Abstract
Whole animal studies have indicated that Ca2+ uptake by the gastrointestinal tract is regulated by the action of parathyroid hormone-related peptide (PTHrP) in teleost fish. We have characterised PTH receptors (PTHR) in piscine enterocytes and established, by using amino-terminal PTHrP peptides, the amino acid residues important for receptor activation and for stabilising the ligand/receptor complex. Ligand binding of 125I-(1–35tyr) PTHrP to the membrane fraction of isolated sea bream enterocytes revealed the existence of a single saturable high-affinity receptor (K D=2.59 nM; B max=71 fmol/mg protein). Reverse transcription/polymerase chain reaction with specific primers for sea bream PTH1R and PTH3R confirmed the mRNA expression of only the later receptor. Fugu (1–34)PTHrP increased cAMP levels in enterocytes but had no effect on total inositol phosphate accumulation. The amino-terminal peptides (2–34)PTHrP, (3–34)PTHrP and (7–34)PTHrP bound efficiently to the receptor but were severely defective in stimulating cAMP in enterocyte cells indicating that the first six residues of piscine (1–34)PTHrP, although not important for receptor binding, are essential for activation of the adenylate cyclase/phosphokinase A (AC-PKA)-receptor-coupled intracellular signalling pathway. Therefore, PTHrP in teleosts acts on the gastrointestinal tract through PTH3R and the AC-PKA intracellular signalling pathway and might regulate Ca2+ uptake at this site. Ligand-receptor binding and activity throughout the vertebrates appears to be allocated to the same amino acid residues of the amino-terminal domain of the PTHrP molecule.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parathyroid hormone-related peptide (PTHrP) was recently identified, characterised and cloned in the sea bream (Sparus auratus; Flanagan et al. 2000). Multiple sequence alignment of the amino acid sequence from man, chicken, mouse, rat, dog, pig and sea bream shows that the N-terminus is conserved and that the fish protein shares 52% identity with higher vertebrate PTHrP in this region (Flanagan et al. 2000). Results from recent physiological studies with sea bream larvae in seawater have revealed that N-terminal 1–38 pufferfish or fugu (Takifugu rubripes) PTHrP (Power et al. 2000) promotes an increase in calcium uptake from the surrounding medium (Guerreiro et al. 2001), indicating a possible hypercalcaemic action for this part of the peptide. Moreover, in common with observations in mammals, the N-terminal region of (1–34)PTHrP has been suggested to contain the major determinants necessary for this action.
In mammals, PTHrP is produced by most cell types and gives rise to a family of mature secretory forms arising from post-translational endoproteolytic cleavage of the initial translation product. Most of these secretory forms of PTHrP have been shown to act as paracrine or autocrine factors (for reviews, see Jans et al. 2003; Papp and Stewart 1993; Wysolmerski and Stewart 1998). The activity of PTHrP has been shown to be mediated by its binding to a group of seven specific transmembrane G-protein-coupled receptors that signal via the cAMP/protein kinase A(PKA) and phospholipase-C/PKC pathway (Fujimori et al. 1992; Juppner et al. 1991).
PTH/PTHrP receptors have been isolated from zebrafish (Danio rerio) and the three receptors identified have been classified as PTH1R, PTH2R and PTH3R on the basis of sequence homology with mammalian receptors and the binding characteristics of mammalian ligands to the cloned receptors (Rubin and Juppner 1999; Rubin et al. 1999). However, the presence of these receptors in other teleosts, the tissue specific-distribution of the receptors and their in vitro binding characteristics with piscine PTHrP remain to be characterised.
For sea bream in full seawater, approximately 60% of calcium uptake is calculated to take place via the intestine; this uptake can be modified by PTHrP (Guerreiro et al. 2001) suggesting that the intestine might be a good candidate tissue in which to identify and characterise PTH receptors. The objective of the present study has been to establish whether PTH receptors are expressed in the sea bream intestine, to determine the ligand-binding relationships of the receptor(s) to N-terminal PTHrP peptides and to identify the amino acid residues important for receptor activation and those important for stabilising the ligand/receptor complex.
Materials and methods
Peptides
The amino-terminally truncated forms of pufferfish “fugu” (Takifugu rubripes) (1–34)PTHrP, (2–34)PTHrP, (3–34)PTHrP and (7–34)PTHrP and sea bream (79–93)PTHrP and (100–125)PTHrP were synthesized by Genemed Synthesis (San Francisco, Calif.). Sea bream and fugu (1–34)PTHrP only differ at amino acid residues 19 and 26. Flounder (1–35tyr)PTHrP, which only differs from sea bream at amino acid residue 25, was synthesized at the University of Sheffield (Krebs Institute Molecular Synthesis Service, on an Applied Biosystems Model 476A peptide synthesizer). Human (1–34)PTH and human (1–34)PTHrP were purchased from Sigma-Aldrich. Radiolabelling and purification of the flounder [Tyr35]PTHrP(1–35) peptide (hereafter referred to as piscine [Tyr35]PTHrP(1–35)) was performed by using 125I-Na (2,200 Ci/mmol, Amersham Pharmacia Biosciences, Lisbon, Portugal) and 1,3,4,6-tetrachloro-3α-6α diphenylglucoluril (Pierce, USA) as previously described (Rotllant et al. 2003).
Animals
Immature male sea bream weighing 80–130 g were obtained from a commercial source (Viveiro Vilanova, Vilanova de Milfontes, Portugal). The fish were kept under natural conditions of photoperiod (14L:10D) and water temperature (18–24°C), in 1,000-l tanks at a density of 4 kg.m−3. The water was continuously renewed (250 l.h−1) and aerated. Fish were fed daily with the equivalent of 1% of their body weight in commercial dry pellets (PROVIMI, Alverca, Portugal). Prior to sampling, fish were deprived of food for 24 h.
Enterocyte isolation and membrane fraction preparation
The preparation of intestinal cells enriched in enterocytes was carried out with a modification of the method described by Del Castillo (1987) and Dopido et al. (2004) and yielded approximately 97% enrichment. In brief, the fish were killed by decapitation and the intestines were rapidly removed and rinsed in a sterile Ringer saline solution. Subsequently, a more thorough wash was carried out by filling the intestines with solution 1 (7 mM K2SO4, 44 mM K2HPO4, 9 mM NaHCO3, 10 mM HEPES-TRIS, and 180 mM glucose at pH 7.4) and incubating them for 10 min at 20°C. This procedure was repeated twice. The intestine was then filled with solution 2, which had an identical composition to solution 1 but also contained 0.5 mM dithiothreitol and 0.2 mM EDTA, and the two ends of the intestine were clamped with forceps. The segment was then gently massaged for 3 min. The luminal contents, which contained the isolated cells, were diluted in two volumes of solution 3 (116 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgSO4, 10 mM NaHCO3, 1 mM NaH2PO4, 10 mM K2SO4, 10mM HEPES, pH: 7.4), filtered through a nylon membrane of 60–100 μm pore size and then centrifuged at 1500 rpm for 15 min at 4°C. The supernatant was discarded and the pellet containing the cells was resuspended in 10 ml solution 3 supplemented with 1 mg collagenase type IV (Sigma-Aldrich, Madrid, Spain) and incubated at 25°C with gentle agitation for 15 min. The resulting cell suspension was diluted in 20 ml solution 3, filtered through a 60–100 μm pore size nylon membrane and centrifuged at 1500 rpm for 15 min at 4°C. The pellet was then resuspended in the desired volume of L-15 medium (Sigma-Aldrich) and the cells were maintained in this medium at 4°C without agitation until used in experiments (generally 1 h after their preparation). Viability of the isolated enterocytes was determined by using the trypan blue exclusion method. Cell viability was found to be 94±0.8% at the end of the isolation procedure.
Membrane fractions were prepared according to Yasuoka et al. (1996). Briefly, the enterocyte cells were collected in TE buffer (50 mM TRIS-HCl, 1 mM EDTA, pH 7.4) supplemented with sucrose (0.25 M). The cell suspension was homogenized in a Teflon-glass homogenizer and centrifuged at 3,000 rpm for 10 min at 4°C and the supernatant was collected. The pellet was resuspended in TE buffer and centrifuged at 3,000 rpm for 10 min at 4°C. The supernatants were combined and centrifuged at 30,000 rpm for 30 min at 4°C. The pellet was washed by gentle mixing in 15 volumes of TE buffer. The mixture was centrifuged at 30,000 rpm for 30 min at 4°C and the resulting pellet corresponding to the membrane fraction was resuspended in TE buffer. Aliquots were stored at −80°C. The protein concentration of the membranes was determined by using the Lowry method (Lowry et al. 1951).
Binding assay
Binding assays were performed as previously reported for avian cell membranes (Ieda et al. 2000; Yasuoka et al. 1996). All assays were carried out in triplicate at 4°C for 2 h in microcentrifuge tubes pre-treated with TE supplemented with 2% bovine serum albumin (Sigma-Aldrich) and with 30 μg protein/tube of membrane fractions in TE buffer to give a final incubation volume of 300 μl. For saturation binding experiments, the membrane fractions were incubated with increasing concentrations of piscine 125I-[Tyr35]PTHrP(1–35) in the presence or absence of 1 μM unlabelled fugu (1–34)PTHrP. For competitive binding experiments, membrane fractions were incubated with labelled piscine 125I-[Tyr35]PTHrP(1–35) (25–30×103 cpm) and various concentrations of unlabelled peptide fragments of fugu PTHrP [(1–34)PTHrP, (2–34)PTHrP, (3–34)PTHrP, (7–34)PTHrP], flounder (1–34)PTHrP, sea bream (79–93)PTHrP and sea bream (100–125)PTHrP. Reactions were stopped by centrifuging tubes at 10,000 rpm for 10 min at 4°C. The resulting pellet was rinsed in 1 ml TE buffer and centrifuged (10,000 rpm for 10 min at 4°C) and the radiolabelled piscine 125I-[Tyr35]PTHrP(1–35) bound to the pellet was measured by counting in a gamma counter (Wallac, 1470 Wizard, Pharmacia).
The means of triplicate determinations of four independent experiments were used as individual points for the Scatchard analysis (Scatchard 1949) in order to calculate the equilibrium dissociation constant (Kd) and the maximum binding capacity (Bmax).
RNA isolation and reverse transcription/polymerase chain reaction
Sea bream intestinal cells, scales and a fragment of liver and kidney were collected and immediately frozen in liquid nitrogen. Total RNA was extracted using TRI-reagent (Sigma-Aldrich). After extraction, the RNA concentration was determined in a spectrophotometer (GeneQuant, Amersham Pharmacia Biosciences) followed by size fractionation on a TBE buffer/1.5% agarose gel (TBE buffer = 0.09 M TRIS-borate, 0.002 M EDTA, pH 8.3) to evaluate RNA quality.
First-strand cDNA was synthesised from 3 μg total RNA in a 40-μl reaction volume (0.05 M TRIS-HCl pH 8.3, 0.075 M KCl, 3 mM MgCl2, 5 mM dithiothreitole, 0.25 mM dNTP mix, 4 μg random hexamers (pd(N)6), 8U RNAse Guard) with reverse transcriptase (40 U M-MLV; Gibco-BRL, Barcelona, Spain), for 1 h 30 min at 37°C. In negative control reactions, the enzyme was omitted and confirmed the absence of genomic contamination in the subsequent polymerase chain reaction (PCR) amplification (results not shown). Specific reverse transcription/PCRs (RT-PCRs) for PTHrP, PTH3R and PTH1R detection were carried out in an iCycler thermocycler (BioRad, Lisbon, Portugal) with cDNA (0.5 μg) prepared from intestinal cells, scale, liver and kidney as template in a 50-μl reaction volume (10 mM TRIS-HCl pH 9.0, 50 mM KCl, 0.1% Triton X-100, 2 mM MgCl2, 0.2 mM dNTP mix) containing 10 pmol of each of the forward and reverse primers and Taq DNA polymerase (1.25 U; Promega, Madison, USA). In negative control reactions, cDNA was substituted by sterile water. In positive control reactions, cloned target gene cDNA was amplified with the specific primer pairs used in PCRs (results not shown). Sea bream specific primers used for amplification of sea bream PTHrP were PTHrPfw (5′-GAATTCAGGAGGTCAGTGAGCCAC-3′) and PTHrPrv (5′-AACAGACCGTGCCCGCCTCCTCTTCTTGTC-3′) and yielded a single 371-bp product. The thermocycle used was as follows: 2 min at 95°C, 35 cycles of 1 min at 95°C, 1 min at 62°C and 1 min at 72°C and, finally, 5 min at 72°C. The sea-bream-specific primers utilised to amplify PTH3R were designed by using the sequence of a partial clone of the receptor (accession no. AY547261). The primers employed were PTH3Rfw (5′-ACATCCACATTCACTTCTTCAC-3′) and PTH3Rrv (5′-CTTCATGGCTTCCTCTCTGACA-3′); they amplified a product of 250 bp. The thermocycling protocol was 2 min at 96°C, 40 cycles of 1 min at 96°C, 1 min 30 s at 63°C, 2 min at 72°C and, finally, 5 min extension at 72°C. The sea-bream-specific primers utilised to amplify PTH1R were designed by using the sequence of a partial clone of the receptor (accession no. AJ619023). The primers used to amplify PTH1R were PTH1Rfw (5′-TCACCAACGTCACTGCCAGAGGA-3′) and PTH1Rrv (5′-TGTCCCGACGAGGGTATCGAGTT-3′) and amplified a 142-bp fragment. The thermocycle was identical to that used for PTHrP amplification with the exception of the annealing temperature, which was 55°C. Amplification of the three target genes was carried out by using the same cDNA synthesis reaction and RT-PCR was repeated with several different samples to confirm reproducibility. The quantity of cDNA used in PCRs was standardised and was confirmed by amplification of ribosomal RNA 18S. The primer pair utilised was 18sFw: 5′-TCAAGAACGAAAGTCGGAGG-3′ and 18sRv: 5′-GGACATCTAAGGGCATCACA-3′ and the reaction conditions and thermocycling protocol were: a denaturing step at 95°C for 2 min, followed by 20 cycles at 95°C for 30 s, 55°C for 30 s, 72°C for 45 s and a final elongation step at 72°C for 5 min.
PCR products of PTHrP, PTH1R and PTH3R were purified with GFX PCR DNA and a Gel Band Purification Kit (Amersham Biosciences, Lisbon, Portugal) and cloned into pGem-T-easy plasmid (Promega). Plasmid DNA was prepared by using the alkaline lysis method and the inserts were sequenced with T7 or SP6 primers (Macrogen, Seoul, South Korea). DNA sequences were analysed with Genedoc (Nicholas et al. 1997) and their probable identity was confirmed by searching GenBank (http://www.ncbi.nih.nlm.gov) with BLASTX (Altschul et al. 1990).
Measurements of cAMP production
To measure cAMP production, enterocytes were plated in 24-well plates at 2×106 cells.well−1 per millilitre in minimal essential media (MEM; Sigma-Aldrich). Cells were incubated for 30 min in MEM containing 1 mM 3-isobutyl-1-methylxanthine (IMBX; Sigma-Aldrich), a phosphodiesterase inhibitor that prevents enzymatic degradation of cAMP. Cells were subsequently incubated for 30 min in MEM-IMBX in the presence or absence of 10−6 M, 10−7 M, 10−8 M, 10−9 M fugu (1–34)PTHrP, 10−6 M fugu (2–34)PTHrP, fugu (3–34)PTHrP and fugu (7–34)PTHrP, 10−6 M flounder (1–34)PTHrP, 10−6M human (1–34)PTHrP and 10−6M human (1–34)PTH. To stop reactions, microplates were centrifuged (3,000 rpm for 10 min at 4°C), the medium was aspirated and the cell pellet was resuspended in 0.01 M PBS with 4 mM EDTA. Subsequently, the samples were sonicated, an aliquot was taken for total protein determination and the remainder was boiled and centrifuged at 5,000 rpm for 5 min. The supernatant was collected and stored at −20°C until cAMP determination with the Biotrak TRK 432 kit (Amersham Biosciences). Total protein was determined by using the Lowry method. Results are reported as picomoles cAMP per milligram total protein. A selective adenylyl-cyclase activator forskolin (1 μM) was used as a positive control as it increases intracellular concentrations of cAMP. Furthermore, an adenylyl-cyclase-specific inhibitor (SQ-22536, Sigma-Aldrich) at a concentration of 10−4M (Rotllant et al. 2005) was used to block the increase in the intracellular level of cAMP in sea bream enterocytes. Cell viability decreased from 94±0.8% to 92±0.5% after 30 min of incubation at 20°C, indicating that only a small reduction in cell viability had occurred. The results presented are the means of triplicate determinations of four independent experiments.
Measurement of [H3]myo-inositol incorporation
[H3]Myo-inositol incorporation was evaluated following the method of Ramirez et al. (1999). Enterocytes were plated in 24-well plates at 2×106 cells.well−1 per millilitre MEM. After 1 h pre-incubation in MEM, cells were incubated for 30 min in MEM containing [H3]myo-inositol (1 μCi.ml−1; 14 Ci.mmol−1; 37 MBq.ml−1; Amersham Bioscience, UK) in the absence (control) or presence of fugu (1–34)PTHrP, fugu (2–34)PTHrP, fugu (3–34)PTHrP, fugu (7–34)PTHrP and 10−6 M flounder (1–34)PTHrP. The incubates were centrifuged (5,000 rpm for 5 min), the supernatant was removed, the pellet was resuspended in 500 μl ice-cold 10% trichloroacetic acid and the contents were placed in Eppendorf tubes and sonicated. An aliquot was taken for total protein determination and the remainder was centrifuged at 12,000 rpm for 15 min at 4°C. The supernatant, which contained inositol phosphates (IPs), was collected and measured in a scintillation counter (Model LS6000IC, Beckman Instruments, Fullerton, USA). Chloroform-methanol (2:1, v/v) was then added to the pellet to extract the phosphoinositides (PIPs). The mixture was centrifuged at 12,000 rpm for 15 min at 4°C and the supernatant was also counted. Results obtained for IPs and PIPs showed a parallel synthesis profile and therefore counts obtained for both were added together; the results are reported as the total [H3]myo-inositol incorporated in each sample and expressed as CPM.mg−1 protein. The results presented are the means of triplicate determinations of four independent experiments.
Statistics
Results are presented as mean±SEM. Scatchard plots and competitive binding were analysed with the pharmacology module of SigmaPlot (version 8.0, SPSS, Chicago, USA). The effect of peptides and inhibitors on intracellular cAMP synthesis and [H3]myo-inositol incorporation was analysed by one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls (SNK) test (INSTAT tm, GraphPad Software V2.04a). Significance was considered at P<0.05.
Results
Scatchard analysis of piscine 125I-[Tyr35]PTHrP(1–35) binding to isolated sea bream enterocyte membrane preparations indicated the existence of a single class of binding sites (Fig. 1). The Bmax of the receptor in sea bream enterocytes is 71 fmol.mg−1 protein and has an apparent Kd of 2.59 nM (Fig. 1). Binding of 125I-(1–35Tyr) PTHrP to the enterocyte membrane was reduced by the addition of 10–1,000 M excess of the unlabelled peptides fugu (1–34)PTHrP, fugu (2–34)PTHrP, fugu (3–34)PTHrP, fugu (7–34)PTHrP and flounder (1–34)PTHrP. All these peptides were equipotent in their capacity to displace piscine 125I-[Tyr35]PTHrP(1–35) from enterocyte membrane fractions indicating that amino-terminal deletions in teleost PTHrP, such as removal of up to the first six residues (7–34)PTHrP did not affect the ability of these truncated forms to bind to enterocyte membrane fractions (Fig. 2). In contrast, neither (79–93)PTHrP or (100–125)PTHrP were able to displace piscine 125I-[Tyr35]PTHrP(1–35) from enterocyte membrane fractions (Fig. 1). These results suggested that the first six amino acids of the N-terminus of PTHrP or the amino acids in the mid- and carboxy-terminal regions of intact PTHrP were unimportant for stabilising the interaction between ligand and receptor.
Binding of N-terminal (1–34)PTHrP and truncated forms of (79–93)PTHrP and (100–125)PTHrP to sea bream enterocyte membrane fractions. Competitive binding of 125I-(1–35Tyr) PTHrP to enterocyte membrane fractions was carried out in the presence of increasing concentrations of competing unlabelled ligands fugu (1–34)PTHrP, fugu (2–34)PTHrP, fugu (3–34)PTHrP, fugu (7–34)PTHrP, flounder (1–34)PTHrP, sea bream (79–93)PTHrP and sea bream (100–125)PTHrP. Binding is expressed as the percent specific binding of radioligand added. Each value is the mean±SEM of four experiments in triplicate determinations
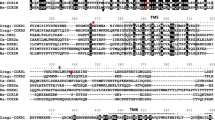
The results of the ligand-binding assays demonstrating the presence of a single PTHrP receptor in sea bream enterocyte membranes was confirmed by the results of RT-PCR with specific primers for PTH1R and PTH3R. A single type of PTHR was amplified in the intestine and only primers specific for PTH3R yielded a product (Fig. 3). Sequencing of the amplified product, followed by a BLAST search against the non-redundant translated nucleotide database in GeneBank, established that it shared greatest amino acid sequence similarity (48%) to zebrafish PTH3R (Fig. 4). As the amplified product encompasses extracellular loop 1 of the PTHRs, which is in general poorly conserved (Rubin et al. 1999), this may explain the limited homology with zebrafish PTH3R.
RT-PCR amplification products of sea bream PTHrP, PTH1R and PTH3R in mRNA extracts from scales (S), intestinal cells (I), kidney (K) and liver (L). Ribosomal RNA 18S amplification was used to standardise the quantity of total RNA in PCRs (− negative control in which cDNA was omitted from the reaction)
Amino acid sequence alignment of the TM2/TM3 region from zebrafish PTHR3 receptor and the isolated sea bream cDNA clone. BlastX interrogation of the non-redundant nucleotide database in GenBank with the seabream cDNA clone yielded 48% amino acid sequence identity with zPTH3R sequence (black boxes identical amino acid residues)
PTH3R was not amplified from scales, kidney or liver, although PTH1R transcripts were detected in these tissues but not in the intestine (Fig. 3). Transcripts encoding PTHrP were amplified in enterocytes (Fig. 3). No amplified product was detected in either of the control reactions indicating that PCR products did not arise from genomic contamination or reaction contamination (Fig. 3).
To evaluate PTHrP induced signal transduction in enterocyte cells, measurements of cAMP production and [H3]myo-inositol incorporation were determined in intact enterocyte cells incubated in the presence of fugu (1–34)PTHrP. Forskolin (1 μM), a selective activator of adenylyl-cyclase, greatly increased the intracellular level of cAMP in sea bream enterocytes confirming the viability and responsiveness of the cells (Fig. 5a). Fugu (1–34)PTHrP at 10−6 M stimulated the accumulation of cAMP in enterocytes (Fig. 5a) and this accumulation could be blocked by 10−4 M SQ-22536, an adenylyl-cyclase-specific inhibitor (Fig. 5b). Similar results were obtained with flounder (1–34)PTHrP (not shown). These results suggest that the receptor signals via the adenylyl-cyclase pathway. Based on the preceding results, a 10−6 M peptide concentration was used to evaluate the capacity of truncated PTHrP forms to activate the secondary messenger signalling pathways. As illustrated in Fig. 6a, no significant increase (P>0.05) above control levels of cAMP production was detected when intact enterocytes were incubated in the presence of 10−6 M fugu (2–34)PTHrP, (3–34)PTHrP and (7–34)PTHrP. Similar results were found when intact enterocytes were incubated in the presence of 10−6 M human (1–34)PTH or human (1–34)PTHrP or in the absence of peptide: control, 15.2±2.35; human PTHrP, 18.96±2.89; human PTH, 23.86±4.2 pmol cAMP. mg−1 protein (mean±SEM). The present results suggest that, in sea bream, removal of the first N-terminal amino acid of piscine PTHrP is sufficient to cause the loss of activation of cAMP production by the receptor and thus the biological activity stimulated via this pathway by N-terminal PTHrP peptides. In addition, no [H3]myo-inositol incorporation was detectable in enterocytes challenged with any of the ligands (Fig. 6b). The failure of human PTHrP to significantly stimulate cAMP accumulation in sea bream enterocytes can in part be explained by the substitution of the first N-terminal amino acid from Ser in fish to Ala in human, particularly since this position has been shown to be critical for receptor activation.
a Effect of various concentrations of fugu (1–34)PTHrP on cAMP production by sea bream enterocytes (C control, Fk forskolin). **P<0.01 vs. control, ***P<0.001 vs. control. b Effect of fugu (1–34)PTHrP at 10−6 M on cAMP production from dispersed sea bream enterocytes, and its reversal by SQ-22536 (10−4M). Each bar is the mean±SEM of duplicated determinations of four separate experiments. **P<0.01 vs. control
Discussion
The present study firmly establishes enterocytes isolated from the duodenum as a target for amino-terminal peptides of PTHrP in sea bream and provides strong evidence for evolutionary conservation of receptor-binding characteristics and signalling pathways.
Zebrafish, the only teleost in which PTH receptors have been isolated, possesses two receptor subtypes sharing sequence similarity respectively to mammalian PTH1R and PTH2R (Rubin and Juppner 1999; Rubin et al. 1999) together with a novel receptor, zPTH3R (Rubin and Juppner 1999), which does not appear to have a mammalian homologue. No information about the activity that the receptors mediate, their tissue distribution or their abundance is available in fish. In the present study, a single class of receptors has been identified in sea bream enterocytes and biochemical and sequence analysis suggest that it is most like zPTH3R. In mammals, PTH1R is the most frequently characterised receptor in non-skeletal tissues that interacts with amino-terminal fragments of PTHrP (Orloff et al. 1989, 1994); this is also the case for the intestine (Li et al. 1995), including the duodenum (Gentili et al. 2003; Watson et al. 2000).
The role of intestinal PTH receptors in mammals has not yet been demonstrated but is most probably related to the regulation of calcium absorption from the diet, possibly indirectly through its action on 1,25(OH)2 vitamin D3 (Potts et al. 1997; Silverberg et al. 1999). This is supported by recent studies showing that injections of PTHrP in piglets stimulate active duodenal calcium absorption (Klein et al. 2001). Moreover, in chicks, an N-terminal fragment of PTHrP (1–40) stimulates the rapid transport of Ca2+ in the perfused intestine; this effect can be inhibited by the Ca2+ channel inhibitor nifedipine, which is known to abolish the effect elicited by 1,25(OH)2 vitamin D3 (Zhou et al. 1992). The presence of PTH receptors in fish enterocytes, a site for mineral and nutrient exchange, suggests that this hormone may also act to modulate calcium transport across the intestinal wall. Previous studies in the sea bream indicate that intestinal calcium absorption may account for 60%–70% of total calcium uptake (Guerreiro et al. 2002). Although fugu (1–38)PTHrP administered to sea bream larvae as a bath strongly stimulates whole body calcium uptake, it also causes a reduction in drinking, so that specific effects in intestinal calcium transport could not be established (Guerreiro et al. 2001).
Ligand binding of fugu 125I-(1–35Tyr) PTHrP with sea bream enterocyte membrane fractions shows that the receptor has an apparent Kd of 2.59 nM, which is the same order of magnitude as those of zebrafish PTH1R and PTH3R when (1–36)Tyr36PTHrP derived from Fugu rubripes is used to displace [125I]-(Tyr36)human PTHrP-(1–36) (Rubin and Juppner 1999). In sea bream enterocytes, the single binding site characterised must reflect the presence of a PTH3R subtype of receptor, since RT-PCR amplifies a single product with the strongest sequence similarity to that of zPTH3R. PTH receptors signal principally via the adenylyl cyclase/protein kinase A (AC-PKA) signalling pathway and secondarily via the phospholipase-C/PKC intracellular Ca2+ signalling pathway (for a review, see Gardella and Juppner 2001). The receptor identified in sea bream enterocytes appears to activate a single pathway, viz. that of AC-PKA. A similar signalling mechanism has also been reported when COS-7 cells are transfected with zebrafish PTH3R and stimulated with mammalian PTHrP (Rubin et al. 1999). Additionally, cAMP accumulation is stimulated by fugu (1–34)PTHrP but not by human PTH or human PTHrP. The difference in response to human PTHrP observed between fish enterocytes and COS cells transfected with the zebrafish PTH3R (Rubin et al. 1999) is perplexing and may be a consequence of a range of factors. However, differences in the amino acid sequence of the receptors in sea bream and zebrafish may be a determinant factor in ligand binding and therefore receptor activation, as might the characteristics of the assays utilised to study receptor activation (e.g. transfected mammalian cells versus fish primary cell culture). Further work will be required to resolve this question.
Nevertheless, taken together, the similarity of the signalling profile between the sea bream enterocyte PTH receptor and zPTH3R, the specific amplification of a PTH3R-like product by RT-PCR and the ligand-binding affinity suggests that the enterocyte receptor and zPTH3R are most probably the same PTH receptor subtype. Therefore, in fish, PTH3R may be important in the regulation of calcium uptake across the gut. The PTH3R receptor, which is apparently absent in tetrapods, may have arisen during the teleost-specific genome duplication that has been proposed to have occurred (Meyer and Schartl 1999; Ohno et al. 1968) and the receptor may have evolved and acquired a specific function in fish.
Amino-terminal deletions, such as fugu (2–34)PTHrP, (3–34)PTHrP and (7–34)PTHrP do not affect the ability of these truncated forms to displace [125I]-(1–35tyr)PTHrP bound to enterocyte membrane fractions, indicating that the first six amino acids of the N-terminus of PTHrP are not important in stabilising the interaction between ligand and receptor. The (15–34) truncated form of human PTH and PTHrP can inhibit the binding of the corresponding 1–34 peptides to human PTH1R, thus demonstrating that this domain contains the principal determinants of receptor binding (Abou-Samra et al. 1989; Caulfield et al. 1990). Gardella et al. (1993) have shown that substitutions of Leu24 and Leu28 in PTH(1–34) reduce the binding affinity 100-fold, indicating that the hydrophobic face formed principally by Trp23, Leu24 and Leu28 plays a key role in receptor binding. In all known teleost PTHrP, these same residues (Trp23, Leu24 and Leu28) are conserved, suggesting that this region is a key domain for the receptor-ligand interaction across vertebrates.
With regard to the ligand determinants of receptor signalling, the N-terminal region of PTHrP is crucial for interactions with the common PTH/PTHrP receptor in bone and kidney of terrestrial vertebrates (Juppner et al. 1991), thereby evoking hypercalcemic effects. Furthermore, N-terminal truncated PTH or PTHrP analogues, such as (3–34)PTH, (7–34)PTH and (7–34)PTHrP, although binding to PTH1R with high affinity, have been shown to elicit little or no increase in cAMP accumulation (Gardella and Juppner 2001), thus demonstrating the importance of the first six amino acids in the determination of biological activity. Our own findings demonstrate that this is also the case in fish enterocytes and confirm the strong conservation of the structure and function of the PTH system between fish and higher vertebrates.
In conclusion, the results from the present study are consistent with PTHrP being a potential factor regulating gastrointestinal Ca2+ uptake via PTH3R and the AC-PKA intracellular signalling pathway. Ligand receptor binding and activity appears to be allocated throughout the vertebrates to the same amino acid residues of the amino-terminal domain of the PTHrP molecule.
References
Abou-Samra AB, Uneno S, Jueppner H, Keutmann H, Potts JT Jr, Segre GV, Nussbaum SR (1989) Non-homologous sequences of parathyroid hormone and the parathyroid hormone related peptide bind to a common receptor on ROS 17/2.8 cells. Endocrinology 125: 2215–2217
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Caulfield MP, McKee RL, Goldman ME, Duong LT, Fisher JE, Gay CT, DeHaven PA, Levy JJ, Roubini E, Nutt RF (1990) The bovine renal parathyroid hormone (PTH) receptor has equal affinity for two different amino acid sequences: the receptor binding domains of PTH and PTH-related protein are located within the 14–34 region. Endocrinology 127:83–87
Del Castillo JR (1987) The use of hyperosmolar, intracellular-like solutions for the isolation of epithelial cells from guinea-pig small intestine. Biochim Biophys Acta 901:201–208
Dópido R, Rodríguez C, Goméz T, Acosta NG, Díaz M (2004) Isolation and characterization of enterocytes along the intestinal tract of the gilthead seabream (Sparus aurata L.). Comp Biochem Physiol A 139:21–31
Flanagan JA, Power DM, Bendell LA, Guerreiro PM, Fuentes J, Clark MS, Canario AV, Danks JA, Brown BL, Ingleton PM (2000) Cloning of the cDNA for sea bream (Sparus aurata) parathyroid hormone-related protein. Gen Comp Endocrinol 118:373–382
Fujimori A, Cheng SL, Avioli LV, Civitelli R (1992) Structure-function relationship of parathyroid hormone: activation of phospholipase-C, protein kinase-A and -C in osteosarcoma cells. Endocrinology 130:29–36
Gardella TJ, Juppner H (2001) Molecular properties of the PTH/PTHrP receptor. Trends Endocrinol Metab 12:210–217
Gardella TJ, Wilson AK, Keutmann HT, Oberstein R, Potts JT Jr, Kronenberg M, Nussbaum SR (1993) Analysis of parathyroid hormone's principal receptor-binding region by site-directed mutagenesis and analog design. Endocrinology 132:2024–2030
Gentili C, Morelli S, de Boland AR (2003) Characterization of PTH/PTHrP receptor in rat duodenum: effects of ageing. J Cell Biochem 88:1157–1167
Guerreiro PM, Fuentes J, Power DM, Ingleton PM, Flik G, Canario AV (2001) Parathyroid hormone-related protein: a calcium regulatory factor in sea bream (Sparus aurata L.) larvae. Am J Physiol 281:R855–R860
Guerreiro PM, Fuentes J, Canario AV, Power DM (2002) Calcium balance in sea bream (Sparus aurata): the effect of oestradiol-17b. J Endocrinol 173:377–385
Ieda T, Takahashi T, Saito N, Yasuoka T, Kawashima M, Shimada K (2000) Changes in parathyroid hormone-related peptide receptor binding in the shell gland of laying hens (Gallus domesticus) during the oviposition cycle. Gen Comp Endocrinol 117:182–188
Jans DA, Thomas RJ, Gillespie MT (2003) Parathyroid hormone-related protein (PTHrP): a nucleocytoplasmic shuttling protein with distinct paracrine and intracrine roles. Vitam Horm 66:345–384
Juppner H, Abou-Samra A, Freeman M, Kong X, Schipani E, Richards J, Kolakowski L, Hock J, Potts J, Kronenberg H (1991) A G protein-linked receptor for parathyroid hormone and parathyroid hormone-relatyed peptide. Science 254:1024–1026
Klein C, Abbas SK, Stewart AF, Care A, Harmeyer J (2001) PTHrP (38–94NH2) probably stimulates active absorption of Ca2+ from the duodenum of piglets. 1st Joint Meeting: International Bone and Mineral Society and European Calcified Tissue Society, Comparative Endocrinology of Calcium Regulation, Madrid, p 13
Li H, Seitz PK, Thomas ML, Selvanayagam P, Rajaraman S, Cooper CW (1995) Widespread expression of the parathyroid hormone-related peptide and PTH/PTHrP receptor genes in intestinal epithelial cells. Lab Invest 73:864–870
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Meyer A, Schartl M (1999) Gene and genome duplications in vertebrates: the one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr Opin Cell Biol 11:699–704
Nicholas KB, Nicholas HB Jr, Deerfield DWI (1997) GeneDoc: analysis and visualization of genetic variation. EMBNEW.NEWS 4:14
Ohno S, Wolf U, Atkin NB (1968) Evolution from fish to mammals by gene duplication. Hereditas 59:169–187
Orloff JJ, Wu TL, Stewart AF (1989) Parathyroid hormone-like proteins: biochemical responses and receptor interactions. Endocr Rev 10:476–495
Orloff JJ, Reddy D, de Papp AE, Yang KH, Soifer NE, Stewart AF (1994) Parathyroid hormone-related protein as a prohormone: post-translational processing and receptor interactions. Endocr Rev 15:40–60
Papp A, Stewart A (1993) Parathyroid hormone-related protein, a peptide of diverse physiologic functions. TEM 4:181–187
Potts JT Jr, Gardella TJ, Juppner H, Kronenberg H (1997) The history of parathyroid hormone and its receptor: structure-based design of parathyroid hormone analogues. Osteoporos Int 7(Suppl 3):S169–S173
Power DM, Ingleton PM, Flanagan J, Canario AV, Danks J, Elgar G, Clark MS (2000) Genomic structure and expression of parathyroid hormone-related protein gene (PTHrP) in a teleost, Fugu rubripes. Gene 250:67–76
Ramirez JL, Castano JP, Torronteras R, Martinez-Fuentes AJ, Frawley LS, Garcia-Navarro S, Gracia-Navarro F (1999) Growth hormone (GH)-releasing factor differentially activates cyclic adenosine 3',5'-monophosphate- and inositol phosphate-dependent pathways to stimulate GH release in two porcine somatotrope subpopulations. Endocrinology 140:1752–1759
Rotllant J, Worthington GP, Fuentes J, Guerreiro PM, Teitsma CA, Ingleton PM, Balment RJ, Canario AVM, Power DM (2003) Determination of tissue and plasma concentrations of PTHrP in fish: development and validation of a radioimmunoassay using a teleost 1–34 N-terminal peptide. Gen Comp Endocrinol 133:146–153
Rotllant J, Guerreiro PM, Anjos L, Redruello B, Canario AVM, Power DM (2005) Stimulation of cortisol release by the N-terminus of teleost parathyroid hormone-related protein in interrenal cells in vitro. Endocrinology 146:71–76
Rubin DA, Juppner H (1999) Zebrafish express the common parathyroid hormone/parathyroid hormone-related peptide receptor (PTH1R) and a novel receptor (PTH3R) that is preferentially activated by mammalian and fugufish parathyroid hormone-related peptide. J Biol Chem 274:28185–28190
Rubin DA, Hellman P, Zon LI, Lobb CJ, Bergwitz C, Juppner H (1999) A G protein-coupled receptor from zebrafish is activated by human parathyroid hormone and not by human or teleost parathyroid hormone-related peptide—implications for the evolutionary conservation of calcium-regulating peptide hormones. J Biol Chem 274:23035–23042
Scatchard G (1949) The attractions of proteins for small molecules and ions. Ann N Y Acad Sci 51:660–672
Silverberg SJ, Shane E, Dempster DW, Bilezikian JP (1999) The effects of vitamin D insufficiency in patients with primary hyperparathyroidism. Am J Med 107:561–567
Watson PH, Fraher LJ, Hendy GN, Chung UI, Kisiel M, Natale BV, Hodsman AB (2000) Nuclear localization of the type 1 PTH/PTHrP receptor in rat tissues. J Bone Miner Res 15:1033–1044
Wysolmerski J, Stewart A (1998) The physiology of parathyroid hormone-related protein: an emerging role as a development factor. Annu Rev Physiol 60:431–460
Yasuoka T, Kawashima M, Takahashi T, Iwata A, Oka N, Tanaka K (1996) Changes in parathyroid hormone receptor binding affinity during egg laying—implications for calcium homeostasis in chicken. J Bone Miner Res 11:1913–1920
Zhou LX, Nemere I, Norman AW (1992) A parathyroid-related peptide induces transcaltachia (the rapid, hormonal stimulation of intestinal Ca2+ transport). Biochem Biophys Res Commun 186:69–73
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was carried out with the financial support of the Commission of the European Union, Quality of Life and Management of Living Resources specific RTD programme (FISHCAL, Q5RS-2001-02904). J.R. and P.M.G. were in receipt of fellowships (SFRH/BPD/1524/2000, Fulbright-MECD-FU2003-1026, and BPD/9464/02).
Rights and permissions
About this article
Cite this article
Rotllant, J., Guerreiro, P.M., Redruello, B. et al. Ligand binding and signalling pathways of PTH receptors in sea bream (Sparus auratus) enterocytes. Cell Tissue Res 323, 333–341 (2006). https://doi.org/10.1007/s00441-005-0070-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-005-0070-7