Abstract
Growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) is a new member of the transforming growth factor beta (TGF-β) superfamily, which has most recently been found in activated macrophages (MΦ). We have now investigated GDF-15/MIC-1 in human MΦ after exposure to oxidized low-density lipoproteins (oxLDL) related mediators in vitro and in arteriosclerotic carotid arteries. Using RT-PCR and Western blotting a pronounced induction of GDF-15/MIC-1 expression by oxLDL, C6-ceramide, tumor necrosis factor (TNFα) and hydrogen peroxide (H2O2) was found in cultured human MΦ. In 11 human arteriosclerotic carotid arteries, immunohistochemical analyses supported by computer-assisted morphometry and regression analyses demonstrated a significant colocalization of GDF-15/MIC-1 immunoreactivity (IR) with oxLDL IR and manganese superoxide dismutase (MnSOD) IR in CD68 immunoreactive (ir) MΦ, which were also expressing AIF-IR (apoptosis-inducing factor), caspase-3-IR (CPP32), PARP-IR, c-Jun/AP-1-IR and p53-IR. Our data suggest that GDF-15/MIC-1 is inducible in human MΦ by oxLDL and its mediators in vitro and is supposed to contribute to oxidative stress dependent consequences in arteriosclerotic plaques, e.g. modulating apoptosis and inflammatory processes in activated MΦ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transforming growth factor betas (TGF-βs) have been shown to be strongly implicated in a number of pathophysiological processes including chronic vascular diseases (Topper 2000). Furthermore, TGF-βs constitute inflammatory markers of advanced arteriosclerosis (Erren et al. 1999; Czernichow and Hercberg 2001) and seem to be involved in the pathogenesis of ischemic heart disease in humans (Tashiro et al. 1997). High concentrations of TGF-βs have been described in early atherosclerotic lesions (fatty streaks), supporting the hypothesis that TGF-βs contribute to the pathogenesis of lipid-rich atherosclerotic lesions by activating proteolytic mechanisms in macrophages (MΦ) (Bobik et al. 1999).
MΦ-inhibitory cytokine-1 (MIC-1), which is identical to growth differentiation factor-15 (GDF-15) (Bootcov et al. 1997; Fairlie et al. 1999; Bauskin et al. 2000), a divergent member of the TGF-β superfamily (Strelau et al. 2000), is widely distributed in adult tissues, being most strongly expressed in epithelial cells and MΦ (Böttner et al. 1999b). GDF-15/MIC-1 expression is upregulated in mononuclear cells by a variety of stimuli including interleukin-1β, interleukin-2, tumor necrosis factor alpha (TNFα), phorbol myristate acetate or MΦ colony-stimulating factor and, thus, seems to be associated with MΦ activation (Bootcov et al. 1997; Bauskin et al. 2000; Fairlie et al. 2001; Schober et al. 2001). Moreover, GDF-15/MIC-1 has most recently been shown to be induced by p53, suggesting a role in apoptosis, too (Kannan et al. 2000). In the lesioned CNS, GDF-15/MIC-1 may have important anti-inflammatory functions, supplementing the roles of other members of the TGF-β superfamily. However, localization of GDF-15/MIC-1 in neurons within the lesion site has raised questions of putative pro-apoptotic or anti-apoptotic functions of this protein (Schober et al. 2001; Subramaniam et al. 2003).
Activation of monocytes to MΦ is known to play a key role in normal and pathological processes of the arteriosclerotic vessel wall, including immune and inflammatory responses, largely by their capacity to phagocyte detrimental material and to secrete biologically active molecules. Apoptosis of these activated MΦ in arteriosclerotic lesions may considerably modulate their inflammatory response, affecting the plaque stability and growth, as has been suggested from human subjects and animal models (Libby et al. 1996; Kockx and Herman 2000; van Vlijmen et al. 2001). Most recently, MΦ have been shown to undergo apoptosis in conditions of lipid-rich plaque, pointing to a key role of lipid content and inflammatory cell viability in determining plaque thrombogenicity (Hutter et al. 2004). OxLDL and its mediators ceramide or TNFα are known to initiate apoptosis in human MΦ in vitro (Jovinge et al. 1996; Kinscherf et al. 1997a, b, 1998, 1999; Deigner et al. 2001). Signal transduction of oxLDL induced apoptosis includes activation of caspase-3 (CPP32) (Wintergerst et al. 2000), induction of manganese superoxide dismutase (MnSOD) as well as increase of p53 (Kinscherf et al. 1997b, 1998) which all have also been shown in human arteriosclerotic plaques (Ihling et al. 1997; Kinscherf et al. 1997b; Mallat et al. 1997; Ihling et al. 1998; Kinscherf et al. 1998, 1999). In addition, poly(ADP-ribose) polymerase (PARP), c-Jun-AP-1 or apoptosis inducing factor (AIF) have also been detected in apoptotic cells (Grand et al. 1995; Martinet et al. 2002; Zhang et al. 2003).
Because the plasma concentration of GDF-15/MIC-1 has most recently been found to be a potential marker for cardiovascular events in women (Brown et al. 2002), we were interested about its presence in arteriosclerotic plaques and its induction and involvement in oxLDL-mediated apoptosis of human MΦ in vitro.
Materials and methods
In vitro experiments: isolation of peripheral blood mononuclear cells and cell culture of MΦ
Buffy coats of healthy human volunteers were obtained from the center of blood donation of the University of Heidelberg. PBMCs were isolated by Histopaque (Sigma, Taufkirchen, Germany) density gradient centrifugation and cultured in supplemented RPMI 1640 medium at 37°C in humified CO2 (5%) atmosphere as described earlier (Kinscherf et al. 1997b, 1998; Schober et al. 2001). The differentiated MΦ were either kept in supplemented RPMI 1640 medium (control medium) or were incubated in lipoprotein-deficient serum for 24 h before exposition (4 h) to oxLDL (50 μg/ml), C6-ceramide (30 μM), recombinant human TNFα (3 ng/ml) or H2O2 (100 μM) (Sigma, Taufkirchen, Germany) as described earlier (Kinscherf et al. 1997b, 1998; Deigner et al. 2001).
Measurement of apoptotic MΦ
Apoptotic cells were identified by YO-PRO-1 staining (Idziorek et al. 1995) in combination with the Hoechst 33342 dye (Mobitec Company, Goettingen) as previously published (Deigner et al. 2001). The percentage of apoptotic cells was counted using an inverse fluorescence microscope and a computer-assisted morphometry system developed by our group (VIBAM 0.0-VFG 1 frame grabber) (Kinscherf et al. 1997b, 1998; Deigner et al. 2001).
Reverse transcription-polymerase chain reaction
RNA extraction from 1×106 cells using Trizol LS reagent as well as denaturating agarose gel electrophoresis and RT-PCR were performed routinely as previously described (Kinscherf et al. 1997b, 1998; Deigner et al. 2001; Schober et al. 2001).
After reverse transcription, cDNA samples were subjected to PCR amplification using sequence-specific primers based on the human coding sequence of GDF-15/MIC-1: 5′-ACT GCT GGC AGA ATC TTC GT-3′ (forward) and 5′-AAT GAG CAC CAT GGG ATT GT-3′ (reverse), generating a 352 base pair (bp) fragment. Amplification of part of the β-microglobulin gene was used as a positive control using primers 5′-TGT CGG ATT GAT GAA ACC CAG-3′ (forward) and 5′-CTC GCG CTA CTC TCT CTT TCT-3′ (reverse). A PCR reaction profile with initial denaturation for 3 min at 94°C, followed by 30 s 94°C, 30 s 50°C, 45 s 72°C 32–36 cycles and a final extension step for 10 min at 72°C, was performed using a Genius thermal cycler (Techne Inc., Cambridge, UK). The O’Range Ruler 50 bp DNA Ladder (MBI Fermentas, St-Leon-Rot, Germany) was used. Amplification products were separated on a 1.5% agarose gel and stained with ethidium bromide.
Western blotting
GDF-15/MIC-1 expression in cultured MΦ was measured by Western blotting as recently described (Schober et al. 2001) with minor modifications: MΦ were scraped off, boiled and the protein concentration was quantified. After SDS-PAGE gel electrophoresis, blotting was performed with a semi-dry system (Milliblot; Millipore, Eschborn, Germany) and PVDF membrane for ECL (Amersham Pharmacia, Freiburg, Germany). After protein transfer non-specific sites were blocked with TRIS-buffered saline, and rabbit anti-human GDF-15/MIC-1 antibodies were added (Schober et al. 2001). Protein loading was tested by the use of a monoclonal mouse anti-human α-tubulin antibody (1:200; Dianova, Hamburg, Germany). A polyclonal sheep anti-rabbit or anti-mouse IgG horseradish peroxidase conjugate and the chemiluminescence ECL detection kit (Amersham Pharmacia) were used for visualization. A hyperfilm ECL (Amersham Pharmacia) served for documentation. Washing steps after every incubation period were performed using TBS.
Human arteriosclerotic tissue
Specimen of human arteriosclerotic carotid arteries (n=11) obtained at surgery were shock-frozen in liquid nitrogen-cooled isopentane and kept in a −70°C freezer until used (Kinscherf et al. 1997b, 1998, 1999). The patients provided informed written consent before participation.
Immunohistochemistry and computer-assisted morphometry
Immunohistochemistry of human arteriosclerotic carotid arteries was routinely performed as described earlier (Kinscherf et al. 1997b, 1998, 1999). Cryostat sections (6 μm) were exposed to acetone (10 min, −20°C) and dried thereafter (30 min). Non-specific sites were blocked with 1% normal swine serum (Life Technologies) in PBS. The following primary monoclonal mouse (mab) and polyclonal antibodies were used: polyclonal rabbit anti-human GDF-15/MIC-1 (1:100), mab anti-human MnSOD (1:100; Alexis, Grünberg, Germany), mab anti-human p53 (Ab5, 1:50; Calbiochem, Bad Soden/Schwalbach, Germany), polyclonal rabbit anti-human oxLDL (1:2,000; Immundiagnostik, Bensheim, Germany), polyclonal rabbit anti-human c-Jun/AP-1 (1:10; Calbiochem, Schwalbach, Germany), mab anti-human AIF (1:100; Chemicon, Hofheim, Germany), mab anti-human CPP32 (caspase-3) (1:100; Transduction Laboratories, Lexington, Ky., USA), mab anti-human PARP (1:50; BioMol, Hamburg, Germany), mab anti-CD68 (1:1,000; DakoCytomation, Glostrup, Denmark), mab anti-human α-actin (1:800; Roche Mannheim, Germany) and mab anti-human CD31 (1:1000; Immunotech, Marseille, France).
Incubation times at room temperature and secondary antibody dilutions were as follows: (1) single staining was performed by incubation of primary antibody with biotinylated anti-rabbit IgG (1:100; 2 h; Vector Lab, USA); endogenous peroxidase activity was suppressed with 3% H2O2 in PBS (5 min); afterwards the sections were incubated with peroxidase-conjugated streptavidin (1:100, 2 h) and staining reaction was achieved with diamino-benzidine (DAB) solution (Pierce, Rockford, Ill., USA). Nuclei were counterstained with hematoxylin. (2) Double-stainings were performed by adding two primary antibodies sequentially to the same section and using Cy2-conjugated or Cy3-conjugated streptavidin (1:1000, 2 h; Jackson ImmunoRes. Lab, USA) for visualization. Anti-GDF-15/MIC-1 antibodies were applied in a three-step procedure using Cy3 (or Cy2) fluorochrome followed by a two step procedure for CD68, MnSOD, oxLDL, CPP32, AIF, PARP, c-Jun/AP-1, α-actin or CD31 visualized by Cy2 (or Cy3)-conjugated anti-rabbit or anti-mouse IgG (1:500, Jackson ImmunoRes.). Between first and second staining, sections were rinsed in PBS. Nuclei were counterstained with DAPI (Sigma, Munich, Germany). Finally, all sections were rinsed 3 times in PBS and embedded in fluorescent mounting medium. Negative controls for the GDF-15/MIC-1 polyclonal antibody were performed as described most recently (Schober et al. 2001), using routine methods, i.e. omission of the first antibody, which abolished the immunoreactivity completely (e.g. Kinscherf et al. 1997b). Furthermore irrelevant, isotype-matched monoclonal antibodies as well as mouse/rabbit serum (of non-immunized animals) were used to exclude a cross-reaction of the antibodies due to a Fc-receptor-mediated reaction. The use of this number of antibodies did not reveal any labeling of plaque cells.
Immunoreactive cells in arteriosclerotic carotid arteries were recorded by a video camera (Olympus HCC-3600 P high gain) and quantified using a computer-assisted image analysis system developed by our group (VIBAM 0.0-VFG 1 frame grabber) (e.g. Kinscherf et al. 1997b, 1999). Finally, sections were photographed using an Axioplan2 imaging microscope (Carl Zeiss GmbH, Jena, Germany) and the digital high resolution imaging system AxioCam/AxioVision (Carl Zeiss). Digitalized images were processed, arranged and lettered by standard imaging software (Adobe Photoshop 6.0/Illustrator 10.0).
Statistical analyses
The results were calculated as mean±SEM. Statistical procedures were performed by the Mann–Whitney U–Wilcoxon Rank Sum W-test or by the Student’s t-test for unpaired data using the SPSS Base 11.5 for windows. Correlation between different parameters was graphically described by scatterplots and linear regression lines. The dependency was quantitatively assessed by Pearson’s product correlation coefficient r and by a statistical test for the existence of a positive or negative slope. A P value of 0.05 or less was chosen for statistical significance.
Results
Induction of apoptosis and GDF-15/MIC-1 expression in human MΦ
After exposure (4 h) to oxLDL (50 μg/ml), C6-ceramide (30 μM), TNFα (3 ng/ml) or H2O2 (100 μM), the number of apoptotic MΦ significantly increased about 3.41±0.013-fold, 2.91±0.032-fold, 2.34±0.015 or 3.20±0.034-fold in comparison to the control, which revealed an absolute apoptosis rate of 9.2%±1.3% (five independent experiments). As shown by RT-PCR (Fig. 1A) and Western blotting (Fig. 1B), a pronounced GDF-15/MIC-1 expression in human MΦ was simultaneously induced by oxLDL, C6-ceramide, TNFα or H2O2.
GDF-15/MIC-1 expression in human MΦ. RT-PCR A and Western blot B analyses showing pronounced GDF-15/MIC-1 induction in cultured human MΦ after exposure to oxLDL (50 μg/ml), C6-ceramide (30 μM), TNFα (3 ng/ml) or H2O2 (100 μM) for 4 h in comparison to medium (control). For Western blot analyses, recombinant GDF-15/MIC-1 protein (band refers to 200 ng), produced in our laboratory, was used as positive control. α-Tubulin or β-microglobulin was used as an internal control
Localization of GDF-15/MIC-1 IR in apoptotic MΦ in arteriosclerotic lesions of human carotid arteries
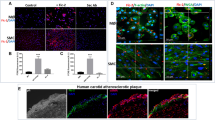
Immunohistology shows that GDF-15/MIC-1 ir cells are located in arteriosclerotic lesions of human carotid arteries (Fig. 2). GDF-15/MIC-1 ir cells were found to be localized in superficial and deeper regions of the plaques. Double immunostainings revealed a colocalization of GDF-15/MIC-1 IR in CD68-ir MΦ (Fig. 3A–C), but neither in α-actin ir smooth muscle cells (Fig. 3D–F) nor in CD31 ir endothelial cells (not shown). GDF-15/MIC-1 IR was also colocalized in oxLDL ir MΦ (Fig. 4A–C) and MnSOD ir MΦ (Fig. 5A–C). Moreover, GDF-15/MIC-1 ir was also found in apoptotic, CPP32 (caspase-3)-ir (Fig. 5D–F) AIF-ir, c-Jun/AP-1-ir, PARP-ir or p53-ir MΦ (not shown).
Double staining of MΦ in an arteriosclerotic plaque of a human carotid artery. CD68 ir (A; arrows) is colocalized with GDF-15/MIC-1 ir (B; arrows) in most MΦ as seen in the overlay C (yellow color; arrows). α-Actin ir (D; arrows; =smooth muscle cells) is not colocalized with GDF-15/MIC-1 ir (E; arrows) as also demonstrated in the overlay F. Nuclei are counterstained with DAPI. Arrowheads show cells, which are neither CD68-actin nor α-actin or GDF-15/MIC-1 ir. Bar 25 μm
Double staining of MΦ in an arteriosclerotic plaque of a human carotid artery. OxLDL ir (A; arrows) is colocalized with GDF-15/MIC-1 ir (B; arrows) in most MΦ, as seen in the overlay (C; yellow color; arrows). Nuclei are counterstained with DAPI. Arrowheads show cells, which are neither oxLDL ir nor GDF-15/MIC-1 ir. Bar 25 μm
Double staining of MΦ in an arteriosclerotic plaque of a human carotid artery. MnSOD ir (A; arrows) and GDF-15/MIC-1 ir (B; arrows) are colocalized in most MΦ as seen in the overlay C (yellow color; arrows). GDF-15/MIC-1 (D; arrows) ir is colocalized with CPP32 (caspase-3) ir (E; arrows) in most MΦ as seen in the overlay (C; yellow color; arrows). Nuclei are counterstained with DAPI. Cells that are neither MnSOD nor GDF-15/MIC-1 ir or CPP32 ir are also seen (arrowheads). Bar 25 μm
Morphometric analyses of immunoreactivities in arteriosclerotic human carotid arteries demonstrated a significant positive correlation between the percentage of GDF-15/MIC-1-ir and CD68-ir, oxLDL-ir, MnSOD-ir, CPP32-ir, AIF-ir, c-Jun/AP-1-ir, PARP-ir, or p53-ir MΦ (Fig. 6).
Discussion
GDF-15/MIC-1 expression and oxidative stress in human MΦ in vitro
GDF-15/MIC-1, a member of the TGF-β superfamily, has recently been induced in phorbolester-stimulated, activated MΦ (Bootcov et al. 1997; Schober et al. 2001). The present study, which focused on cell associated GDF-15/MIC-1, demonstrates for the first time that GDF-15/MIC-1 expression in human MΦ is upregulated by oxLDL and its mediators C6-ceramide, TNFα or H2O2. Simultaneously, the expression of several other substances (cytokines) like TNFα, macrophage migration inhibitory factor or transcription factors, e.g. nuclear factor kappa B, as well as processes such as apoptosis and inflammation, have been found to be enhanced (Jovinge et al. 1996; Kinscherf et al. 1997b, 1998; Mikita et al. 2001; Burger-Kentischer et al. 2002). OxLDL and its mediators are known to increase oxidative stress in MΦ, as indicated, e.g. by an induction of the MnSOD and p53 expression (Kinscherf et al. 1997b, 1998). We therefore assume that oxidative stress might also be involved in the increased GDF-15/MIC-1 expression. This hypothesis is supported by the finding that phorbol ester, which stimulates superoxide anion production in microglia cells or MΦ (Colton et al. 1998; Wagner et al. 2000), or H2O2 alone (this study), induce GDF-15/MIC-1 expression. Recent findings showing the regulation of GDF-15/MIC-1 gene induction by, e.g. TNFα, LPS and p53 (Bootcov et al. 1997; Kannan et al. 2000), which all are known to induce or to be associated with oxidative stress, lend further support (De la Fuente and Victor 2001). Based on these findings, GDF-15/MIC-1 has been supposed to limit the later phases of MΦ activation (Bootcov et al. 1997). A recent analysis of the rat promoter of the GDF-15/MIC-1 gene has indicated the presence of multiple regulatory elements, including a TATA-like sequence as well as several SP1, AP-1 and AP-2 sites (Böttner et al. 1999a), which are all well-known to be redox-regulated (Das et al. 1995; Deigner et al. 2001; Zhao et al. 2001).
GDF-15/MIC-1 ir apoptotic MΦ in human arteriosclerotic lesions
TGF-βs have already been shown to be multifunctional cytokines playing key roles in cell cycle control, development, repair processes, and apoptosis (e.g. Böttner et al. 2000; Unsicker and Strelau 2000; Schuster and Krieglstein 2002). Several earlier studies report that many members of the TGF-β superfamily are involved in inflammatory processes, such as those in arteriosclerotic lesions (Corradin et al. 1993; Letterio and Roberts 1998; Bobik et al. 1999; Reckless et al. 2001). We here show for the first time GDF-15/MIC-1-ir cells in human arteriosclerotic carotid arteries, which have been identified as MΦ. Double staining procedures excluded smooth muscle or endothelial cells to contain GDF-15/MIC-1. Most of the GDF-15/MIC-1-ir MΦ revealed a colocalization with MnSOD IR, which has been used as a marker for increased oxidative stress (Kinscherf et al. 1997b, 1998, 1999; Martinet et al. 2002; Zhang et al. 2003). We further found a significant colocalization with oxLDL IR, which was supported by a strong positive statistical correlation between the percentage of GDF-15/MIC-1-IR and CD68-IR or oxLDL IR in MΦ of the arteriosclerotic plaques. In the context with our in vitro experiments, we assume an induction of GDF-15/MIC-1 (as well as of MnSOD) by oxLDL (or its mediators) in MΦ of human arteriosclerotic lesions (Kinscherf et al. 1997b, 1998). GDF-15/MIC-1 IR is also colocalized with apoptosis markers like PARP, c-Jun/AP-1, CPP32 (caspase-3) or AIF IR. Thus, it might be involved in apoptosis signaling of plaque MΦ, as has already been suggested according to results in several cell lines (Baek et al. 2001; Subramaniam et al. 2003). The colocalization of GDF-15/MIC-1 IR with p53 IR supports further evidence for a direct functional link, as has been supposed for the TGF-β superfamily (Li et al. 2000). Moreover, GDF-15/MIC-1 is suggested as an important downstream mediator of p53 function, while acting as a target of cellular stress signaling (Albertoni et al. 2002). The death program might modulate inflammatory processes within MΦ of the atherosclerotic vessel wall (Schlittenhardt, Kinscherf unpublished results), however, putative cross talks between these pathways need to be addressed in more detail by future experiments. Potential autocrine effects of this protein on MΦ remain as long speculative as GDF-15/MIC-1 receptors have not been identified.
In summary, we found a pronounced induction of GDF-15/MIC-1 expression by oxLDL, C6-ceramide, TNFα and H2O2 in cultured human MΦ. In human arteriosclerotic carotid arteries, GDF-15/MIC-1 IR was exclusively localized in MΦ and colocalized with oxLDL-IR, MnSOD-IR, AIF-IR, caspase-3-IR (CPP32), PARP-IR, c-Jun/AP-1-IR, and p53-IR. GDF-15/MIC-1 is supposed to contribute to modulation of apoptosis and inflammatory processes of activated MΦ.
References
Albertoni M, Shaw PH, Nozaki M, Godard S, Tenan M, Hamou MF, Fairlie DW, Breit SN, Paralkar VM, de Tribolet N, Van Meir EG, Hegi ME (2002) Anoxia induces macrophage inhibitory cytokine-1 (MIC-1) in glioblastoma cells independently of p53 and HIF-1. Oncogene 21:4212–4219
Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE (2001) Cyclooxygenase inhibitors regulate the expression of a TGF-β superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol 59:901–908
Bauskin AR, Zhang HP, Fairlie WD, He XY, Russell PK, Moore AG, Brown DA, Stanley KK, Breit SN (2000) The propeptide of macrophage inhibitory cytokine (MIC-1), a TGF-beta superfamily member, acts as a quality control determinant for correctly folded MIC-1. EMBO J 19:2212–2220
Bobik A, Agrotis A, Kanellakis P, Dilley R, Krushinsky A, Smirnov V, Tararak E, Condron M, Kostolias G (1999) Distinct patterns of transforming growth factor-beta isoform and receptor expression in human atherosclerotic lesions. Colocalization implicates TGF-beta in fibrofatty lesion development. Circulation 99:2883–2891
Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN (1997) MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA 94:11514–11519
Böttner M, Laaff M, Schechinger B, Rappold G, Unsicker K, Suter-Crazzolara C (1999a) Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1). Gene 237:105–111
Böttner M, Suter-Crazzolara C, Schober A, Unsicker K (1999b) Expression of a novel member of the TGF-beta superfamily, growth/differentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res 297:103–110
Böttner M, Krieglstein K, Unsicker K (2000) The TGF-βs: structure signalling and roles in nervous system development and function. J Neurochem 75:2227–2240
Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, Ridker PM (2002) Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet 59:2159–2163
Burger-Kentischer A, Goebel H, Seiler R, Fraedrich G, Schaefer HE, Dimmeler S, Kleemann R, Bernhagen J, Ihling C (2002) Expression of macrophage migration inhibitory factor in different stages of human atherosclerosis. Circulation 105:1561–1566
Colton CA, Snell-Callanan J, Chernyshev ON (1998) Ethanol induced changes in superoxide anion and nitric oxide in cultured microglia. Alcohol Clin Exp Res 22:710–716
Corradin SB, Buchmuller-Rouiller Y, Smith J, Suardet L, Mauel J (1993) Transforming growth factor beta 1 regulation of macrophage activation depends on the triggering stimulus. J Leukoc Biol 54:423–429
Czernichow S, Hercberg S (2001) Interventional studies concerning the role of antioxidant vitamins in cardiovascular diseases: a review. J Nutr Health Aging 5:188–195
Das KC, Lewis-Molock Y, White CW (1995) Activation of NF-kappa B and elevation of MnSOD gene expression by thiol reducing agents in lung adenocarcinoma (A549) cells. Am J Physiol 269:L588–L602
De la Fuente M, Victor VM (2001) Ascorbic acid and N-acetylcysteine improve in vitro the function of lymphocytes from mice with endotoxin-induced oxidative stress. Free Radic Res 35:73–84
Deigner HP, Claus R, Bonaterra GA, Gehrke C, Bibak N, Blaess M, Cantz M, Metz J, Kinscherf R (2001) Ceramide induces aSMase expression: implications for oxLDL-induced apoptosis. FASEB J 15:807–814
Erren M, Reinecke H, Junker R, Fobker M, Schulte H, Schurek JO, Kropf J, Kerber S, Breithardt G, Assmann G, Cullen P (1999) Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler Thromb Vasc Biol 19:2355–2363
Fairlie WD, Moore AG, Bauskin AR, Russell PK, Zhang HP, Breit SN (1999) MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. J Leukoc Biol 65:2–5
Fairlie WD, Russell PK, Moore AG, Zhang HP, Brown PK, Breit SN (2001) Epitope mapping of the transforming growth factor-β superfamily protein, macropahage inhibitory cytokine-1 (MIC-1): identification of at least five distinct epitope specificities. Biochemistry 40:65–73
Grand RJ, Milner AE, Mustoe T, Johnson GD, Owen D, Grant ML, Gregory CD (1995) A novel protein expressed in mammalian cells undergoing apoptosis. Exp Cell Res 218:439–451
Hutter R, Valdiviezo C, Sauter BV, Savontaus M, Chereshnev I, Carrick FE, Bauriedel G, Lüderitz B, Fallon JT, Fuster V, Badimon JJ (2004) Caspase-3 and tissue factor expression in lipid-rich plaque macrophages: evidence for apoptosis as link between inflammation and atherothrombosis. Circulation 109:2001–2008
Idziorek T, Estaquier J, De Bels F, Ameisen JC (1995) YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J Immunol Meth 185:249–258
Ihling C, Menzel G, Wellens E, Monting JS, Schaefer HE, Zeiher AM (1997) Topographical association between the cyclin-dependent kinases inhibitor P21, p53 accumulation, and cellular proliferation in human atherosclerotic tissue. Arterioscler Thromb Vasc Biol 17:2218–2224
Ihling C, Haendeler J, Menzel G, Hess RD, Fraedrich G, Schaefer HE, Zeiher AM (1998) Co-expression of p53 and MDM2 in human atherosclerosis: implications for the regulation of cellularity of atherosclerotic lesions. J Pathol 185:303–312
Jovinge S, Ares MP, Kallin B, Nilsson J (1996) Human monocytes/macrophages release TNF-alpha in response to Ox-LDL. Arterioscler Thromb Vasc Biol 16:1573–1579
Kannan K, Amariglio N, Rechavi G, Givol D (2000) Profile of gene expression regulated by induced p53: connection to the TGF-beta family. FEBS Lett 470:77–82
Kinscherf R, Claus R, Deigner HP, Nauen O, Gehrke C, Hermetter A, Russwurm S, Daniel V, Hack V, Metz J (1997a) Modified low density lipoprotein delivers substrate for ceramide formation and stimulates the sphingomyelin-ceramide pathway in human macrophages. FEBS Lett 405:55–59
Kinscherf R, Deigner HP, Usinger C, Pill J, Wagner M, Kamencic H, Hou D, Chen M, Schmiedt W, Schrader M, Kovacs G, Kato K, Metz J (1997b) Induction of manganese superoxide dismutase by oxidized-LDL—its relevance in atherosclerosis of humans and heritable hyperlipidemic rabbits. FASEB J 11:1317–1328
Kinscherf R, Claus R, Wagner M, Gehrke C, Kamencic H, Hou D, Chen M, Nauen O, Schmiedt W, Kovacs G, Pill J, Metz J, Deigner HP (1998) Apoptosis caused by oxidized-LDL is manganese superoxide dismutase and p53-dependent. FASEB J 12:461–467
Kinscherf R, Wagner M, Kamencic H, Bonaterra GA, Hou D, Schiele RA, Deigner HP, Metz J (1999) Characterization of apoptotic macrophages in atheromatous tissue of humans and heritable hyperlipidemic rabbits. Atherosclerosis 144:33–39
Kockx MM, Herman AG (2000) Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc Res 45:736–746
Letterio JJ, Roberts AB (1998) Regulation of immune responses by TGF-beta. Annu Rev Immunol 16:137–161
Li PX, Wong J, Ayed A, Ngo D, Brade AM, Arrowsmith C, Austin RC, Klamut HJ (2000) Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. J Biol Chem 275:20127–20135
Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT (1996) Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol 7:330–335
Mallat Z, Ohan J, Leseche G, Tedgui A (1997) Colocalization of CPP-32 with apoptotic cells in human atherosclerotic plaques. Circulation 96:424–428
Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM (2002) Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation 106:927–932
Mikita T, Porter G, Lawn RM, Shiffman D (2001) Oxidized low density lipoprotein exposure alters the transcriptional response of macrophages to inflammatory stimulus. J Biol Chem 276:45729–45739
Reckless J, Rubin EM, Verstuyft JB, Metcalfe JC, Grainger DJ (2001) A common phenotype associated with atherogenesis in diverse mouse models of vascular lipid lesions. J Vasc Res 38:256–265
Schober A, Böttner M, Strelau J, Kinscherf R, Bonaterra GA, Barth M, Schilling L, Fairlie WD, Breit SN, Unsicker K (2001) Expression of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in the perinatal, adult, and injured rat brain. J Comp Neurol 439:32–45
Schuster N, Krieglstein K (2002) Mechanisms of TGF-β-mediated apoptosis. Cell Tissue Res 307:1–14
Strelau J, Böttner M, Lingor P, Suter-Crazzolara C, Galter D, Jaszai J, Sullivan A, Schober A, Krieglstein K, Unsicker K (2000) GDF-15/MIC-1 a novel member of the TGF-beta superfamily. J Neural Transm Suppl 60:273–276
Subramaniam S, Strelau J, Unsicker K (2003) Growth differentiation factor-15 prevents low potassium-induced cell death of cerebellar granule neurons by differential regulation of Akt and ERK pathways. J Biol Chem 278:8904–8912
Tashiro H, Shimokawa H, Yamamoto K, Momohara M, Tada H, Takeshita A (1997) Altered plasma levels of cytokines in patients with ischemic heart disease. Coron Artery Dis 8:143–147
Topper JN (2000) TGF-beta in the cardiovascular system: molecular mechanisms of a context-specific growth factor. Trends Cardiovasc Med 10:132–137
Unsicker K, Strelau J (2000) Functions of transforming growth factor-beta isoforms in the nervous system. Cues based on localization and experimental in vitro and in vivo evidence. Eur J Biochem 267:6972–6975
van Vlijmen BJ, Gerritsen G, Franken AL, Boesten LS, Kockx MM, Gijbels MJ, Vierboom MP, van Eck M, van De Water B, van Berkel TJ, Havekes LM (2001) Macrophage p53 deficiency leads to enhanced atherosclerosis in APOE*3-Leiden transgenic mice. Circ Res 88:780–786
Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M (2000) Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol 20:61–69
Wintergerst ES, Jelk J, Rahner C, Asmis R (2000) Apoptosis induced by oxidized low density lipoprotein in human monocyte-derived macrophages involves CD36 and activation of caspase-3. Eur J Biochem 267:6050–6059
Zhang W, Shokeen M, Li D, Mehta JL (2003) Identification of apoptosis-inducing factor in human coronary artery endothelial cells. Biochem Biophys Res Commun 301:147–151
Zhao Y, Xue Y, Oberley TD, Kiningham KK, Lin SM, Yen HC, Majima H, Hines J, St-Clair D (2001) Overexpression of manganese superoxide dismutase suppresses tumor formation by modulation of activator protein-1 signaling in a multistage skin carcinogenesis model. Cancer Res 61:6082–6088
Acknowledgements
The authors are grateful to Mrs. U. Traut for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
The last two authors are senior authors.
Rights and permissions
About this article
Cite this article
Schlittenhardt, D., Schober, A., Strelau, J. et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res 318, 325–333 (2004). https://doi.org/10.1007/s00441-004-0986-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-0986-3