrow). e Higher magnification of d showing the separation of the dedifferentiating myocyte from the apical coelomic epithelial layer (arrowheads). f Another part of d at higher magnification showing the plasmalemmal invaginations (arrow) together with the dedifferentiated contractile system residue (arrowhead). Bars 4 μm (a, c, d), 2 μm (b), 0.5 μm (e), 1 μm (f)
SLSs are also present within the cytoplasm of phagocytic amoebocytes in the connective tissue layer of the mesentery (Fig. 6a,b). These amoebocytes appear actively to degrade many of the contractile machinery residues. Furthermore, myofilament-containing projections can be found near amoebocytes in the connective tissue layer (Fig. 6c); these are not observed in the mesentery of noneviscerated animals. After 5 days of regeneration, amoebocytes in the connective tissue layer (Fig. 6d) in regenerating blastema-like structures can be observed to have accumulated, in their cytoplasm, an increased number of lysosomal vacuoles containing SLSs and electron-dense nerve vesicles. Other changes that occur in the mesentery of regenerating animals during the first week of regeneration include the appearance of cells with a high nucleus-to-cytoplasm ratio in the connective tissue (Fig. 7a) and a small number of cells undergoing apoptosis in the mesothelium (not shown).
Cross sections of the regenerating mesentery and blastema-like structure at various regeneration times showing phagocytic amoebocytes; transmission electron microsocpy (BL basal lamina, CE coelomic epithelium, CT connective tissue). a An amoebocyte containing an SLS (arrowhead) is present in the connective tissue of the second-day regenerating mesentery, near the mesothelium. b As muscle dedifferentiation continues, SLSs (arrowheads) and lysosomes with muscle cell components accumulate in the cytoplasm of phagocytic amoebocytes. c Projections that contain disorganized myofilaments (arrow) are present near amoebocytes in the connective tissue of third-day regenerating mesentery. SLSs (arrowheads) can still be observed in the mesothelium of the mesentery. d In the connective tissue of the 5-day regenerating blastema-like structure, phagocytic amoebocytes accumulate, in their cytoplasm, SLSs (arrowheads) and electron-dense vesicles from nerve fibers. Bars 2 μm (a), 4 μm (b, c, d)
Cross sections of seventh-day and fourteenth-day regenerating mesentery. Myocyte dedifferentiation occurs during the first 2 weeks of regeneration; transmission electron microsocpy (BL basal lamina, CE coelomic epithelium, CT connective tissue, IS intercellular space, NP nerve plexus, SLS spindle-like structure). a An SLS is present within a coelomic epithelial (CE) cells in the seventh-day regenerating mesentery. An undifferentiated cell (arrowhead) with a high nucleus-to-cytoplasm ratio and an oval nucleus is also observed in the connective tissue below the mesothelium. b After the second week of regeneration, the number of myocytes within the mesentery has greatly diminished, and SLSs (arrowhead) are still present in the coelomic epithelia. c In the fourteenth-day regenerating mesentery, a cell that appears to be migrating basally presents characteristics of myoepithelial cells: an apical intercellular junction (arrowhead) with the apical peritoneocyte and a basal process containing myofilaments (arrow). d, e Areas of c at higher magnification showing (d) myofilament-containing basal process (arrow) and (e) a desmosome-like structure connecting the cell with an apical coelomic epithelial cell (arrow). Bars 4 μm (a,b), 2 μm (c), 1 μm (d), 0.25 μm (e)
Disruption of the mesentery muscle layer with its associated formation of SLSs continues for the first 2 weeks of regeneration, suggesting that not all myocytes undergo dedifferentiation simultaneously. In the seventh-day regenerating animals, both intact myocytes and SLSs are observed in the mesentery (Fig. 7a). Moreover, peritoneocytes with euchromatic nuclei and well-developed Golgi complexes contain extruded SLSs and lysosomal inclusions with muscle components in their cytoplasm. Similarly, at 14 days of regeneration, myofilament disruption is still present among the small number of myocytes present (Fig. 7b). The changes that occur in the muscle layer during regeneration can be quantified by measuring the muscle area (this is mainly the contractile apparatus, as seen in cross section). During the first 2 weeks of regeneration, the muscle area undergoes a dramatic decrease from approximately 19.3±1.6 μm2 (mean±SE, n=65) in the second-day regenerating mesentery compared with 3.6±0.7 μm2 (mean±SE, n=25) in the fourteenth-day regenerating mesentery.
Events leading to the formation of the new mesentery muscle layer can also be observed in the fourteenth-day regenerating animal: primarily, the appearance of cells that are located basal to the apical coelomic epithelium and are characterized by uncommon processes that extend toward the mesothelial basal lamina and contain myofilaments (Fig. 7c,d). These cells appear to be in the process of acquiring myocyte characteristics that include not only morphological features such as the presence of myofilaments in their basal process, but also their basal localization. Nerve fibers containing dense-core vesicles of various electron densities are located near the myofilament-containing processes (Fig. 7d), and desmosome-like structures lie between the apical coelomic epithelial cells and the myofilament-containing cells (Fig. 7e).
Discussion
Immunocytological observations
Previous experiments have indicated that dramatic changes occur in the contractile tissue of the mesentery and regenerating intestine during the regeneration process in Holothuria glaberrima. First, during the first regeneration week, few or no muscle can be found within the mesenterial thickenings that give rise to the new intestine either by histological stains, immunocytochemistry, or phalloidin labeling (García-Arrarás et al. 1998; Murray and García-Arrarás 2004). Second, a loss of muscle-associated laminin is observed not only in the regenerating intestine, but also in the associated mesentery (Quiñones et al. 2002). Finally, preliminary evidence has indicated that myocytes have almost completely disappeared from the mesentery by the end of the first week of regeneration (García-Arrarás and Greenberg 2001).
We have now shown that a dramatic change does indeed occur in the mesentery muscle. At the immunocytochemical level, the changes involve a disorganization of the muscle layer, in which the cells appear to contract and acquire a smaller and irregular morphology. Moreover, at least some cells appear to migrate from their position in the mesothelium and move into the internal connective tissue of the mesentery. The outcome of these changes is that myocytes are lost from the mesentery during the first week of regeneration in a process that occurs with a spatial gradient from the edge of the mesentery to the body wall. This spatial loss coincides with the loss observed in muscle-associated laminin (Quiñones et al. 2002).
The loss in muscle components has been confirmed by the use of Western blots showing that, during the first week of regeneration, the principal epitope (8-kDa band) labeled by a muscle-specific antibody disappears. Because the disappearance of the labeled epitope does not necessarily result from disappearance of the cells, we have used transmission electron microscopy to extend our study and to follow the specific changes that occur in the myocytes.
Muscle cells undergo dedifferentiation
Our ultrastructural analysis has confirmed that the changes observed in the muscle layer are attributable to myocyte dedifferentiation. Dedifferentiation involves genomic changes in the nuclei indicative of nucleic activation (for example, development of nucleoli) and the loss of differentiated cell components that, in the case of myocytes, include cell processes that attach the muscle to the basal lamina and to adjacent cells, and the loss of cellular junctions. More dramatically, it involves the loss of myocyte contractile elements, which become SLS. These SLSs are a definite signal of muscle dedifferentiation, as has been shown in other instances in echinoderm regenerative processes (Dolmatov 1992; Dolmatov et al. 1996; Dolmatov and Ginanova 2001; Mashanov and Dolmatov 2001; Candia-Carnevali and Bonasoro 2001).
The two best-documented cases of SLS formation and their relationship to muscle dedifferentiation have been presented by Dolmatov and colleagues (Dolmatov 1992; Dolmatov et al. 1996; Dolmatov and Ginanova 2001), and Candia-Carnevali and colleagues (Candia-Carnevali et al. 1998; Candia-Carnevali and Bonasoro 2001). Dolmatov describes dedifferentiation processes that occur not only during visceral muscle regeneration of the acquapharyngeal complex in the sea cucumber Eupentacta fraudatrix (Dolmatov 1992), but also during longitudinal body muscle band regeneration in E. fraudatrix and Apostichopus japonicus (Dolmatov and Ginanova 2001). As in our case, muscle cells have been shown to contain highly developed nucleoli and to lose differentiated cell components including, most conspicuously, their contractile apparatus, with the formation and gradual elimination of SLSs. Candia-Carnevali and colleagues (Candia-Carnevali et al. 1998; Candia-Carnevali and Bonasoro 2001) have described the dedifferentiation of myocytes that occurs during crinoid arm-explant regeneration. In this preparation, myocytes at different stages of dedifferentiation can be observed in the intermediate part of the stump. In some cases, dedifferentiated contractile systems similar to SLSs together with many undifferentiated cells have been observed in the coelomic canals of the arms during the first 2 weeks of regeneration. Therefore, in all these cases, as with the present example, muscle dedifferentiation results in the formation and eventual elimination of SLSs.
Muscle cell dedifferentiation is not a process unique to echinoderms. This process appears to play a key role in tissue and organ regeneration in both invertebrates (Fontes et al. 1983; Cornec et al. 1987) and vertebrates (Hay 1959; Lo et al. 1993; Kumar et al. 2000; Brockes and Kumar 2002; Echeverri et al. 2001; Echeverri and Tanaka 2002; Tanaka 2003). For example, during annelid anterior traumatic regeneration, muscle cells undergo dedifferentiation by shedding their contractile machinery (Fontes et al. 1983). This is characterized by the presence of sarcolemmal vesicles and plasmalemmal invaginations that separate the nucleated part of the cell from the part that contains the myofilaments. Disruption of the contractile systems results in the formation of sarcolytes (their equivalent of SLSs), which are then phagocytosed by coelomocytes or released into the coelom (Fontes et al. 1983; Cornec et al. 1987; Vetvicka et al. 1994).
In the case of vertebrates, muscle cell dedifferentiation has been demonstrated to occur in amphibian limb and tail regeneration by the use of both ultrastructural studies (Hay 1959) and transplantation experiments (Lo et al. 1993; Kumar et al. 2000; Echeverri et al. 2001). In this model system, cells of limb skeletal muscle have been shown to dedifferentiate by losing their myofibrils. Indeed, during the first week of regeneration, cells can be observed in the process of dedifferentiation with the separation of the cytoplasm surrounding the nuclei from the contractile apparatus. Therefore, dedifferentiation of muscle cells observed as the disruption of the muscle cell contractile apparatus and its separation from myocyte nuclei are common mechanisms associated with regenerative processes.
The extent of the role(s) played by epimorphic and morphollactic mechanisms in regeneration processes, particularly within the echinoderms, is controversial (García-Arrarás et al. 1998; García-Arrarás and Greenberg 2001; Sanchez Alvarado 2000). In this respect, the finding that muscle dedifferentiation occurs during the initial weeks of intestinal regeneration provides support for a morphollactic mechanism. Moreover, since muscle dedifferentiation appears to occur in animals from different phyla, morphollactic mechanisms may be common to the regenerating processes of different metazoa.
Possible fates of dedifferentiated muscle cells
What is the fate of the dedifferentiated myocytes? If these myocytes do indeed undergo dedifferentiation, their fate should be determined. However, this is not easily established, and various possibilities can be proposed.
First, dedifferentiated myocytes may lose their differentiated cell characteristics to proceed into cell division, form new cells needed for the regenerating structure, and later differentiate into new myocytes producing new myofilaments. This possibility is favored by the finding that, as the mesentery thickenings are formed and continue to grow during the second and third stages of regeneration, new myocytes are needed to form part of the mesothelial lining. In addition, previous experiments from our group have shown that cell division mainly occurs in the mesothelium and peaks during the second week of regeneration (García-Arrarás et al. 1998), thus suggesting that the dedifferentiated cells in this tissue are also those that proliferate. Holothurian studies in Cuvierian tubule regeneration have also indicated that undifferentiated mesothelial cells probably divide and give rise to new myocytes (VandenSpiegel et al. 2000).
A second possibility is that dedifferentiated cells give rise to undifferentiated cells that might play an important role in regeneration processes. Dedifferentiating myocytes appear to detach from the apical coelomic epithelial cells and show a high nucleus-to-cytoplasm ratio. Their membranes exhibit plasmalemmal invaginations that are directed to the area of separation between the nucleated part of the cell and the contractile area, and that do not contain any traces of myofibrils in their cytoplasm. Similar observations have been made during the regeneration of holothurian longitudinal muscle bands (Dolmatov and Ginanova 2001). Therefore, dedifferentiated myocytes with a high nucleus-to-cytoplasm ratio might become the undifferentiated cells with a high nucleus-to-cytoplasm ratio observed during the second regeneration stage, as Candia-Carnevali and Bonasoro (2001) have suggested occurs during crinoid arm-explant regeneration (García-Arrarás and Greenberg 2001). Finally, undifferentiated cells might migrate into the regenerating structure and give rise to muscle and other cellular phenotypes. However, the degree of multipotentiality of these cells is not known.
The formation of undifferentiated cells by muscle dedifferentiation has also been shown to occur during vertebrate limb and tail regeneration and annelid traumatic regeneration. In the case of vertebrate regeneration, muscle dedifferentiation results in the formation of undifferentiated mesenchymal cells (also called mononucleated cells) characterized by the loss of all myofibrils, a high nucleus-to-cytoplasm ratio (resulting from the loss of cytoplasm by fragmentation), large round to oval nuclei with nucleoli, and scant cytoplasm (Hay 1959; Lo et al. 1993; Kumar et al. 2000; Echeverri et al. 2001). Moreover, the dedifferentiation of annelid muscle cells results in the formation of dividing undifferentiated cells that migrate to form the blastema (Fontes et al. 1983). Therefore, a common finding in regenerative systems seems to be the contribution that dedifferentiation of myocytes make to the formation of undifferentiated cells with a high nucleus-to-cytoplasm ratio.
Finally, a third but nonetheless important possibility is that some myocytes undergo apoptosis. Indeed, a small number of cells in the mesothelium of the mesentery have been observed to undergo apoptosis, as shown by their nuclei with condensed chromatin and invaginated nuclear membrane. However, the number of apoptotic cells observed is extremely small, and some of them might be apoptotic peritoneocytes. Apoptosis has previously been shown to occur during regeneration. For example, some apoptotic cells have been observed in crinoid arm-regeneration studies (Candia Carnevali and Bonasoro 2001). Furthermore, studies of urodele limb regeneration have suggested that a number of dedifferentiated muscle cells near the area of amputation or of severe tissue damage undergo apoptosis instead of contributing to the regenerating structure (Lo et al. 1993; Echeverri et al. 2001). Therefore, in all regenerating systems, a percentage of muscle cells undergoes apoptosis instead of contributing to the formation of other cell populations that have a definite role in the regenerating process.
Formation of new mesentery muscle cells
Our results also shed some light on the origin of the new muscle layer. Previous immunofluorescence experiments have indicated that, in the regenerating intestine, some cells containing myofibrils in their cytoplasm migrate from the coelomic epithelia into the underlying area in which the muscle layer will be formed (Murray and García-Arrarás 2004); therefore, the muscle layers in the regenerating structure might be derived from dividing coelomic epithelial cells. Ultrastructural results also support the idea that the new muscle layer originally comes from the coelomic epithelium by showing that cells with uncommon basal processes that contain myofilaments are also connected to apical coelomic epithelial cells by spot desmosomes. According to Rieger and Lombardi (1987), spot desmosomes are commonly found connecting adjacent myocytes. In the case of the holothurian myoepithelium, Rieger and Lombardi (1987) report the presence of spot desmosomes between adjacent coelomic epithelial cells. As no desmosomes are observed to connect coelomic epithelial cells to adjacent myocytes, this suggests that this type of cell has an intermediate morphology typical of myoepithelial cells (Rieger and Lombardi 1987) and eventually forms the basal myocytes. Formation of the new muscle layer by the coelomic epithelium has also been reported previously in other echinoderm species. This includes the regeneration of the longitudinal muscle band in E. fraudatrix and A. japonicus (Dolmatov and Ginanova 2001) and the regeneration of the muscle layer in the Cuvierian tubules of Holothuria forskali (VandenSpiegel et al. 2000).
Concluding remarks
Our proposed model of intestinal regeneration is as follows. During the first week of regeneration, myocytes close to the ruptured edge of the intestine undergo a process of dedifferentiation in which muscle nuclei become activated, cell components are lost, and the contractile components become disrupted, forming SLSs. These SLSs are possibly degraded in the dedifferentiated myocyte cytoplasm or discarded from the cell. SLSs are also acquired by the coelomic epithelial cells and eliminated into the coelom and by phagocytic amoebocytes that phagocytose connective tissue and nerve fiber debris. Dedifferentiated myocytes either enter cell division and produce new myofibrils or might become cells with a high nucleus-to-cytoplasm ratio and scant cytoplasm. Meanwhile, a small number of dedifferentiated cells undergo apoptosis. Muscle dedifferentiation occurs gradually during the first 2 weeks of regeneration. Eventually, mesentery coelomic epithelial cells begin to form the new mesentery muscle layer.
Although previous regeneration studies in other echinoderms have shown the importance of muscle dedifferentiation during regenerative processes, this is the first time that this process has been shown to occur during holothurian intestinal regeneration, especially that involving the dedifferentiation of mesentery muscle cells. Furthermore, these results provide additional evidence that muscle dedifferentiation, including myocyte disruption and fragmentation and the formation of SLSs, is a general mechanism in both vertebrate and invertebrate regenerative processes.
References
Brockes JP, Kumar A (2002) Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev 3:566–574
Byrne M (1985) Evisceration behavior and the seasonal incidence of evisceration in the holothurian Eupentacta quinquesemita (Selenka). Ophelia 24:91–101
Byrne M (2001) The morphology of autotomy structures in the sea cucumber Eupentacta quinquesemita before and during evisceration. J Exp Biol 204:849–863
Candia-Carnevali MD, Bonasoro F (2001) Microscopic overview of crinoid regeneration. Microsc Res Tech 55:403–426
Candia-Carnevali MD, Bonasoro F, Patrono M, Thorndyke MC (1998) Cellular and molecular mechanisms of arm regeneration in crinoid echinoderms: the potencial of arm explants. Dev Genes Evol 208:421–430
Cornec JP, Cresp J, Delye P, Hoarau F, Reynaud G (1987) Tissue responses and organogenesis during regeneration in the oligochete Limnodrilus hoffmeisteri (Clap.). Can J Zool 65:403–414
Dolmatov IY (1992) Regeneration of aquapharyngeal complex in the holothurian Eupentacta fraudatrix (Holothuria, Dendrochitrota). Monogr Dev Biol 23:40–50
Dolmatov IY, Ginanova TT (2001) Muscle regeneration in holothurians. Microsc Res Tech 55:452–463
Dolmatov IY, Eliseikina MG, Bulgakov AA, Ginanova TT, Lamash NE, Korchagin VP (1996) Muscle regeneration in the holothurian Stichopus japonicus. Rouxs Arch Dev Biol 205:486–493
Echeverri K, Tanaka EM (2002) Mechanisms of muscle dedifferentiation during regeneration. Semin Cell Dev Biol 13:353–360
Echeverri K, Clarke JDW, Tanaka EM (2001) In vivo imaging indicates muscle fiber dedifferentiation is a major contributor to the regenerating tail blastema. Dev Biol 236:151–164
Emson RH, Wilkie IC (1980) Fission and autotomy in echinoderms. Oceanogr Mar Biol Annu Rev 18:155–250
Feral JP, Massin C (1982) Digestive systems: Holothuroidea. In: Jangoux M, Lawrence JM (eds) Echinoderm nutrition. Balkema, Rotterdam, pp 191–212
Fontes M, Coulon J, Delgrossi MH, Thouveny Y (1983) Muscle dedifferentiation and contractile protein synthesis during post-traumatic regeneration by Owenia fusiformis (polychaete annelid). Cell Differ 13:267–282
García-Arrarás JE, Greenberg MJ (2001) Visceral regeneration in holothurians. Microsc Res Tech 55:438–451
García-Arrarás JE, Estrada-Rodgers L, Santiago R, Torres II, Díaz-Miranda L, Torres-Avillán I (1998) Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea: Echinodermata). J Exp Zool 281:288–304
García-Arrarás JE, Díaz-Miranda L, Torres-Vázquez I, File S, Jiménez L, Rivera-Bermudez K, Arroyo E, Cruz W (1999) Regeneration of the enteric nervous system in the sea cucumber Holothuria glaberrima. J Comp Neurol 406:461–475
García-Arrarás JE, Rojas-Soto M, Jiménez LB, Díaz-Miranda L (2001) The enteric nervous system of echinoderms: unexpected complexity revealed by neurochemical analysis. J Exp Biol 204:865–873
Hay ED (1959) Electron microscopic observations of muscle dedifferentiation in regeneration Amblystoma limbs. Dev Biol 1:555–585
Hyman L (1955) The invertebrates: Echinodermata. McGraw-Hill, New York
Kumar A, Velloso CP, Imokawa Y, Brockes JP (2000) Plasticity of retrovirus-labelled myotubes in the newt limb regeneration blastema. Dev Biol 218:125–136
Leibson NL (1992) Regeneration of digestive tube in holothurians Stichopus japonicus and Eupentacta fraudatrix. Monogr Dev Biol 23:51–61
Lo DC, Allen F, Brockes JP (1993) Reversal of muscle differentiation during urodele limb regeneration. Proc Natl Acad Sci USA 90:7230–7234
Mashanov VS, Dolmatov IY (2001) Regeneration of digestive tract in the pentactulae of the far-eastern holothurian Eupentacta fraudatrix (Holothuroidea: Dendrochirota). Invert Reprod Dev 39:143–151
Murray G, García-Arrarás JE (2004) Myogenesis during holothurian intestinal regeneration. Cell Tissue Res 318:515–524
Quiñones JL, Rosas R, Ruiz DC, García-Arrarás JE (2002) Extracellular matrix remodeling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Dev Biol 250:181–197
Rieger RM, Lombardi J (1987) Ultrastructure of the coelomic lining in echinoderm podia: significance for concepts in the evolution of muscle and peritoneal cells. Zoomorphology 107:191–208
Sánchez Alvarado A (2000) Regeneration in the metazoans: why does it happen? BioEssays 22:578–590
Smiley S (1994) Holothuroidea. In: Harrison F, Chia FS (eds) Microscopic anatomy of invertebrates, vol 14. Wiley Liss, New York, pp 401–471
Tanaka EM (2003) Cell differentiation and cell fate during urodele tail and limb regeneration. Curr Opin Genet Dev 13:497–501
VandenSpiegel D, Jangoux M, Flammang P (2000) Maintaining the line of defense: regeneration of Cuvierian tubules in the sea cucumber Holothuria forskali (Echinodermata, Holothuroidea). Biol Bull 198:34–49
Vetvicka V, Sima P, Cooper EL, Bilej M, Roch P (1994) Immunology of annelids. CRC Press, Boca Raton
Acknowledgements
We thank Griselle Valentin for technical assistance with the immunocyochemical experiments and Mr. Camillo Cangani for assistance with the transmission electron microscope.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by NSF (IBN-0110692) and NIH-MBRS (S06GM08102). We also acknowledge partial support from RCMI (RRO-3641-01), the Department of Biology, and the University of Puerto Rico.
Rights and permissions
About this article
Cite this article
Candelaria, A.G., Murray, G., File, S.K. et al. Contribution of mesenterial muscle dedifferentiation to intestine regeneration in the sea cucumber Holothuria glaberrima . Cell Tissue Res 325, 55–65 (2006). https://doi.org/10.1007/s00441-006-0170-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0170-z