Abstract
Several studies have emphasized the role of DNA methylation in vitiligo. However, its profile in human skin of individuals with vitiligo remains unknown. Here, we aimed to study the DNA methylation profile of vitiligo using pairwise comparisons of lesions, peri-lesions, and healthy skin. We investigated DNA methylation levels in six lesional skin, six peri-lesional skin, and eight healthy skin samples using an Illumina 850 K methylation chip. We then integrated DNA methylation data with transcriptome data to identify differentially methylated and expressed genes (DMEGs) and analyzed their functional enrichment. Subsequently, we compared the methylation and transcriptome characteristics of all skin samples, and the related genes were further studied using scRNA-seq data. Finally, validation was performed using an external dataset. We observed more DNA hypomethylated sites in patients with vitiligo. Further integrated analysis identified 264 DMEGs that were mainly functionally enriched in cell division, pigmentation, circadian rhythm, fatty acid metabolism, peroxidase activity, synapse regulation, and extracellular matrix. In addition, in the peri-lesional skin, we found that methylation levels of 102 DMEGs differed prior to changes in their transcription levels and identified 16 key pre-DMEGs (ANLN, CDCA3, CENPA, DEPDC1, ECT2, DEPDC1B, HMMR, KIF18A, KIF18B, TTK, KIF23, DCT, EDNRB, MITF, OCA2, and TYRP1). Single-cell RNA analysis showed that these genes were associated with cycling keratinocytes and melanocytes. Further analysis of cellular communication indicated the involvement of the extracellular matrix. The expression of related genes was verified using an external dataset. To the best of our knowledge, this is the first study to report a comprehensive DNA methylation profile of clinical vitiligo and peri-lesional skin. These findings would contribute to future research on the pathogenesis of vitiligo and potential therapeutic strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Vitiligo is a chronic autoimmune skin disease with refractory depigmentation, affecting approximately 1.3% of the adult population (Bibeau et al. 2022). It is characterized by systemic skin or mucous membranes with limitations or generalized depigmentation spots (Bergqvist and Ezzedine 2020; Frisoli et al. 2020). When depigmentation spots occur on the face or exposed parts of the body, they cause a great psychological burden to patients (Ezzedine et al. 2021). The pathological characteristic of vitiligo is the selective loss of epidermal melanocytes, which is linked to multifactorial causation, including the dysfunction of melanocytes and changes in the external microenvironment (Chen et al. 2021; Ezzedine et al. 2015). Vitiligo is generally considered to be caused by the combined effects of environmental and genetic factors.
Recently, increasing attention has been paid to the role of epigenetic modifications in skin diseases. During skin aging and tumorigenesis, epigenetic mechanisms such as DNA methylation become deregulated, resulting in impaired cellular function and identity (Köhler and Rodríguez-Paredes 2020). However, in some autoimmune, allergic, and inflammatory skin disorders, the role of epigenetic regulation has only recently been recognized and investigated. In systemic lupus erythematosus and systemic sclerosis, (Javierre et al. 2010; Lei et al. 2009) the expression of genes involved in the immune response and cell activation are decreased. In other common autoimmune skin diseases such as alopecia areata and pemphigus, (Zhao et al. 2012 Feb; Zhao et al. 2012) increased global DNA methylation levels have been observed. In addition, DNA methylation has also been reported to play a role in the pathogenesis of inflammatory skin diseases, such as psoriasis and atopic dermatitis (Zhou et al. 2016; Chen et al. 2022).
As vitiligo is thought to be caused by a combination of genetic and environmental influences, several studies have focused on the potential role of epigenetic modifications in its formation and development. Zhao et al. reported increased genomic DNA methylation in peripheral blood mononuclear cells of patients with vitiligo (Zhao et al. 2010). Furthermore, our previous study on the DNA methylation in vitiligo melanocyte cell lines also identified its importance (Pu et al. 2021). However, there is still a lack of studies directly analyzing DNA methylation levels in the lesions of patients with vitiligo.
The present study analyzed the DNA methylation profiles of lesions and peri-lesions from patients with vitiligo. Our comparative and integrated analyses were aimed at elucidating methylation and transcriptome changes during vitiligo progression. We identified 2 functional modules involving 16 genes whose methylation levels changed separately from the transcriptome levels. We further confirmed their distribution using single-cell RNA sequencing and validated their expression using an external dataset. To our knowledge, this is the first study on DNA methylation in vitiligo skin lesions and the findings suggest a subclinical vitiligo methylated state in the peri-lesions, which are clinically non-depigmented. We expect our findings to provide new ideas and targets for treatment and prevention of vitiligo.
Methods
Study samples
This study followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (No.:2023-126). All patients and control subjects who participated in skin biopsies for DNA methylation analysis signed an informed consent form. The inclusion criteria for the case group were as follows: no previous oral or topical medication taken for vitiligo, no other serious skin conditions, and no contraindications for skin biopsy. Consequently, six patients with non-segmental vitiligo, who were diagnosed using the Wood’s lamp examination, were included in the case group. From each patient, two 4 mm skin lesions were obtained using skin biopsy. One was from the vitiligo lesion, and the other was from the peri-lesion, defined as 5 mm outside the vitiligo lesion. Based on sex and age matching, eight healthy participants served as the control group and a 4 mm skin lesion was taken from each participant.
The mRNA profiles of ten patients with non-segmental vitiligo and ten healthy controls were obtained from the Gene Expression Omnibus database GSE65127 (Regazzetti et al. 2015). Similarly, the lesions obtained for mRNA profiling were vitiligo lesions and peri-lesions, which were derived from a 5 mm area outside the vitiligo lesions. Lesions from healthy participants served as controls. In addition, scRNA-seq data were obtained from six patients with vitiligo from the GSE203262 dataset, including six skin lesions and six matched non-lesional skin samples (Shiu et al. 2022).
DNA methylation chip analysis
DNA was isolated from each skin sample using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). Using EZ DNA Methylation Kits (Zymo Research, USA), 500 ng of DNA from each sample was used for bisulfite conversion and then placed into 850 K BeadChips according to the manufacturer’s instructions (Illumina). Later, using the ChAMP package in R, β values were calculated to represent the DNA methylation level. The BMIQ method was then used to normalize the β value matrix, and Combat was applied to correct the batch effect caused by the BeadChip Slide and array. The adjusted p values were computed using the Benjamini–Hochberg method.
Differential DNA methylation analysis
CpGs with |Δβ|≥ 0.10 and adjusted p values ≤ 0.05 were considered as differential methylation sites (DMSs), including hyper- and hypo-DMSs. Pairwise comparisons were performed for lesions vs. healthy controls (L vs. HC), peri-lesions vs. healthy controls (PL vs. HC), and lesions vs. peri-lesions (L vs. PL). Moreover, strong differential methylation was recognized as |Δβ|≥ 0.2 and adjusted p values ≤ 0.05.
To further analyze the differentially methylated genes (DMGs) mapped from DMSs and exclude universally expressed genes, we used weighted gene co-expression network analysis (WGCNA) to analyze the mRNA profiles of vitiligo-related gene modules. After intersecting the modules with our DMGs, we performed Gene Ontology (GO) analysis to reveal the functional enrichment of DMGs in each comparison.
Differentially methylated and expressed genes identification in vitiligo lesions
We identified differentially expressed genes (DEGs) in the mRNA profile GSE65127 by pairwise comparisons with the threshold of |log2FC|≥ 0.5 and adjusted p value < 0.05. Subsequently, by integrating DMGs in the DNA methylation data of the L vs. HC comparison and DEGs in the corresponding mRNA profile, we identified differentially methylated and expressed genes (DMEGs) between L vs. HC, which were regarded as upregulated and hypomethylated or downregulated and hypermethylated genes. Venn diagrams were used to visualize overlaps. Subsequently, based on the findings for the DMEGs, we conducted functional enrichment analysis, protein–protein interaction (PPI) network construction, and functional module identification in Cytoscape using the MCODE app software package.
Differentially methylated genes preceding differential expression in peri-lesions
In pairwise comparisons of the DEGs and DMGs, some DMEGs were differentially methylated before being differentially expressed in the peri-lesions. We named these DMEGs “pre-DMEGs”. These DMEGs were identified following visualization using a Venn diagram. Functional enrichment analysis, PPI network, and module identification were performed. Genes in important functional modules were identified as key pre-DMEGs. The correlation between genes in different important functional modules was explored by calculating the Pearson’s correlation coefficient and visualized using a correlation heat map.
Distribution of pre-DMEGs in scRNA-seq data
The Seurat R package was used to analyze the scRNA-seq data from GSE203262. After data normalization using the SCTransform method, clusters were generated, and the best resolution was chosen from the clustering tree. We then manually annotated cell clusters based on published cell markers (Shiu et al. 2022; Gellatly et al. 2021), including spinous keratinocytes (high KRT1 and KRT10 expression), basal keratinocytes (high KRT5 and KRT14 expression), granular keratinocytes (high KRT2 and KLK11 expression), cycling keratinocytes (high TOP2A expression), melanocytes (high PMEL expression), DCs (high CD207 expression), and T cells (high CD3D expression). Uniform Manifold Approximation and Projection (UMAP) plots were used to visualize the cell clusters. Next, we examined the distribution of key pre-DMEGs in different cell clusters and illustrated them using violin diagrams. Furthermore, we applied the CellChat R package to explore ligand-receptor interactions and intercellular communications in vitiligo, especially in the cell populations of interest.
Validation in an external dataset
We collected external mRNA data from the GSE75819 dataset, which contained 15 lesions and 15 controls, as our validation set (Singh et al. 2017). We studied the differences in expression levels of key pre-DMEGs in lesions and controls and further explored the relevance between key pre-DMEGs and immune cells in the GSE75819 dataset. We performed immune cell infiltration analysis of the samples using the CIBERSORT algorithm and calculated the Pearson’s correlation coefficient between the immune cell infiltration score of each sample and the expression level of key pre-DMEGs. Additionally, in order to reduce data dimension and prediction error, the least absolute shrinkage and selection operator (LASSO) algorithm were employed to obtain biomarkers using the glmnet R package. Receiver operating characteristic (ROC) curves were drawn and the area under curve (AUC) values were calculated to examine the performance of each biomarker in validation set.
Statistical analyses
All bioinformatics and statistical analyses were processed using R (version 4.3.1) from Statistical Computing, Vienna, Austria. All correlation analyses were performed by calculating the Pearson’s correlation coefficient. Student’s t tests were performed to assess the significance between the cases and controls. Results with p values < 0.05 were considered statistically significant.
Results
Global DNA methylation profile of L, PL, and HC
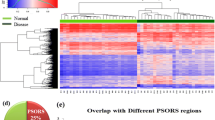
A total of 705,399 sites were obtained across all pairwise comparisons, and the number of DMSs identified is shown in Fig. 1A. Hypomethylated sites were more abundant than hypermethylated sites in vitiligo lesions. Compared with healthy controls, the number of differential methylated sites of vitiligo lesions was similar to that of peri-lesions when |Δβ| was either ≥ 0.10 or ≥ 0.20. The Venn diagram showed considerable overlap (78,591 sites) of methylated sites in the vitiligo and peri-lesions (Fig. 1B). In addition, 21,743, 20,931, and 1058 DMGs were mapped from the DMSs of L vs. HC, PL vs. HC, and L vs. PL groups, respectively. WGCNA analysis confirmed eight vitiligo-related gene modules (p values < 0.05) represented as green, grey60, light green, yellow, light yellow, blue, tan, and turquoise in Fig. 1C; these modules contained 5867, 5665, and 357 DMGs for the L vs. HC, PL vs. HC, and L vs. PL groups, respectively. Further GO analysis based on DMGs in vitiligo-related modules showed high similarity between lesions and peri-lesions (Fig. 1D–F).
A Distribution of differentially methylated sites in the pairwise comparisons of L, PL, and HC. B Venn plots of the overlap of DMSs in the pairwise comparisons. C Relationship between modules and traits in the weighted gene co-expression network analysis. D Display of the tope terms of gene ontology enrichment obtained upon intersecting the genes in vitiligo-related modules with the differentially methylated genes in L vs. HC. E Display of the top terms of gene ontology enrichment obtained upon intersecting the genes in vitiligo-related modules with the differentially methylated genes in PL vs. HC. F Display of gene ontology enrichment of the top terms obtained upon intersecting the genes in vitiligo-related modules with the differentially methylated genes in L vs. PL. L lesion; HC healthy control; PL peri-lesion
DMEGs in vitiligo and their functional enrichment
There were 520, 374, and 80 DEGs between the L vs. HC, PL vs. HC, and L vs. PL groups, respectively (Fig. S1A). The functional enrichment results of the GO analysis implied that peri-lesions were more similar to the healthy skin samples at the transcriptional level than to the vitiligo lesions, especially in terms of pigmentation and development (Fig. S1B–D).
We then intersected the DEGs and DMGs of L vs. HC and found 167 hyper methylated genes with downregulated expression, and 97 hypomethylated genes with upregulated expression (Fig. 2A). Furthermore, these DMEGs were mainly concentrated in the GO terms “pigmentation” and “collagen-containing extracellular matrix” (Fig. 2B). The PPI network results are shown in Fig. S2A. Furthermore, MCODE enrichment analysis identified eight functional modules: module 1 was related to the cell cycle and cell division; module 2 was involved in the process of pigmentation; module 3 was associated with the circadian rhythm; modules 4 and 5 were both related to fatty acid metabolism; module 6 was associated with peroxidase activity; module 7 was linked to the regulation of synapses; and module 8 was associated with the extracellular matrix (Fig. 2C).
A Venn plots of the overlap between DEGs and DMGs. B Gene ontology enrichment of DMEGs. C Functional modules of the protein–protein interaction network obtained using the MCODE method. DMEGs differentially methylated and expressed genes; DEGs differentially expressed genes; DMGs differentially methylated genes
Identification of pre-DMEGs
The results of DEGs and DMGs (Figs. 1 and S1) suggested that peri-lesions were more closely related to lesions at the methylation level but were closer to healthy skin at the transcriptional level. Thus, we assumed that during the development of vitiligo, the methylation levels of some genes changed prior to differences in transcriptome levels, and that these genes played a role in vitiligo progression. Using Venn diagrams, we identified 102 pre-DMEGs (Fig. 3A). The PPI network of these 102 pre-DMEGs was shown in Fig. S2B. Moreover, functional enrichment analysis highlighted the close link of these pre-DMEGs to “cell division” and “pigmentation,” which consisted of 11 genes (ECT2, DEPDC1, HMMR, CDCA3, KIF18A, KIF18B, TTK, KIF23, ANLN, CENPA, and DEPDC1B) in module 1, and five genes (TYRP1, EDNRB, DCT, OCA2, and MITF) in module 2 (Fig. 3B,and C). We further analyzed the correlation between the expression levels of modules 1 and 2, which revealed an overall negative correlation with p values < 0.05 (Fig. 3D).
A Venn plots for identification of pre-DMEGs. B Gene ontology enrichment of pre-DMEGs. C Functional modules of pre-DMEGs obtained using the MCODE method. D Correlation heat map between the expression levels of genes in module 1 and module 2. The grid with a white background indicates no significant difference (p values > 0.05)
ScRNA-seq data processing and distribution of pre-DMEGs
In 6 pairs of skin lesions, we identified 19 cell clusters using unsupervised clustering analysis (Fig. 4A). The clusters were divided into seven cell types by matching published markers from the dataset (Fig. 4B and C). We then examined the distribution and expression of pre-DMEGs in the scRNA-seq data and found that “cell division”-related genes were expressed in cycling keratinocytes and “pigmentation”-related genes were expressed in melanocytes (Fig. 4D). Subsequently, the CellChat analysis of intercellular communications showed that, compared with the control groups, the communication of cells in vitiligo samples was enhanced, including between cycling keratinocytes and melanocytes (Fig. 5). We further examined the potential communication network of cycling keratinocytes and melanocytes, and ligand–receptor interactions in both vitiligo lesions and controls (Table 1). The results showed that the enhancement of cellular communication was mainly related to extracellular matrix components such as collagen and laminin.
A UMAP plot of 19 clusters in all cells. B Marker gene of each cluster. C UMAP plot of different cell types. D Violin plot of the expression and distribution of “cell division”-related genes in different cell types. The red violins represent the vitiligo group while the brown violins represent the control group. E Violin plot of the expression and distribution of “pigmentation”-related genes in different cell types. The pink violins represent the vitiligo group while the brown violins represent the control group
A Inferred cellular communication networks of all cell types in non-lesions. B Inferred cellular communication networks of cycling keratinocytes in non-lesions. C Inferred cellular communication networks of melanocytes in non-lesions. D Inferred cellular communication networks of all cell types in lesions. E Inferred cellular communication networks of cycling keratinocytes in lesions. F Inferred cellular communication networks of melanocytes in lesions
Validation in the external dataset
We verified the expression levels of pre-DMEGs in the GSE75819 dataset. Figure 6A shows that most genes were significantly differentially expressed, except CDCA3 and MITF. KIF18B was not detected because of the use of different platforms. Most “cell division-related genes exhibited significantly upregulated expression (p value < 0.05) in vitiligo lesions, while “pigmentation”-related genes had significantly downregulated expression (p value < 0.05). More detailed information is listed in Table S1. Moreover, the immune cell infiltration analysis suggested that the proportions of activated dendritic cells, M1 and M2 macrophages, resting mast cells, and regulatory T cells were significantly different between the vitiligo lesions and controls (Fig. 6B). These immune cells were significantly correlated with most pre-DMEGs (p value < 0.05), as displayed by a correlation heat map (Fig. 6C). Additionally, analysis using the LASSO algorithm yielded four genes, DCT, EDNRB, OCA2, and TTK, as biomarkers (Figure S3). The performance assessment of each biomarker in the validation dataset using ROC analysis revealed high AUC values for these biomarkers, suggesting that their use is promising (Fig. 6D).
A Expression levels of “cell division”-related genes and “pigmentation”-related genes in vitiligo lesions and controls. B Analysis of immune cell infiltration using CIBERSORT. C Correlation heat map between the expression levels of key pre-DMEGs and immune infiltration scores of cells. D ROC curves for predicted biomarkers in the validation dataset. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns no significance, ROC receiver operating characteristic
Discussion
To the best of our knowledge, this is the first study to report a genome-wide analysis of the DNA methylation profile of vitiligo in the human skin. We observed that vitiligo lesions generally had more hypomethylated sites than hypermethylated sites. By integrating the transcriptome data, we identified 264 DMEGs and eight functional modules. We conducted a pairwise comparison of lesions, peri-lesions, and controls and found that during the development of vitiligo, changes in DNA methylation seemed to precede transcriptome changes. Further analysis using single-cell data confirmed that this might be related to enhanced communication between cycling keratinocytes and melanocytes.
Our previous study on DNA methylation of vitiligo melanocyte cell lines preliminarily revealed the role of DNA methylation modifications in vitiligo and identified three epigenetic modules: cell cycle and cell division (involving five genes: CDK1, ECT2, HELLS, KIF23, and PBK), melanogenesis and pigmentation (involving four genes: MC1R, MLANA, SOX10, and TYRP1), and the regulation of cellular responses to stress (involving three genes: BCL2L1, PTGS2, and HSP90AA1) (Pu et al. 2021). These results from cell lines are consistent with the results of the present study of clinical skin samples. The eight functional modules identified in the present study were related to “cell division,” “the process of pigmentation,” “circadian rhythm,” “fatty acid metabolism,” “peroxidase activity,” “the regulation of synapses,” and “extracellular matrix.” The circadian rhythm plays a role in CD4 + cells and natural killer cell from the peripheral blood of patients with vitiligo (Mozzanica et al. 1989, 1990, 1992,). Furthermore, some studies preliminarily explored the characteristics of fatty acid metabolism in vitiligo and a potential relationship between fatty acid-binding proteins and the diagnosis of vitiligo was identified (Ye et al. 2022; Farag et al. 2022). Moreover, oxidative stress is widely known to lead to melanocyte death through dysregulation of molecules and immune responses (Xuan et al. 2022). The modules and genes identified here may aid further research on these aspects.
We found that in the peri-lesions, which are clinically the same as normal skin, the methylation level of some genes was altered before the transcription level. However, in vitiligo lesions, these genes showed significant differences at both the methylation and transcription levels. Therefore, we concluded that there was subclinical vitiligo-methylation in the peri-lesions, which might be useful for predicting vitiligo progression and identifying therapeutic targets. The characteristics of non-lesional skin in patients with skin diseases have been studied. In psoriasis, a chronic inflammatory immunological skin disease, many differential DNA methylation sites have been found in the uninvolved epidermis compared with healthy controls, which prompt a pre-psoriatic state in uninvolved skin (Verma et al. 2018; Roberson et al. 2012). Additionally, some microRNAs and cytokines showed a robust trend of increased expression from non-lesions to peri-lesions, and then to paired lesions (Delić et al. 2020; Rasheed et al. 2016). Moreover, patients with squamous cell carcinoma, a common form of skin malignancy, have been reported to exhibit enhanced metabolic activity in peri-tumoral regions, characterized by increased consumption of glucose and glutamine (Mei et al. 2022). For vitiligo, previous studies have reported some factors found in the non-depigmented skin of patients with vitiligo and their potential pathogenic role. Interestingly, Regazzetti et al. found that the expression of CXCL10 increased in peri-lesions and non-depigmented skin rather than in vitiligo lesions (Regazzetti et al. 2015). Moreover, in non-lesional skin, scRNA-seq analysis of vitiligo patients revealed a subclinical activated state of T cells and a subclinical inflammatory state caused by the cell type-specific responses to IFN-γ (Gellatly et al. 2021). In the present study, we also proposed a subclinical vitiligo-methylation state after analyzing DNA methylation data as well as transcriptome data. Therefore, investigating non-depigmented skin, especially peri-lesions, might be a direction for future research on skin diseases and could serve as a starting point for identifying biomarkers of dermatosis-prone skin before onset.
In this study, subclinical vitiligo-methylation state-related genes were mainly associated with cell division and pigmentation. Further analysis of the scRNA-seq data revealed that they were distributed in cycling keratinocytes and melanocytes. In general, keratinocytes control the proliferation behavior and localization of epidermal melanocytes (Brandner and Haass 2013). In addition, keratinocyte-derived factors and proteins have been found to be related to melanogenesis (López et al. 2015). A previous study suggested that proliferating keratinocytes are involved in the proliferation, pigmentation, and dendritogenesis of melanoblasts or melanocytes, which might be related to the secretion of some mitogens and melanogens by keratinocytes in the epidermis (Hirobe et al. 2002). However, the mechanism underlying this occurrence is still unclear. Herein, we predicted the cellular communication between cycling keratinocytes and melanocytes and found that their enhanced communication may be mainly associated with the extracellular matrix (ECM). The ECM plays a vital role in cell migration, homeostasis, the physical framework of cellular constituents, and urgent biomechanical initiation (Abedin and King 2010; Michel et al. 2010). It contains many fibrous proteins such as collagens, fibronectin, and laminins, and adhesion components such as E-cadherin and integrins. Interest in the role of the ECM in vitiligo has increased in recent years. Several studies have highlighted differential expression of ECM components in vitiligo lesions (Rani et al. 2023; Bastonini et al. 2016; Poole et al. 1997). They observed aberrant expression of collagen, elastin, fibronectin, E-cadherin, and integrins, which can inhibit the adhesion and binding of melanocytes and lead to the loss of melanocytes in vitiligo. However, the specific role of DNA methylation in the ECM of vitiligo skin is unknown and may be a valuable topic for future investigations.
Although we preliminarily analyzed the DNA methylation profile in vitiligo skin samples in this study, there are some limitations. First, owing to data limitations, our external validation dataset only covered the expression of genes in vitiligo, rather than the expression and methylation status of genes in peri-vitiligo. Although our DNA methylation dataset, which included six patients and eight controls, and the transcriptome dataset, which included ten patients and ten controls, appear to be sufficient for this analysis, larger multi-omics datasets and external comprehensive validation datasets are still needed. Moreover, it will be valuable to ascertain whether changes in DNA methylation levels are related to the progression of vitiligo, which could shed light on the treatment of this condition. Furthermore, it is worth experimentally verifying the association between cycling keratinocytes and melanocytes in vitiligo and whether the ECM plays an important role in the pathogenesis of vitiligo. The role of DNA methylation in the ECM may become the next hot topic in vitiligo research, and it would be interesting to explore this in future studies.
Conclusions
In conclusion, we performed a preliminary DNA methylation study on human vitiligo skin samples and found that hypomethylated sites were more abundant than hypermethylated sites. Further integrative analysis of transcriptome data identified 264 DMEGs and their enrichment in cell division, pigmentation, circadian rhythm, fatty acid metabolism, peroxidase activity, synapse regulation, and the ECM. After comparative analysis of DNA methylation and transcriptome profiles of vitiligo lesions, peri-lesions, and healthy controls, misalignment of DNA methylation and transcriptional changes in peri-lesions was observed. The related genes were found to be distributed in cycling keratinocytes and melanocytes, and the potential role of the ECM was identified in scRNA-seq data analysis. This study provides new insights into the occurrence and development of vitiligo and direction for potential therapeutic developments.
Availability of data and materials
They are available from the corresponding author upon reasonable request.
References
Abedin M, King N (2010) Diverse evolutionary paths to cell adhesion. Trends Cell Biol 20(12):734–742
Bastonini E, Kovacs D, Picardo M (2016) Skin pigmentation and pigmentary disorders: focus on epidermal/dermal cross-talk. Ann Dermatol 28(3):279–289
Bergqvist C, Ezzedine K (2020) Vitiligo: a review. Dermatology (Basel, Switzerland) 236(6):571–592
Bibeau K, Pandya AG, Ezzedine K, Jones H, Gao J, Lindley A et al (2022) Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol 36(10):1831–1844
Brandner JM, Haass NK (2013) Melanoma’s connections to the tumour microenvironment. Pathology 45(5):443–452
Chen J, Li S, Li C (2021) Mechanisms of melanocyte death in vitiligo. Med Res Rev 41(2):1138–1166
Chen KD, Huang YH, Guo MM, Chang LS, Chu CH, Bu LF et al (2022) DNA methylation array identifies golli-MBP as a biomarker for disease severity in childhood atopic dermatitis. J Invest Dermatol 142(1):104–113
Delić D, Wolk K, Schmid R, Gabrielyan O, Christou D, Rieber K et al (2020) Integrated microRNA/mRNA expression profiling of the skin of psoriasis patients. J Dermatol Sci 97(1):9–20
Ezzedine K, Eleftheriadou V, Whitton M, van Geel N (2015) Vitiligo. Lancet (Lond, Engl) 386(9988):74–84
Ezzedine K, Eleftheriadou V, Jones H, Bibeau K, Kuo FI, Sturm D et al (2021) Psychosocial effects of vitiligo: a systematic literature Review. Am J Clin Dermatol 22(6):757–774
Farag AGA, Badr EAE, El-Shafey AES, Elshaib ME (2022) Fatty acid-binding protein 4 circulating levels in non-segmental vitiligo. Anais Bras De Dermatol 97(1):28–36
Frisoli ML, Essien K, Harris JE (2020) Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol 26(38):621–648
Gellatly KJ, Strassner JP, Essien K, Refat MA, Murphy RL, Coffin-Schmitt A et al (2021) scRNA-seq of human vitiligo reveals complex networks of subclinical immune activation and a role for CCR5 in T(reg) function. Sci Transl Med 13(610):eabd8995
Hirobe T, Furuya R, Akiu S, Ifuku O, Fukuda M (2002) Keratinocytes control the proliferation and differentiation of cultured epidermal melanocytes from ultraviolet radiation B-induced pigmented spots in the dorsal skin of hairless mice. Pigment Cell Res 15(5):391–399
Javierre BM, Fernandez AF, Richter J, Al-Shahrour F, Martin-Subero JI, Rodriguez-Ubreva J et al (2010) Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res 20(2):170–179
Köhler F, Rodríguez-Paredes M (2020) DNA methylation in epidermal differentiation, aging, and cancer. J Invest Dermatol 140(1):38–47
Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK (1997) Tenascin is overexpressed in vitiligo lesional skin and inhibits melanocyte adhesion. Br J Dermatol 137(2):171–178
Lei W, Luo Y, Lei W, Luo Y, Yan K, Zhao S et al (2009) Abnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositis. Scand J Rheumatol 38(5):369–374
López S, Alonso S, García de Galdeano A, Smith-Zubiaga I (2015) Melanocytes from dark and light skin respond differently after ultraviolet B irradiation: effect of keratinocyte-conditioned medium. Photodermatol Photoimmunol Photomed 31(3):149–158
Mei L, Ying L, Wang H, Xu G, Ye X, Yang G (2022) (1)H NMR-based metabolomics of skin squamous cell carcinoma and peri-tumoral region tissues. J Pharm Biomed Anal 1(212):114643
Michel G, Tonon T, Scornet D, Cock JM, Kloareg B (2010) The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol 188(1):82–97
Mozzanica N, Frigerio U, Negri M, Tadini G, Villa ML, Mantovani M et al (1989) Circadian rhythm of natural killer cell activity in vitiligo. J Am Acad Dermatol 20(4):591–596
Mozzanica N, Frigerio U, Finzi AF, Cattaneo A, Negri M, Scaglione F et al (1990) T cell subpopulations in vitiligo: a chronobiologic study. J Am Acad Dermatol 22(2 Pt 1):223–230
Mozzanica N, Villa ML, Foppa S, Vignati G, Cattaneo A, Diotti R et al (1992) Plasma alpha-melanocyte-stimulating hormone, beta-endorphin, met-enkephalin, and natural killer cell activity in vitiligo. J Am Acad Dermatol 26(5 Pt 1):693–700
Pu Y, Chen X, Chen Y, Zhang L, Chen J, Zhang Y et al (2021) Transcriptome and differential methylation integration analysis identified important differential methylation annotation genes and functional epigenetic modules related to vitiligo. Front Immunol 12:587440
Rani S, Pervaiz N, Parsad D, Kumar R (2023) Differential expression of extracellular matrix proteins in the lesional skin of vitiligo patients. Arch Dermatol Res. https://doi.org/10.1007/s00403-023-02628-z
Rasheed H, El-Komy M, Hegazy RA, Gawdat HI, AlOrbani AM, Shaker OG (2016) Expression of sirtuins 1, 6, tumor necrosis factor, and interferon-γ in psoriatic patients. Int J Immunopathol Pharmacol 29(4):764–768
Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P et al (2015) Transcriptional analysis of vitiligo skin reveals the alteration of WNT pathway: a promising target for repigmenting vitiligo patients. J Invest Dermatol 135(12):3105–3114
Roberson ED, Liu Y, Ryan C, Joyce CE, Duan S, Cao L et al (2012) A subset of methylated CpG sites differentiate psoriatic from normal skin. J Invest Dermatol 132(3 Pt 1):583–592
Shiu J, Zhang L, Lentsch G, Flesher JL, Jin S, Polleys C et al (2022) Multimodal analyses of vitiligo skin identify tissue characteristics of stable disease. JCI Insight. https://doi.org/10.1172/jci.insight.154585
Singh A, Gotherwal V, Junni P, Vijayan V, Tiwari M, Ganju P et al (2017) Mapping architectural and transcriptional alterations in non-lesional and lesional epidermis in vitiligo. Sci Rep 7(1):9860
Verma D, Ekman AK, BivikEding C, Enerbäck C (2018) Genome-wide DNA methylation profiling identifies differential methylation in uninvolved psoriatic epidermis. J Invest Dermatol 138(5):1088–1093
Xuan Y, Yang Y, Xiang L, Zhang C (2022) The role of oxidative stress in the pathogenesis of vitiligo: a culprit for melanocyte death. Oxid Med Cell Longev 2022:8498472
Ye Z, Chen J, Du P, Ni Q, Li B, Zhang Z et al (2022) Metabolomics signature and potential application of serum polyunsaturated fatty acids metabolism in patients with vitiligo. Front Immunol 13:839167
Zhao M, Gao F, Wu X, Tang J, Lu Q (2010) Abnormal DNA methylation in peripheral blood mononuclear cells from patients with vitiligo. Br J Dermatol 163(4):736–742
Zhao M, Huang W, Zhang Q, Gao F, Wang L, Zhang G et al (2012) Aberrant epigenetic modifications in peripheral blood mononuclear cells from patients with pemphigus vulgaris. Br J Dermatol 167(3):523–531
Zhao M, Liang G, Wu X, Wang S, Zhang P, Su Y et al (2012) Abnormal epigenetic modifications in peripheral blood mononuclear cells from patients with alopecia areata. Br J Dermatol 166(2):226–273
Zhou F, Shen C, Xu J, Gao J, Zheng X, Ko R et al (2016) Epigenome-wide association data implicates DNA methylation-mediated genetic risk in psoriasis. Clin Epigenetics 8:131
Acknowledgements
We are very grateful to the patients and volunteers involved in this study.
Funding
The National Natural Science Foundation of China (82073462). Chongqing Natural Science Foundation (CSTB2023NSCQ- MSX0664 and CSTB2023NSCQ- MSX0075).
Author information
Authors and Affiliations
Contributions
Conceptualization, LL and YX. Data collection and processing, YL, XP, and YC. Interpretation of data, YH. Software, TC, XS, and JZ. LL is responsible for writing the initial article. Revision and finalization, YP and JC. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
None declared.
Ethical approval and consent to participate
For patient samples, written informed consent was obtained from each patient and the study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (No.:2023-126). The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
439_2023_2630_MOESM1_ESM.tif
Supplementary file1 Figure S1. (A) Venn plots of the overlap of DEGs in the pairwise comparisons of L, PL, and HC. Gene ontology enrichment of DEGs of (B) L vs. HC, (C) PL vs. HC, and (D) L vs. PL. L, lesion; HC, healthy control; PL, peri-lesion. (TIF 2120 kb)
439_2023_2630_MOESM2_ESM.tif
Supplementary file2 Figure S2. (A) PPI network of 264 DMEGs. (B) PPI network of 102 pre-DMEGs. PPI, Protein-Protein Interaction; DMEGs, differentially methylated and expressed genes. (TIF 3803 kb)
439_2023_2630_MOESM3_ESM.tif
Supplementary file3 Figure S3. LASSO logistic regression algorithm. (A) LASSO coefficient profiles. (B) Cross-validation for tuning parameter selection in the LASSO model. (TIF 1183 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Xue, Y., Li, Y. et al. Genome-wide DNA methylation of lesional and peri-lesional skin in vitiligo: a comparative and integrated analysis of multi-omics in Chinese population. Hum. Genet. 143, 137–149 (2024). https://doi.org/10.1007/s00439-023-02630-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-023-02630-5