Abstract
We investigated the relationship between neuroticism and 16 mental and 18 physical traits using summary results of genome-wide association studies for these traits. LD score regression was used to investigate genetic correlations between neuroticism and the 34 health outcomes. Mendelian randomization was performed to investigate mutual causal relationships between neuroticism and the 34 health outcomes. Neuroticism genetically correlates with a majority of health-related traits and confers causal effects on 12 mental traits (major depressive disorder (MDD), insomnia, subjective well-being (SWB, negatively), schizophrenia, attention-deficit/hyperactivity disorder, alcohol dependence, loneliness, anorexia nervosa, anxiety disorder, bipolar disorder, obsessive–compulsive disorder, and psychiatric disorders) and two physical diseases (cardiovascular disease and hypertensive disease). Conversely, MDD, SWB, and insomnia have a causal effect on neuroticism. We highlighted key genes contributing to the causal associations between neuroticism and MDD, including RBFOX1, RERE, SOX5, and TCF4, and those contributing to the causal associations between neuroticism and cardiovascular diseases, including MAD1L1, ARNTL, RERE, and SOX6. The present study indicates that genetic variation mediates the causal influences of neuroticism on mental health and cardiovascular diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroticism is one of the five higher order factors taxonomically defining one’s personality. The modern conception of neuroticism was initially introduced by Hans Eysenck and his contemporaries when it was defined in Freudian theory terms (Ormel et al. 2013). Currently, the consensus has neuroticism as the tendency to experience negative emotions, including anxiety, fear, sadness, anger, guilt, disgust, irritability, loneliness, worry, self-consciousness, dissatisfaction, hostility, embarrassment, reduced self-confidence, and feelings of vulnerability, in reaction to various types of stress, and to place themselves into situations that foster negative affect (Jeronimus et al. 2014; Specht et al. 2011). The individual differences in neuroticism scores show moderate to high stability across much of the adult life course (Wray et al. 2007).
Mounting evidence suggests that neuroticism has profound significance for mental health (Lahey 2009; Ormel et al. 2013). Specifically, people who score higher in the neuroticism dimension have a greater propensity for psychiatric conditions, such as anxiety, somatoform, obsessive–compulsive disorder (OCD), as well as schizophrenia (SZ), bipolar disorder (BD), major depression disorder (MDD), and attention-deficit hyperactivity disorder (ADHD) (Kendler and Myers 2010; Kotov et al. 2010; Lonnqvist et al. 2009; Michielsen et al. 2014; Schirmbeck et al. 2015; Van Os and Jones 2001). Furthermore, several studies linked higher levels of neuroticism to an increase in all-cause mortality (Gale et al. 2016). Neuroticism also contributes to other phenomena that correlate strongly with psychological distress, for example, persistent low subjective well-being (SWB), and physical health problems (Heller et al. 2004; Okbay et al. 2016). Previous studies had quantified neuroticism’s cross-sectional association with common mental disorders (CMDs), but also revealed the strong predictive effect of neuroticism with CMDs in prospective association (Kotov et al. 2010; Lahey 2009; Lonnqvist et al. 2009; Van Os and Jones 2001). Discovered connections between neuroticism and CMDs have promoted the assumption that neuroticism is an independent, etiologically informative risk factor, which may actively contribute to the progress of CMDs.
A vital role of neuroticism in mental health has long been documented, but whether the association of neuroticism with psychopathology is determined by inherited genetic variants remains elusive. Previous studies indicated that the possible explanation for associations between neuroticism and these various mental disorders may be due to shared genetic components. Genetic correlations have been identified between neuroticism and anorexia nervosa, MDD, and SZ, and attributed to the genes with pleiotropic effects (Gale et al. 2016; Hill et al. 2019). In addition, some of the subfactors of neuroticism were also genetically associated with BD, ADHD, and autism spectrum disorder (ASD) (Hill et al. 2019). Furthermore, previous studies reported moderate to high genetic correlations between neuroticism and obsessive–compulsive symptoms (Bergin et al. 2014; Taylor et al. 2011). Twin studies have shown considerable overlaps between the genetic factors of neuroticism and those conferring risk for internalizing disorders, such as anxiety and depression (Hettema et al. 2006).
Recently, genome-wide association studies (GWAS) have begun to map the loci that are associated with neuroticism (Luciano et al. 2018; Smith et al. 2016), as well as ones contributing both to neuroticism and psychiatric disorders (Barlow et al. 2014; Lo et al. 2017; Smeland et al. 2017). Current evidence strongly supports the importance of neuroticism in the genetic etiology of mental disorders. However, causal relationships between neuroticism and a wide range of human health outcomes, especially physical conditions, remain largely unknown.
Mendelian randomization (MR) is an analytic technique that uses genetic variants as instrumental variables to test for causative association between an exposure and an outcome (Lawlor et al. 2008). MR is becoming increasingly efficient and cost-effective when applied to ever-growing data curated from recent GWAS. Recently, the GSMR (Generalized Summary-data-based MR) had been developed by leveraging power from multiple genetic variants accounting for linkage disequilibrium (LD) between the variants (Zhu et al. 2018).
Here, we hypothesize that genetic liability of neuroticism may confer risk to mental health and some kinds of physical diseases. In the present study, we used LD score regression to trace the genetic correlations of neuroticism to a panel of mental and physical conditions. We further used the GSMR to perform a multi-SNP MR analysis on summary results presented in GWAS datasets to test the causal associations between neuroticism and these conditions. Finally, we curated and compared genome-wide risk genes for neuroticism and 13 traits causally influenced by neuroticism. For further dissection, we have concentrated on the connection of neuroticism to MDD and cardiovascular diseases (CVDs), two major causes of morbidity in world populations.
Materials and methods
Datasets and quality control
Summary results of GWAS for 17 mental and 18 physical conditions were included in the analyses (Table 1). The mental conditions were selected based on each condition’s frequency and importance, and the physical conditions were selected based on each condition’s significance within human non-communicative chronic disorders. The trait psychiatric disorders included the collection of psychological/psychiatric problem, mania/bipolar disorder/manic depression, schizophrenia, anxiety/panic attacks. The cardiovascular disease (CVD) included a panel of cardiovascular conditions recruited by the UKB (Supplementary File) (Sudlow et al. 2015). In this study, we used CVD to specifically denote the collection of cardiovascular conditions used the UKB dataset, and used CVDs to denote all cardiovascular conditions. All participants were of European origins. Per condition sample sizes have ranged from 7556 to 694,649. Detailed information of these included studies is summarized in Table 1. We compared SNP alleles between the neuroticism dataset and each of the other datasets and performed the following quality control steps: (1) SNPs were fileted based on INFO ≥ 0.90 if it exists; (2) each SNP was compared between the two datasets and SNPs with conflicting alleles between each pair of datasets were excluded; (3) the alleles and effects of the datasets were harmonized. For palindromic SNPs, we employed allele frequency (FRQ) filtering to determine whether the alleles from the two GWASs were derived from the same reference panel or not (SNPs with FRQ difference between the two GWASs ≥ 0.20 were deemed as a mapping from opposite reference panels).
Genetic correlation analysis
GWAS summary results were utilized to analyze the genetic correlations of neuroticism with the 34 health conditions using LD score regression (Bulik-Sullivan et al. 2015a; b). The 1000 Genome project phase 3 (Genomes Project et al. 2015) was used to estimate the LD structure for European populations, which was obtained from the LD score regression website (Bulik-Sullivan et al. 2015a, b; Finucane et al. 2015). SNPs were filtered by 1.1 million variants, a subset of 1000 Genomes and HapMap3, with MAF above 0.05. Adjustment for multiple tests was achieved by calculating the false discovery rate (FDR).
Bidirectional MR analysis
Bidirectional causal associations between neuroticism and the 34 traits were inferred using GSMR (Zhu et al. 2018), and FDR was calculated for the 68 tests. This method utilizes summary-level data to test for putative causal associations between a risk factor (exposure) and an outcome using independent genome-wide significant SNPs as instrumental variables as an index of the exposure. Instrumental variants were selected based on default P ≤ 5 × 10–8 and further pruned using a clumping r2 cutoff of 0.01. When the threshold was surpassed by less than 10 SNPs, a P value threshold of 1 × 10–5 was used; when more than 10,000 SNPs surpassed the default threshold, further analysis was limited to the top 10,000 most significant SNPs. HEIDI outlier detection was used to filter genetic instruments that showed clear pleiotropic effects on the exposure phenotype and the outcome phenotype. We used a P value threshold of 0.01 for the outlier detection analysis in HEIDI, which removes 1% of SNPs by chance if there is no pleiotropic effect.
The method estimates a putative causal effect of the exposure on the outcome (bxy) as a function of the relationship between the SNP’s effects on the exposure (bzx) and the SNP’s effects on the outcome (bzy), given the assumption that the effect of non-pleiotropic SNPs on an exposure (x) should be related to their effect on the outcome (y) in an independent sample only via mediation through the phenotypic causal pathway (bxy). The estimated causal effect coefficients (bxy) are approximately equal to the natural log odds ratio for a case–control trait. An odds ratio of 2 can be interpreted as a doubled risk compared with the population prevalence of a binary trait for every standard deviation increase in the exposure trait. Criteria for significant causal association included both the absolute value of effect size bxy ≥ 0.10 and FDR < 0.05.
To explore the mechanisms underlying the causal associations between neuroticism and MDD, CVD, and HD, we mapped the instrumental SNPs into their host genes. FUMA was used to map SNPs to genes and identify LD-independent genomic regions (Watanabe et al. 2017). All genes located within 10 kb vicinity of each variant were mapped. Therefore, these mapped genes contributed to the causal effects between neuroticism and MDD, CVD, and HD.
Curation of GWAS genes and gene overlapping analysis
We obtained GWAS results (including meta-analysis) of the 14 traits with a causal association with neuroticism from the GWAS Catalog database (https://www.ebi.ac.uk/gwas/) (Buniello et al. 2019). Quality control steps: (1) studies with sample size of ≥ 2000 were retained; (2) studies with mixed phenotypes were removed; (3) genes in MHC regions were removed due to the high LD within the MHC region, which may inflate the gene overlapping analysis; (4) pharmacogenetic studies were excluded. The results of quality control steps are listed in Supplementary Table 1.
Analysis of overlaps between gene sets of each trait with those of neuroticism was conducted using R package SuperExactTest (Wang et al. 2015), with the total gene number in the genome being set as 30,000.
All the statistical analyses were conducted in R 3.6.1 or Python 3.7 environment. A more detailed description of the methods is provided in the Supplementary File.
Results
Genetic correlations
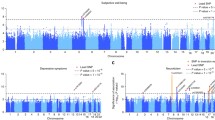
Genetic correlation analyses indicated that neuroticism has a significant genetic correlation with all the 16 mental conditions, and with 8 out of the 18 physical conditions studied (Supplementary Table 2, Fig. 1a). In particular, a relatively high genetic correlation was detected between neuroticism and SWB (rg = − 0.68) and MDD (0.67), followed by anxiety disorder, loneliness, psychiatric disorders, insomnia, alcohol dependence, and OCD (rg in range of 0.31–0.54). The average absolute rg for correlations between neuroticism and mental conditions was 0.34 ± 0.18, while the average absolute rg between neuroticism and physical conditions was 0.11 ± 0.05.
Relationships of neuroticism to each of correlating traits. AD Alzheimer’s disease, ADHD attention-deficit/hyperactivity disorder, ASD autism spectrum disorder, BD bipolar disorder, BMI waist-to-hip ratio adjusted for body mass index, CAD coronary artery disease, CVD cardiovascular disease, HAC hernia abdominopelvic cavity, IBD inflammatory bowel disease, MDD major depressive disorder, OCD obsessive–compulsive disorder, PTSD posttraumatic stress disorder, PVD peripheral vascular disease, SWB subjective well-being, SZ schizophrenia, T2D type 2 diabetes, TS Tourette syndrome, HAC hernia abdominopelvic cavity, PVD peripheral vascular disease, HD hypertensive disease, PD psychiatric disorders. a Genetic correlation of neuroticism with all traits assessed. Red bars indicate FDR ≤ 0.05, and blue bars indicate FDR > 0.05; the lines in each bar indicate standard error. b Genetic correlation coefficient and Mendelian randomization effect size for each trait. c Mendelian randomization analysis of neuroticism with all traits assessed. Arrowed lines show a causal effect from a source node to a targeted node; red lines show positive coefficient, and blue lines show negative coefficient; the thickness of lines is relative to the absolute value of the coefficient. d Pyramid depicting the hierarchy of the relationship between all traits connected to neuroticism
MR analysis
In the MR analysis, neuroticism displayed a causal effect on 12 out the 16 mental conditions (MDD, insomnia, SWB, SZ, ADHD, alcohol dependence, loneliness, anorexia nervosa, anxiety disorder, BD, OCD, and psychiatric disorders) and two physical conditions (hypertensive disease (HD) and CVD) (Table 2 and Fig. 1c). Conversely, MDD, insomnia, and reduced SWB displayed a causal effect on neuroticism. The SNPs and genes contributing to the causal effect between neuroticism and MDD are listed in Supplementary Tables 3–4. The SNPs and genes contributing to the causal effect of neuroticism on CVD and HD are listed in Supplementary Tables 5–6. Figure 2 depicts outcomes of specific analysis which uncovered genes mediating the causal effects between neuroticism and MDD, CVD, and HD. The genetic correlation coefficient and MR effect size for each trait are shown in Fig. 1b.
The mental and physical traits can be grouped into four classes based on their relationship with neuroticism (see Fig. 1d). At the first level, traits do not have any genetic relationship with neuroticism, while the traits residing at the second level and the neuroticism share some genetic background. Traits at the third level both share a genetic background with neuroticism, and are causally influenced by neuroticism. Fourth-level traits (at the top) display both high genetic correlations and mutually causal relationships with neuroticism.
Gene overlaps
GWAS results of the 14 traits are listed in Supplementary Tables 7–20. Except for OCD and anxiety disorder, each trait showed significant over-representation of overlapped genes with neuroticism (Supplementary Table 21). Especially, 304 out of 590 GWAS risk genes for SWB (significant overlapping, P = 0; FDR = 0) and 218 out of 791 GWAS risk genes for MDD (significant overlapping, P = 1.47 × 10–166; FDR = 1.03 × 10–165) were overlapped with those for neuroticism.
Discussion
The profound significance of neuroticism for public health is evidenced by the fact that the estimated economic costs of neuroticism exceed those of common mental disorders (Cuijpers et al. 2010). Higher scores for neuroticism are associated with an increased risk for various mental disorders, and, somewhat less consistently, with certain aspects of physical health. A well-known case is the type A behavior pattern (TABP) as a coronary risk factor. An overall association between the TABP and CHD has been well established (Smith and MacKenzie 2006). The prevailing model to explain this association is that type A individuals show higher levels of cardiovascular and neuroendocrine responses to stressors compared with their more relaxed Type B counterparts. Type D (i.e., distressed) personality is another kind of personality associated with CHD (Denollet 2005; Denollet et al. 1996). One of the two factors of this construct—negative affectivity—corresponds closely to neuroticism (Denollet 2005).
The evidence supporting the association of neuroticism with mental health and cardiovascular risk is convincing, the emphasis at present is to determine the mechanisms involved. The concept that personality influences health seems straightforward, but the explication of mechanisms underlying mind–body associations is conceptually complex and methodologically challenging due to inconsistent findings and alternative interpretations (Smith and MacKenzie 2006).
We outline the models of mechanisms for neuroticism into two broad categories, physiological reactions to stressors and health-related behavior alterations. Within the psychological reaction category, neuroticism influences the appraisal of potentially stressful life circumstances and coping responses and leads to various negative affections, including depression, anxiety, hostility, and anger, which are also risk factors for CVDs (Barth et al. 2004; Chida and Steptoe 2009). Physiological mechanisms underlying psychological reactions include neuroendocrine responses, inflammation, autonomic function (Schneiderman et al. 2005). Another category concerns patterns of behavioral changes. Personality influences health-relevant daily habits (e.g., smoking, diet, and exercise) relate to negative health behaviors, which could mediate the association between personality and disease (Booth-Kewley and Vickers 1994). Higher neuroticism could lead to unhealthy behaviors such as poor diet, smoking, sleep disturbances, physical inactivity, or lower treatment adherence (Chida and Steptoe 2009). Neuroticism has higher comorbidity with alcohol and drug dependence, conduct disorders, and antisocial personality (Khan et al. 2005). Of note is that the two aspects are not independent and mutually exclusive, but rather act together to link neuroticism to elevated cardiovascular risk.
Our results indicate that neuroticism may confer risk to mental health and CVDs through genetic variation, therefore, reveal a novel mechanism by which neuroticism influences the risk for mental disorders and CVDs. It is a more fundamental way compared with theories derived from psychological effects and health behaviors. The evidence derived from genetic variants as instrumental variables indicates that the mediating effects are most likely etiologically causal in nature compared with other mechanisms. Undoubtedly, the psychological, behavioral, and genetic mechanisms and pathways operate in a synergistic and integrative way to promote cardiovascular changes and related clinical manifestations.
Recently, genetic correlations of neuroticism with some health conditions have been reported. Gale et al. examined genetic correlations of neuroticism with 9 mental traits and 8 physical health-related outcomes, and reported significant positive associations between neuroticism and MDD, anorexia nervosa, and SZ (Gale et al. 2016). Nagel et al. investigated 9 mental traits and 26 other traits, and detected significant positive genetic correlations between neuroticism and 11 health characteristics, including MDD, ADHD, anorexia nervosa, SZ, intracranial volume, and significant negative genetic correlations between neuroticism and SWB, height, age of first child, intelligence quotient (IQ), and educational attainment (Nagel et al. 2018). Using GWAS results from the UK Biobank (UKB), Hill et al. (2019) found that neuroticism significantly correlates with 20 out of the 32 studied conditions; the resultant list of correlates included SZ, MDD, anorexia nervosa, ADHD, and SWB (negatively).
In our study, we found that neuroticism shows a correlation with all mental conditions studied and nearly half of physical conditions and characteristics, with relatively higher correlations between neuroticism and MDD, SWB (negatively), anxiety, loneliness, and insomnia. In addition to replicating previously reported correlations of neuroticism with SZ, anorexia nervosa, AD, and ADHD, our study detected novel genetic connections between neuroticism and loneliness, insomnia, alcohol dependence, OCD, and TS. For physical conditions, our study identified genetic correlations of neuroticism with asthma, CVD, dyslipidemia, osteoarthritis, coronary artery disease, body mass index (BMI), inflammatory bowel disease, HD, and type 2 diabetes. Predictably, the average correlation of neuroticism with mental conditions was stronger than that observed for neuroticism and physical conditions.
Even if previous studies have detected correlations between neuroticism and health-related traits, the causality of these relationships has rarely been tested. Nagel et al. reported a significant causal effect of neuroticism on depression, anxiety disorder, ADHD, SWB, SZ, IQ, and educational attainment, as well as a reverse causal effect of some traits on neuroticism, including depression, ADHD, SWB, SZ, childhood obesity, intracranial volume, height, age of first child, IQ, and educational attainment.
Presented results suggest that neuroticism has a close genetic relationship with all major mental conditions, and in most cases, these relationships are causal. We augmented the study of Nagel et al. by identifying additional traits genetically influenced by neuroticism, including insomnia, alcohol dependence, loneliness, anorexia nervosa, BD, and OCD. Thus, etiological evidence for the previous observation that high scores for neuroticism predate the onset and development of any CMD, and that their effects rarely decline over time (Jeronimus et al. 2016) is provided. Furthermore, our study shows a causal relationship between neuroticism and CVDs, pointing at the strength of the mind–body relationship underlining at least some physical conditions. In this light, it is important to note a handful of genes and protein products recently shown to contribute both to psychological traits, including neuroticism, and physical diseases. The causal role of neuroticism in the development of a physical disease may inform a pathway to truly personalized, prevention-driven medicine.
In agreement with the above results, we observed significant enrichment of overlapping genes between neuroticism and the majority of the 14 traits tested. Especially, half of GWAS risk genes of SWB and more than a quarter of risk genes of MDD were overlapped with those of neuroticism.
Given together, neuroticism showed the closest relationship with MDD and SWB, as evidenced by strong genetic correlation, large bidirectional causal effect, and striking enrichment of overlapping genes between neuroticism and the above two mentioned characteristics. This might probably attribute to the shared genetic factors, and these shared genetic factors have vertical genetics pleiotropy, which may cause some related intermedia phenotypes to be causally correlated.
A strong association between neuroticism and an increased risk of MDD has been previously reported in two large population cohorts (Navrady et al. 2017), consistent with previous cross-sectional observations that neuroticism is most strongly associated with depressive disorders (Kotov et al. 2010). However, molecular mechanisms underlying the associations of neuroticism and MDD remain elusive. In this study, we revealed a panel of genes contributing to both the causal effect of neuroticism on MDD and those contributing to the causal effect of MDD on neuroticism, including DCC, LINC00461, DKFZP686K1684, LOC105377703, RBFOX1, RERE, SOX5, and TCF4. Each of these genes has been previously identified as a genome-wide gene for each of the two traits. Therefore, these genes are plausible key contributors to the bidirectional causal associations of the two conditions.
The transcription factor 4 encoding gene, TCF4, was one of the first genome-wide risk loci for SZ (Stefansson et al. 2009). Later, in some works, this gene was implicated in MDD (Wray et al. 2018), insomnia (Jansen et al. 2019), Pitt–Hopkins syndrome, and other neurodevelopmental disorders (Forrest et al. 2014). It plays a role in lymphoid tissue development and epithelial–mesenchymal transition (Forrest et al. 2014). DCC plays a key role in axon guidance and nerve regeneration (Finci et al. 2015), and is a genome-wide risk gene for MDD (Wray et al. 2018), neuroticism (Nagel et al. 2018; Okbay et al. 2016), intelligence (Savage et al. 2018), cognitive ability (Lee et al. 2018), and educational attainment (Lee et al. 2018). LINC00461 locus encodes one of the most highly conserved lncRNA, which is predominantly expressed in the brain (Deguchi et al. 2017). Previous GWASs have implicated it in MDD (Nagel et al. 2018) and neuroticism (Baselmans et al. 2019; Luciano et al. 2018; Nagel et al. 2018). RBFOX1 regulates tissue-specific alternative splicing, and have being implicated in neurodevelopmental and neuropsychiatric disorders, including MDD (Hyde et al. 2016; Wray et al. 2018), ASD (Grove et al. 2019), and neuroticism (Baselmans et al. 2019; Luciano et al. 2018; Okbay et al. 2016). DKFZP686K1684 and LOC105377703 are two ncRNAs with unknown functions. The function of RERE is discussed later.
SWB is an indicator of happiness or satisfaction. The highest negative correlation between neuroticism and SWB observed in our study was similar to the results of two previous studies (Hill et al. 2019; Nagel et al. 2018), which is not surprising as all three studies, including the present one, utilized the same dataset for SWB (Okbay et al. 2016). The high negative correlation between neuroticism and SWB and the negative causal effect of neuroticism on SWB points towards an overall detrimental effect of neuroticism on mental health.
In addition to its association with mental and physical diseases, neuroticism also influences one’s social life. This effect is evident from a high genetic correlation between neuroticism and loneliness and an extremely high causal effect of neuroticism on loneliness (bxy = 0.74). These results are consistent with the previously reported genetic correlation between neuroticism and social deprivation (Hill et al. 2019), suggesting an extended role of neuroticism in one’s social behavior and activity.
Personality is commonly seen as an inherited trait that remains stable across the lifespan. Due to the general belief that neuroticism is a risk factor for various mental disorders and distress, previous work heavily emphasized the effect of neuroticism on various medical conditions. In contrast, our present work, along with the study of Nagel et al., indicates that the scores for neuroticism may be affected by an individual’s mental status, too, stressing on bidirectionality of the relationship between neuroticism and mental disorders. The discovery of neuroticism-promoting influence on insomnia and reduced SWB is of clinical relevance. In the case of insomnia, the discovery of its bidirectional causal connections to neuroticism may bring hope for the patients seeking personality improvement, since the sleeping problems are more amenable to modification than the neuroticism itself. The negative genetic correlation and the bidirectional negative causal connections between neuroticism and SWB indicate that either mutually detrimental effects or reciprocally inhibiting mechanisms shared between these conditions are at play. It is tempting to speculate that an individual’s neuroticism may be improved by increasing one’s feeling of happiness, which can be achieved by social interventions or in course of psychotherapy. Together, our results point towards a potential avenue for possible amelioration of maladaptive personality traits, and therefore, for decreasing the risks for the relevant mental disorders by improving the quality of sleep, and/or by enhancing the feeling of happiness or satisfaction.
For physical conditions, neuroticism may genetically exert an effect on CVD and HD. CVDs account for a large proportion of the total disability and morbidity (DALYs and Collaborators 2018). Hypertension is a major risk factor for CVDs, contributing to a large proportion of cardiovascular deaths (Lim et al. 2012). Neuroticism has been reported to be correlated with stroke and increased coronary heart disease mortality (Jokela et al. 2014), but biological mechanisms underlying their associations are poorly understood. Our findings may have implications in the prevention and personalized treatment of CVDs. The American Heart Association (AHA) advocated screening depression in all patients with CADs (Lichtman et al. 2008). Our results suggest that it may be useful to screen neuroticism in patients with CVDs, since improved treatment may be reached by including psychological or psychiatric interventions for subgroups with higher neuroticism.
Here, we explored critical biological molecules and potential mechanisms contributing to the links between neuroticism and CVDs. Of particular interest are MAD1L1, ARNTL, RERE, LINC01122, and SOX6. All these genes were found both to mediate the causal effect of neuroticism to CVD, and to confer genome-wide risks for the two conditions. None of these genes is a novice in the field of neurodevelopment. The arginine-glutamic acid dipeptide (RE) repeats gene (RERE) is located in the proximal 1p36 region critical for neurodevelopment (Zoltewicz et al. 2004). Heterozygous variants in the RERE gene have been reported to be associated with a neurodevelopmental disorder with or without anomalies of the brain, eye, or heart (NEDBEH) (Jordan et al. 2018). In addition, RERE is also one of the candidate genes for CHARGE syndrome (Issekutz et al. 2005) and 1p36 deletion syndrome (Zaveri et al. 2014), sharing the features of the developmental delay, intellectual disability, congenital heart disease, cardiovascular malformations, and cardiomyopathy (Fregeau et al. 2016; Issekutz et al. 2005; Jordan et al. 2018; Zaveri et al. 2014). Mouse models implicate RERE in cerebellar foliation and the migration and maturation of Purkinje cells (Kim and Scott 2014) as well as in ventricular septal defects (Kim et al. 2018). LINC01122 is a non-coding RNA that was linked to depression (Nagel et al. 2018), insomnia (Jansen et al. 2019), Tourette syndrome (Yu et al. 2019), neuroticism (Nagel et al. 2018), and CVDs (Kichaev et al. 2019). MAD1L1 play a role in cell cycle control and has been implicated in the genetic susceptibility for MDD (Howard et al. 2018), SZ (Ripke et al. 2013), BD (Psychiatric 2011), neuroticism (Luciano et al. 2018; Nagel et al. 2018), and CVDs (Kichaev et al. 2019). The protein encoded by SOX6 is a transcriptional activator that plays a versatile regulatory role in vertebrate development, including normal development of the central nervous system and maintenance of cardiac and skeletal muscle cells (Hagiwara 2011). GWASs implicated it in neuroticism (Baselmans et al. 2019; Luciano et al. 2018; Nagel et al. 2018), hypertension or blood pressure (Ehret et al. 2016; Giri et al. 2019; Kichaev et al. 2019; Lu et al. 2015; Takeuchi et al. 2018), and CVDs (Kichaev et al. 2019; van der Harst and Verweij 2018), while molecular physiology research pointed at its direct contribution to the prevention of the cardiac hypertrophy (Huang et al. 2015). The presence of both neurodevelopmental and cardiovascular phenotypes in the spectrum of the effects pertained to each of these genes is remarkable.
Our study had several strengths. First, we analyzed the data for a larger set of traits, especially for mental traits. Second, for each trait, only well-powered datasets were included, and a threshold of MR effect coefficient was used to select the strongest factors. Furthermore, to avoid potential population heterogeneity across the studies, we limited our analysis to individuals of European ancestry.
However, several limitations should also be noted. First, to focus attention on mental conditions, a relatively small number of physical traits was included in the study, thus, necessitating future exploring of the association of neuroticism with physical conditions in detail. Second, amounts of samples comprising different datasets varied substantially—from a minimum of 7,556 samples for loneliness to 694,649 samples for BMI; this imbalance may result in uneven power across the traits. Finally, as our analysis was limited to a genetic component of each trait, presented results should be interpreted cautiously, with the understanding that human traits result from a complex web of interactions among a plethora of psycho-social-environmental factors.
Conclusions
In summary, our findings reveal genetic relationships between neuroticism and various health conditions and shed light on mechanisms underlying their phenotypic relationships.
Data availability
The present study was based on a secondary analysis of GWAS data and all data generated during the study are included in this published article.
Change history
09 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00439-021-02306-y
References
Barlow DH, Ellard KK, Sauer-Zavala S, Bullis JR, Carl JR (2014) The Origins of Neuroticism. Perspect Psychol Sci 9:481–496. https://doi.org/10.1177/1745691614544528
Barth J, Schumacher M, Herrmann-Lingen C (2004) Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 66:802–813. https://doi.org/10.1097/01.psy.0000146332.53619.b2
Baselmans BML, Jansen R, Ip HF, van Dongen J, Abdellaoui A, van de Weijer MP, Bao Y, Smart M, Kumari M, Willemsen G, Hottenga JJ, Boomsma DI, de Geus EJC, Nivard MG, Bartels M, consortium B, Social Science Genetic Association C (2019) Multivariate genome-wide analyses of the well-being spectrum. Nat Genet 51:445–451. https://doi.org/10.1038/s41588-018-0320-8
Bergin J, Verhulst B, Aggen SH, Neale MC, Kendler KS, Bienvenu OJ, Hettema JM (2014) Obsessive compulsive symptom dimensions and neuroticism: an examination of shared genetic and environmental risk. Am J Med Genet B Neuropsychiatr Genet 165B:647–653. https://doi.org/10.1002/ajmg.b.32269
Booth-Kewley S, Vickers RR Jr (1994) Associations between major domains of personality and health behavior. J Pers 62:281–298. https://doi.org/10.1111/j.1467-6494.1994.tb00298.x
Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Patterson N, Daly MJ, Price AL, Neale BM, Schizophrenia Working Group of the Psychiatric Genomics C (2015a) LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 47:291–295. https://doi.org/10.1038/ng.3211
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, ReproGen C, Psychiatric Genomics C, Duncan L, Perry JR, Patterson N, Robinson EB, Daly MJ, Price AL, Neale BM, Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control C (2015b) An atlas of genetic correlations across human diseases and traits. Nat Genet 47:1236–1241. https://doi.org/10.1038/ng.3406
Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, McMahon A, Morales J, Mountjoy E, Sollis E, Suveges D, Vrousgou O, Whetzel PL, Amode R, Guillen JA, Riat HS, Trevanion SJ, Hall P, Junkins H, Flicek P, Burdett T, Hindorff LA, Cunningham F, Parkinson H (2019) The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res 47:D1005–D1012. https://doi.org/10.1093/nar/gky1120
Chida Y, Steptoe A (2009) The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. J Am Coll Cardiol 53:936–946. https://doi.org/10.1016/j.jacc.2008.11.044
Cuijpers P, Smit F, Penninx BW, de Graaf R, ten Have M, Beekman AT (2010) Economic costs of neuroticism: a population-based study. Arch Gen Psychiatry 67:1086–1093. https://doi.org/10.1001/archgenpsychiatry.2010.130
DALYs GBD, Collaborators H (2018) Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1859–1922. https://doi.org/10.1016/S0140-6736(18)32335-3
Deguchi S, Katsushima K, Hatanaka A, Shinjo K, Ohka F, Wakabayashi T, Zong H, Natsume A, Kondo Y (2017) Oncogenic effects of evolutionarily conserved noncoding RNA ECONEXIN on gliomagenesis. Oncogene 36:4629–4640. https://doi.org/10.1038/onc.2017.88
Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, Baldursson G, Belliveau R, Bybjerg-Grauholm J, Baekvad-Hansen M, Cerrato F, Chambert K, Churchhouse C, Dumont A, Eriksson N, Gandal M, Goldstein JI, Grasby KL, Grove J, Gudmundsson OO, Hansen CS, Hauberg ME, Hollegaard MV, Howrigan DP, Huang H, Maller JB, Martin AR, Martin NG, Moran J, Pallesen J, Palmer DS, Pedersen CB, Pedersen MG, Poterba T, Poulsen JB, Ripke S, Robinson EB, Satterstrom FK, Stefansson H, Stevens C, Turley P, Walters GB, Won H, Wright MJ, Consortium AWGotPG, Early L, Genetic Epidemiology C, andMe Research T, Andreassen OA, Asherson P, Burton CL, Boomsma DI, Cormand B, Dalsgaard S, Franke B, Gelernter J, Geschwind D, Hakonarson H, Haavik J, Kranzler HR, Kuntsi J, Langley K, Lesch KP, Middeldorp C, Reif A, Rohde LA, Roussos P, Schachar R, Sklar P, Sonuga-Barke EJS, Sullivan PF, Thapar A, Tung JY, Waldman ID, Medland SE, Stefansson K, Nordentoft M, Hougaard DM, Werge T, Mors O, Mortensen PB, Daly MJ, Faraone SV, Borglum AD, Neale BM (2019) Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 51:63–75. https://doi.org/10.1038/s41588-018-0269-7
Denollet J (2005) DS14: standard assessment of negative affectivity, social inhibition, and Type D personality. Psychosom Med 67:89–97. https://doi.org/10.1097/01.psy.0000149256.81953.49
Denollet J, Sys SU, Stroobant N, Rombouts H, Gillebert TC, Brutsaert DL (1996) Personality as independent predictor of long-term mortality in patients with coronary heart disease. Lancet 347:417–421. https://doi.org/10.1016/s0140-6736(96)90007-0
Duncan L, Yilmaz Z, Gaspar H, Walters R, Goldstein J, Anttila V, Bulik-Sullivan B, Ripke S, Eating Disorders Working Group of the Psychiatric Genomics C, Thornton L, Hinney A, Daly M, Sullivan PF, Zeggini E, Breen G, Bulik CM (2017) Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am J Psychiatry 174:850–858. https://doi.org/10.1176/appi.ajp.2017.16121402
Ehret GB, Ferreira T, Chasman DI, Jackson AU, Schmidt EM, Johnson T, Thorleifsson G, Luan J, Donnelly LA, Kanoni S, Petersen AK, Pihur V, Strawbridge RJ, Shungin D, Hughes MF, Meirelles O, Kaakinen M, Bouatia-Naji N, Kristiansson K, Shah S, Kleber ME, Guo X, Lyytikainen LP, Fava C, Eriksson N, Nolte IM, Magnusson PK, Salfati EL, Rallidis LS, Theusch E, Smith AJP, Folkersen L, Witkowska K, Pers TH, Joehanes R, Kim SK, Lataniotis L, Jansen R, Johnson AD, Warren H, Kim YJ, Zhao W, Wu Y, Tayo BO, Bochud M, Absher D, Adair LS, Amin N, Arking DE, Axelsson T, Baldassarre D, Balkau B, Bandinelli S, Barnes MR, Barroso I, Bevan S, Bis JC, Bjornsdottir G, Boehnke M, Boerwinkle E, Bonnycastle LL, Boomsma DI, Bornstein SR, Brown MJ, Burnier M, Cabrera CP, Chambers JC, Chang IS, Cheng CY, Chines PS, Chung RH, Collins FS, Connell JM, Doring A, Dallongeville J, Danesh J, de Faire U, Delgado G, Dominiczak AF, Doney ASF, Drenos F, Edkins S, Eicher JD, Elosua R, Enroth S, Erdmann J, Eriksson P, Esko T, Evangelou E, Evans A, Fall T, Farrall M, Felix JF, Ferrieres J, Ferrucci L, Fornage M, Forrester T, consortium CH-E, consortium C-H, Wellcome Trust Case Control C et al (2016) The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat Genet 48:1171–1184. https://doi.org/10.1038/ng.3667
Finci L, Zhang Y, Meijers R, Wang JH (2015) Signaling mechanism of the netrin-1 receptor DCC in axon guidance. Prog Biophys Mol Biol 118:153–160. https://doi.org/10.1016/j.pbiomolbio.2015.04.001
Finucane HK, Bulik-Sullivan B, Gusev A, Trynka G, Reshef Y, Loh PR, Anttila V, Xu H, Zang C, Farh K, Ripke S, Day FR, Purcell S, Stahl E, Lindstrom S, Perry JR, Okada Y, Raychaudhuri S, Daly MJ, Patterson N, Neale BM, Price AL, ReproGen C, Schizophrenia Working Group of the Psychiatric Genomics C, Consortium R (2015) Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet 47:1228–1235. https://doi.org/10.1038/ng.3404
Forrest MP, Hill MJ, Quantock AJ, Martin-Rendon E, Blake DJ (2014) The emerging roles of TCF4 in disease and development. Trends Mol Med 20:322–331. https://doi.org/10.1016/j.molmed.2014.01.010
Fregeau B, Kim BJ, Hernandez-Garcia A, Jordan VK, Cho MT, Schnur RE, Monaghan KG, Juusola J, Rosenfeld JA, Bhoj E, Zackai EH, Sacharow S, Baranano K, Bosch DGM, de Vries BBA, Lindstrom K, Schroeder A, James P, Kulch P, Lalani SR, van Haelst MM, van Gassen KLI, van Binsbergen E, Barkovich AJ, Scott DA, Sherr EH (2016) De novo mutations of RERE cause a genetic syndrome with features that overlap those associated with proximal 1p36 deletions. Am J Hum Genet 98:963–970. https://doi.org/10.1016/j.ajhg.2016.03.002
Gale CR, Hagenaars SP, Davies G, Hill WD, Liewald DC, Cullen B, Penninx BW, Longevity G, Boomsma DI, Pell J, McIntosh AM, Smith DJ, Deary IJ, Harris SE, International Consortium for Blood Pressure Gwas CCA (2016) Pleiotropy between neuroticism and physical and mental health: findings from 108,038 men and women in UK Biobank. Transl Psychiatry 6:e791. https://doi.org/10.1038/tp.2016.56
Gao J, Davis LK, Hart AB, Sanchez-Roige S, Han L, Cacioppo JT, Palmer AA (2017) Genome-wide association study of loneliness demonstrates a role for common variation. Neuropsychopharmacology 42:811–821. https://doi.org/10.1038/npp.2016.197
Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR (2015) A global reference for human genetic variation. Nature 526:68–74. https://doi.org/10.1038/nature15393
Giri A, Hellwege JN, Keaton JM, Park J, Qiu C, Warren HR, Torstenson ES, Kovesdy CP, Sun YV, Wilson OD, Robinson-Cohen C, Roumie CL, Chung CP, Birdwell KA, Damrauer SM, DuVall SL, Klarin D, Cho K, Wang Y, Evangelou E, Cabrera CP, Wain LV, Shrestha R, Mautz BS, Akwo EA, Sargurupremraj M, Debette S, Boehnke M, Scott LJ, Luan J, Zhao JH, Willems SM, Theriault S, Shah N, Oldmeadow C, Almgren P, Li-Gao R, Verweij N, Boutin TS, Mangino M, Ntalla I, Feofanova E, Surendran P, Cook JP, Karthikeyan S, Lahrouchi N, Liu C, Sepulveda N, Richardson TG, Kraja A, Amouyel P, Farrall M, Poulter NR, Laakso M, Zeggini E, Sever P, Scott RA, Langenberg C, Wareham NJ, Conen D, Palmer CNA, Attia J, Chasman DI, Ridker PM, Melander O, Mook-Kanamori DO, Harst PV, Cucca F, Schlessinger D, Hayward C, Spector TD, Jarvelin MR, Hennig BJ, Timpson NJ, Wei WQ, Smith JC, Xu Y, Matheny ME, Siew EE, Lindgren C, Herzig KH, Dedoussis G, Denny JC, Psaty BM, Howson JMM, Munroe PB, Newton-Cheh C, Caulfield MJ, Elliott P, Gaziano JM, Concato J, Wilson PWF, Tsao PS, Velez Edwards DR, Susztak K, Million Veteran P, O’Donnell CJ, Understanding Society Scientific G, International Consortium for Blood P, Blood Pressure-International Consortium of Exome Chip S et al (2019) Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet 51:51–62. https://doi.org/10.1038/s41588-018-0303-9
Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, Pallesen J, Agerbo E, Andreassen OA, Anney R, Awashti S, Belliveau R, Bettella F, Buxbaum JD, Bybjerg-Grauholm J, Baekvad-Hansen M, Cerrato F, Chambert K, Christensen JH, Churchhouse C, Dellenvall K, Demontis D, De Rubeis S, Devlin B, Djurovic S, Dumont AL, Goldstein JI, Hansen CS, Hauberg ME, Hollegaard MV, Hope S, Howrigan DP, Huang H, Hultman CM, Klei L, Maller J, Martin J, Martin AR, Moran JL, Nyegaard M, Naerland T, Palmer DS, Palotie A, Pedersen CB, Pedersen MG, Poterba TD, Poulsen JB, Pourcain BS, Qvist P, Rehnstrom K, Reichenberg A, Reichert J, Robinson EB, Roeder K, Roussos P, Saemundsen E, Sandin S, Satterstrom FK, Davey Smith G, Stefansson H, Steinberg S, Stevens CR, Sullivan PF, Turley P, Walters GB, Xu X, Stefansson K, Geschwind DH, Nordentoft M, Hougaard DM, Werge T, Mors O, Mortensen PB, Neale BM, Daly MJ, Borglum AD, Autism Spectrum Disorder Working Group of the Psychiatric Genomics C, Bupgen, Major Depressive Disorder Working Group of the Psychiatric Genomics C, andMe Research T (2019) Identification of common genetic risk variants for autism spectrum disorder. Nat Genet 51:431–444. https://doi.org/10.1038/s41588-019-0344-8
Hagiwara N (2011) Sox6, jack of all trades: a versatile regulatory protein in vertebrate development. Dev Dyn 240:1311–1321. https://doi.org/10.1002/dvdy.22639
Heller D, Watson D, Hies R (2004) The role of person versus situation in life satisfaction: a critical examination. Psychol Bull 130:574–600. https://doi.org/10.1037/0033-2909.130.4.574
Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS (2006) A population-based twin study of the relationship between neuroticism and internalizing disorders. Am J Psychiatry 163:857–864. https://doi.org/10.1176/ajp.2006.163.5.857
Hill WD, Weiss A, Liewald DC, Davies G, Porteous DJ, Hayward C, McIntosh AM, Gale CR, Deary IJ (2019) Genetic contributions to two special factors of neuroticism are associated with affluence, higher intelligence, better health, and longer life. Mol Psychiatry. https://doi.org/10.1038/s41380-019-0387-3
Howard DM, Adams MJ, Shirali M, Clarke TK, Marioni RE, Davies G, Coleman JRI, Alloza C, Shen X, Barbu MC, Wigmore EM, Gibson J, Hagenaars SP, Lewis CM, Ward J, Smith DJ, Sullivan PF, Haley CS, Breen G, Deary IJ, McIntosh AM, andMe Research T (2018) Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat Commun 9:1470. https://doi.org/10.1038/s41467-018-03819-3
Huang X, Li Z, Bai B, Li X, Li Z (2015) High expression of microRNA-208 is associated with cardiac hypertrophy via the negative regulation of the sex-determining region Y-box 6 protein. Exp Ther Med 10:921–926. https://doi.org/10.3892/etm.2015.2645
Hyde CL, Nagle MW, Tian C, Chen X, Paciga SA, Wendland JR, Tung JY, Hinds DA, Perlis RH, Winslow AR (2016) Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet 48:1031–1036. https://doi.org/10.1038/ng.3623
Issekutz KA, Graham JM Jr, Prasad C, Smith IM, Blake KD (2005) An epidemiological analysis of CHARGE syndrome: preliminary results from a Canadian study. Am J Med Genet A 133A:309–317. https://doi.org/10.1002/ajmg.a.30560
Jansen PR, Watanabe K, Stringer S, Skene N, Bryois J, Hammerschlag AR, de Leeuw CA, Benjamins JS, Munoz-Manchado AB, Nagel M, Savage JE, Tiemeier H, White T, Tung JY, Hinds DA, Vacic V, Wang X, Sullivan PF, van der Sluis S, Polderman TJC, Smit AB, Hjerling-Leffler J, Van Someren EJW, Posthuma D, andMe Research T (2019) Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet 51:394–403. https://doi.org/10.1038/s41588-018-0333-3
Jeronimus BF, Riese H, Sanderman R, Ormel J (2014) Mutual reinforcement between neuroticism and life experiences: a five-wave, 16-year study to test reciprocal causation. J Pers Soc Psychol 107:751–764. https://doi.org/10.1037/a0037009
Jeronimus BF, Kotov R, Riese H, Ormel J (2016) Neuroticism’s prospective association with mental disorders halves after adjustment for baseline symptoms and psychiatric history, but the adjusted association hardly decays with time: a meta-analysis on 59 longitudinal/prospective studies with 443 313 participants. Psychol Med 46:2883–2906. https://doi.org/10.1017/S0033291716001653
Jokela M, Pulkki-Raback L, Elovainio M, Kivimaki M (2014) Personality traits as risk factors for stroke and coronary heart disease mortality: pooled analysis of three cohort studies. J Behav Med 37:881–889. https://doi.org/10.1007/s10865-013-9548-z
Jordan VK, Fregeau B, Ge X, Giordano J, Wapner RJ, Balci TB, Carter MT, Bernat JA, Moccia AN, Srivastava A, Martin DM, Bielas SL, Pappas J, Svoboda MD, Rio M, Boddaert N, Cantagrel V, Lewis AM, Scaglia F, Kohler JN, Bernstein JA, Dries AM, Rosenfeld JA, DeFilippo C, Thorson W, Yang Y, Sherr EH, Bi W, Scott DA (2018) Genotype-phenotype correlations in individuals with pathogenic RERE variants. Hum Mutat 39:666–675. https://doi.org/10.1002/humu.23400
Kendler KS, Myers J (2010) The genetic and environmental relationship between major depression and the five-factor model of personality. Psychol Med 40:801–806. https://doi.org/10.1017/S0033291709991140
Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS (2005) Personality and comorbidity of common psychiatric disorders. Br J Psychiatry 186:190–196. https://doi.org/10.1192/bjp.186.3.190
Kichaev G, Bhatia G, Loh PR, Gazal S, Burch K, Freund MK, Schoech A, Pasaniuc B, Price AL (2019) Leveraging polygenic functional enrichment to improve GWAS power. Am J Hum Genet 104:65–75. https://doi.org/10.1016/j.ajhg.2018.11.008
Kim BJ, Scott DA (2014) Mouse model reveals the role of RERE in cerebellar foliation and the migration and maturation of Purkinje cells. PLoS ONE 9:e87518. https://doi.org/10.1371/journal.pone.0087518
Kim BJ, Zaveri HP, Jordan VK, Hernandez-Garcia A, Jacob DJ, Zamora DL, Yu W, Schwartz RJ, Scott DA (2018) RERE deficiency leads to decreased expression of GATA4 and the development of ventricular septal defects. Dis Model Mech. https://doi.org/10.1242/dmm.031534
Kotov R, Gamez W, Schmidt F, Watson D (2010) Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol Bull 136:768–821. https://doi.org/10.1037/a0020327
Lahey BB (2009) Public health significance of neuroticism. Am Psychol 64:241–256. https://doi.org/10.1037/a0015309
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G (2008) Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27:1133–1163. https://doi.org/10.1002/sim.3034
Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, Nguyen-Viet TA, Bowers P, Sidorenko J, Karlsson Linner R, Fontana MA, Kundu T, Lee C, Li H, Li R, Royer R, Timshel PN, Walters RK, Willoughby EA, Yengo L, Genetic Association C, Alver M, Bao Y, Clark DW, Day FR, Furlotte NA, Joshi PK, Kemper KE, Kleinman A, Langenberg C, Magi R, Trampush JW, Verma SS, Wu Y, Lam M, Zhao JH, Zheng Z, Boardman JD, Campbell H, Freese J, Harris KM, Hayward C, Herd P, Kumari M, Lencz T, Luan J, Malhotra AK, Metspalu A, Milani L, Ong KK, Perry JRB, Porteous DJ, Ritchie MD, Smart MC, Smith BH, Tung JY, Wareham NJ, Wilson JF, Beauchamp JP, Conley DC, Esko T, Lehrer SF, Magnusson PKE, Oskarsson S, Pers TH, Robinson MR, Thom K, Watson C, Chabris CF, Meyer MN, Laibson DI, Yang J, Johannesson M, Koellinger PD, Turley P, Visscher PM, Benjamin DJ, Cesarini D, Social Science Genetic Association C, andMe Research T (2018) Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet 50:1112–1121. https://doi.org/10.1038/s41588-018-0147-3
Lichtman JH, Bigger JT Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES, American Heart Association Prevention Committee of the Council on Cardiovascular N, American Heart Association Council on Clinical C, American Heart Association Council on E, Prevention, American Heart Association Interdisciplinary Council on Quality of C, Outcomes R, American Psychiatric A (2008) Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 118:1768–1775. https://doi.org/10.1161/CIRCULATIONAHA.108.190769
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F et al (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2224–2260. https://doi.org/10.1016/S0140-6736(12)61766-8
Lo MT, Hinds DA, Tung JY, Franz C, Fan CC, Wang Y, Smeland OB, Schork A, Holland D, Kauppi K, Sanyal N, Escott-Price V, Smith DJ, O’Donovan M, Stefansson H, Bjornsdottir G, Thorgeirsson TE, Stefansson K, McEvoy LK, Dale AM, Andreassen OA, Chen CH (2017) Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nat Genet 49:152–156. https://doi.org/10.1038/ng.3736
Lonnqvist JE, Verkasalo M, Haukka J, Nyman K, Tiihonen J, Laaksonen I, Leskinen J, Lonnqvist J, Henriksson M (2009) Premorbid personality factors in schizophrenia and bipolar disorder: results from a large cohort study of male conscripts. J Abnorm Psychol 118:418–423. https://doi.org/10.1037/a0015127
Lu X, Wang L, Lin X, Huang J, Charles GuC, He M, Shen H, He J, Zhu J, Li H, Hixson JE, Wu T, Dai J, Lu L, Shen C, Chen S, He L, Mo Z, Hao Y, Mo X, Yang X, Li J, Cao J, Chen J, Fan Z, Li Y, Zhao L, Li H, Lu F, Yao C, Yu L, Xu L, Mu J, Wu X, Deng Y, Hu D, Zhang W, Ji X, Guo D, Guo Z, Zhou Z, Yang Z, Wang R, Yang J, Zhou X, Yan W, Sun N, Gao P, Gu D (2015) Genome-wide association study in Chinese identifies novel loci for blood pressure and hypertension. Hum Mol Genet 24:865–874. https://doi.org/10.1093/hmg/ddu478
Luciano M, Hagenaars SP, Davies G, Hill WD, Clarke TK, Shirali M, Harris SE, Marioni RE, Liewald DC, Fawns-Ritchie C, Adams MJ, Howard DM, Lewis CM, Gale CR, McIntosh AM, Deary IJ (2018) Association analysis in over 329,000 individuals identifies 116 independent variants influencing neuroticism. Nat Genet 50:6–11. https://doi.org/10.1038/s41588-017-0013-8
Michielsen M, Comijs HC, Semeijn EJ, Beekman AT, Deeg DJ, Kooij JJ (2014) Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry 22:1623–1632. https://doi.org/10.1016/j.jagp.2014.02.005
Nagel M, Jansen PR, Stringer S, Watanabe K, de Leeuw CA, Bryois J, Savage JE, Hammerschlag AR, Skene NG, Munoz-Manchado AB, White T, Tiemeier H, Linnarsson S, Hjerling-Leffler J, Polderman TJC, Sullivan PF, van der Sluis S, Posthuma D, andMe Research T (2018) Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat Genet 50:920–927. https://doi.org/10.1038/s41588-018-0151-7
Navrady LB, Ritchie SJ, Chan SWY, Kerr DM, Adams MJ, Hawkins EH, Porteous D, Deary IJ, Gale CR, Batty GD, McIntosh AM (2017) Intelligence and neuroticism in relation to depression and psychological distress: Evidence from two large population cohorts. Eur Psychiatry 43:58–65. https://doi.org/10.1016/j.eurpsy.2016.12.012
Okbay A, Baselmans BM, De Neve JE, Turley P, Nivard MG, Fontana MA, Meddens SF, Linner RK, Rietveld CA, Derringer J, Gratten J, Lee JJ, Liu JZ, de Vlaming R, Ahluwalia TS, Buchwald J, Cavadino A, Frazier-Wood AC, Furlotte NA, Garfield V, Geisel MH, Gonzalez JR, Haitjema S, Karlsson R, van der Laan SW, Ladwig KH, Lahti J, van der Lee SJ, Lind PA, Liu T, Matteson L, Mihailov E, Miller MB, Minica CC, Nolte IM, Mook-Kanamori D, van der Most PJ, Oldmeadow C, Qian Y, Raitakari O, Rawal R, Realo A, Rueedi R, Schmidt B, Smith AV, Stergiakouli E, Tanaka T, Taylor K, Thorleifsson G, Wedenoja J, Wellmann J, Westra HJ, Willems SM, Zhao W, LifeLines Cohort S, Amin N, Bakshi A, Bergmann S, Bjornsdottir G, Boyle PA, Cherney S, Cox SR, Davies G, Davis OS, Ding J, Direk N, Eibich P, Emeny RT, Fatemifar G, Faul JD, Ferrucci L, Forstner AJ, Gieger C, Gupta R, Harris TB, Harris JM, Holliday EG, Hottenga JJ, De Jager PL, Kaakinen MA, Kajantie E, Karhunen V, Kolcic I, Kumari M, Launer LJ, Franke L, Li-Gao R, Liewald DC, Koini M, Loukola A, Marques-Vidal P, Montgomery GW, Mosing MA, Paternoster L, Pattie A, Petrovic KE, Pulkki-Raback L, Quaye L, Raikkonen K, Rudan I et al (2016) Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet 48:624–633. https://doi.org/10.1038/ng.3552
Ormel J, Jeronimus BF, Kotov R, Riese H, Bos EH, Hankin B, Rosmalen JGM, Oldehinkel AJ (2013) Neuroticism and common mental disorders: meaning and utility of a complex relationship. Clin Psychol Rev 33:686–697. https://doi.org/10.1016/j.cpr.2013.04.003
Otowa T, Hek K, Lee M, Byrne EM, Mirza SS, Nivard MG, Bigdeli T, Aggen SH, Adkins D, Wolen A, Fanous A, Keller MC, Castelao E, Kutalik Z, der Auwera SV, Homuth G, Nauck M, Teumer A, Milaneschi Y, Hottenga JJ, Direk N, Hofman A, Uitterlinden A, Mulder CL, Henders AK, Medland SE, Gordon S, Heath AC, Madden PA, Pergadia ML, van der Most PJ, Nolte IM, van Oort FV, Hartman CA, Oldehinkel AJ, Preisig M, Grabe HJ, Middeldorp CM Penninx BW, Boomsma D, Martin NG, Montgomery G, Maher BS, van den Oord EJ, Wray NR, Tiemeier H, Hettema JM (2016) Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry 21:1485. https://doi.org/10.1038/mp.2016.11
Psychiatric GCBDWG (2011) Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet 43:977–983. https://doi.org/10.1038/ng.943
Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S, Bergen SE, Collins AL, Crowley JJ, Fromer M, Kim Y, Lee SH, Magnusson PK, Sanchez N, Stahl EA, Williams S, Wray NR, Xia K, Bettella F, Borglum AD, Bulik-Sullivan BK, Cormican P, Craddock N, de Leeuw C, Durmishi N, Gill M, Golimbet V, Hamshere ML, Holmans P, Hougaard DM, Kendler KS, Lin K, Morris DW, Mors O, Mortensen PB, Neale BM, O’Neill FA, Owen MJ, Milovancevic MP, Posthuma D, Powell J, Richards AL, Riley BP, Ruderfer D, Rujescu D, Sigurdsson E, Silagadze T, Smit AB, Stefansson H, Steinberg S, Suvisaari J, Tosato S, Verhage M, Walters JT, Levinson DF, Gejman PV, Kendler KS, Laurent C, Mowry BJ, O’Donovan MC, Owen MJ, Pulver AE, Riley BP, Schwab SG, Wildenauer DB, Dudbridge F, Holmans P, Shi J, Albus M, Alexander M, Campion D, Cohen D, Dikeos D, Duan J, Eichhammer P, Godard S, Hansen M, Lerer FB, Liang KY, Maier W, Mallet J, Nertney DA, Nestadt G, Norton N, O’Neill FA, Papadimitriou GN, Ribble R, Sanders AR, Silverman JM, Walsh D, Williams NM, Wormley B, Arranz MJ, Bakker S, Bender S, Bramon E, Collier D, Crespo-Facorro B, Multicenter Genetic Studies of Schizophrenia C, Psychosis Endophenotypes International C et al (2013) Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet 45:1150–1159. https://doi.org/10.1038/ng.2742
Ruderfer DM, Ripke S, McQuillin A, Boocock J, Stahl EA, Pavlides JM, Mullins N, Charney AW, Ori AP, Loohuis LM, Domenici E. Bipolar D, Schizophrenia Working Group of the Psychiatric Genomics Consortium. Electronic address drve, Bipolar D, Schizophrenia Working Group of the Psychiatric Genomics C (2018) Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell 173:1705–1715. https://doi.org/10.1016/j.cell.2018.05.046
Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, Nagel M, Awasthi S, Barr PB, Coleman JRI, Grasby KL, Hammerschlag AR, Kaminski JA, Karlsson R, Krapohl E, Lam M, Nygaard M, Reynolds CA, Trampush JW, Young H, Zabaneh D, Hagg S, Hansell NK, Karlsson IK, Linnarsson S, Montgomery GW, Munoz-Manchado AB, Quinlan EB, Schumann G, Skene NG, Webb BT, White T, Arking DE, Avramopoulos D, Bilder RM, Bitsios P, Burdick KE, Cannon TD, Chiba-Falek O, Christoforou A, Cirulli ET, Congdon E, Corvin A, Davies G, Deary IJ, DeRosse P, Dickinson D, Djurovic S, Donohoe G, Conley ED, Eriksson JG, Espeseth T, Freimer NA, Giakoumaki S, Giegling I, Gill M, Glahn DC, Hariri AR, Hatzimanolis A, Keller MC, Knowles E, Koltai D, Konte B, Lahti J, Le Hellard S, Lencz T, Liewald DC, London E, Lundervold AJ, Malhotra AK, Melle I, Morris D, Need AC, Ollier W, Palotie A, Payton A, Pendleton N, Poldrack RA, Raikkonen K, Reinvang I, Roussos P, Rujescu D, Sabb FW, Scult MA, Smeland OB, Smyrnis N, Starr JM, Steen VM, Stefanis NC, Straub RE, Sundet K, Tiemeier H, Voineskos AN, Weinberger DR, Widen E, Yu J, Abecasis G, Andreassen OA, Breen G, Christiansen L et al (2018) Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet 50:912–919. https://doi.org/10.1038/s41588-018-0152-6
Schirmbeck F, Boyette LL, van der Valk R, Meijer C, Dingemans P, Van R, de Haan L, Kahn RS, de Haan L, van Os J, Wiersma D, Bruggeman R, Cahn W, Meijer C, Myin-Germeys I, Group (2015) Relevance of Five-Factor Model personality traits for obsessive-compulsive symptoms in patients with psychotic disorders and their un-affected siblings. Psychiatry Res 225:464–470. https://doi.org/10.1016/j.psychres.2014.11.066
Schneiderman N, Ironson G, Siegel SD (2005) Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol 1:607–628. https://doi.org/10.1146/annurev.clinpsy.1.102803.144141
Smeland OB, Wang Y, Lo MT, Li W, Frei O, Witoelar A, Tesli M, Hinds DA, Tung JY, Djurovic S, Chen CH, Dale AM, Andreassen OA (2017) Identification of genetic loci shared between schizophrenia and the Big Five personality traits. Sci Rep 7:2222. https://doi.org/10.1038/s41598-017-02346-3
Smith TW, MacKenzie J (2006) Personality and risk of physical illness. Annu Rev Clin Psychol 2:435–467. https://doi.org/10.1146/annurev.clinpsy.2.022305.095257
Smith DJ, Escott-Price V, Davies G, Bailey ME, Colodro-Conde L, Ward J, Vedernikov A, Marioni R, Cullen B, Lyall D, Hagenaars SP, Liewald DC, Luciano M, Gale CR, Ritchie SJ, Hayward C, Nicholl B, Bulik-Sullivan B, Adams M, Couvy-Duchesne B, Graham N, Mackay D, Evans J, Smith BH, Porteous DJ, Medland SE, Martin NG, Holmans P, McIntosh AM, Pell JP, Deary IJ, O’Donovan MC (2016) Genome-wide analysis of over 106 000 individuals identifies 9 neuroticism-associated loci. Mol Psychiatry 21:749–757. https://doi.org/10.1038/mp.2016.49
Specht J, Egloff B, Schmukle SC (2011) Stability and change of personality across the life course: the impact of age and major life events on mean-level and rank-order stability of the Big Five. J Pers Soc Psychol 101:862–882. https://doi.org/10.1037/a0024950
Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Genetic R, Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA, Outcome in P (2009) Common variants conferring risk of schizophrenia. Nature 460:744–747. https://doi.org/10.1038/nature08186
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R (2015) UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12:e1001779. https://doi.org/10.1371/journal.pmed.1001779
Takeuchi F, Akiyama M, Matoba N, Katsuya T, Nakatochi M, Tabara Y, Narita A, Saw WY, Moon S, Spracklen CN, Chai JF, Kim YJ, Zhang L, Wang C, Li H, Li H, Wu JY, Dorajoo R, Nierenberg JL, Wang YX, He J, Bennett DA, Takahashi A, Momozawa Y, Hirata M, Matsuda K, Rakugi H, Nakashima E, Isono M, Shirota M, Hozawa A, Ichihara S, Matsubara T, Yamamoto K, Kohara K, Igase M, Han S, Gordon-Larsen P, Huang W, Lee NR, Adair LS, Hwang MY, Lee J, Chee ML, Sabanayagam C, Zhao W, Liu J, Reilly DF, Sun L, Huo S, Edwards TL, Long J, Chang LC, Chen CH, Yuan JM, Koh WP, Friedlander Y, Kelly TN, Bin Wei W, Xu L, Cai H, Xiang YB, Lin K, Clarke R, Walters RG, Millwood IY, Li L, Chambers JC, Kooner JS, Elliott P, van der Harst P, Chen Z, Sasaki M, Shu XO, Jonas JB, He J, Heng CK, Chen YT, Zheng W, Lin X, Teo YY, Tai ES, Cheng CY, Wong TY, Sim X, Mohlke KL, Yamamoto M, Kim BJ, Miki T, Nabika T, Yokota M, Kamatani Y, Kubo M, Kato N, International Genomics of Blood Pressure C (2018) Interethnic analyses of blood pressure loci in populations of East Asian and European descent. Nat Commun 9:5052. https://doi.org/10.1038/s41467-018-07345-0
Taylor S, Asmundson GJ, Jang KL (2011) Etiology of obsessive-compulsive symptoms and obsessive-compulsive personality traits: common genes, mostly different environments. Depress Anxiety 28:863–869. https://doi.org/10.1002/da.20859
van der Harst P, Verweij N (2018) Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res 122:433–443. https://doi.org/10.1161/CIRCRESAHA.117.312086
Van Os J, Jones PB (2001) Neuroticism as a risk factor for schizophrenia. Psychol Med 31:1129–1134
Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, Aliev F, Bacanu SA, Batzler A, Bertelsen S, Biernacka JM, Bigdeli TB, Chen LS, Clarke TK, Chou YL, Degenhardt F, Docherty AR, Edwards AC, Fontanillas P, Foo JC, Fox L, Frank J, Giegling I, Gordon S, Hack LM, Hartmann AM, Hartz SM, Heilmann-Heimbach S, Herms S, Hodgkinson C, Hoffmann P, Jan Hottenga J, Kennedy MA, Alanne-Kinnunen M, Konte B, Lahti J, Lahti-Pulkkinen M, Lai D, Ligthart L, Loukola A, Maher BS, Mbarek H, McIntosh AM, McQueen MB, Meyers JL, Milaneschi Y, Palviainen T, Pearson JF, Peterson RE, Ripatti S, Ryu E, Saccone NL, Salvatore JE, Sanchez-Roige S, Schwandt M, Sherva R, Streit F, Strohmaier J, Thomas N, Wang JC, Webb BT, Wedow R, Wetherill L, Wills AG, andMe Research T, Boardman JD, Chen D, Choi DS, Copeland WE, Culverhouse RC, Dahmen N, Degenhardt L, Domingue BW, Elson SL, Frye MA, Gabel W, Hayward C, Ising M, Keyes M, Kiefer F, Kramer J, Kuperman S, Lucae S, Lynskey MT, Maier W, Mann K, Mannisto S, Muller-Myhsok B, Murray AD, Nurnberger JI, Palotie A, Preuss U, Raikkonen K, Reynolds MD, Ridinger M, Scherbaum N, Schuckit MA, Soyka M, Treutlein J, Witt S et al (2018) Transancestral GWAS of alcohol dependence reveals common genetic underpinnings withpsychiatric disorders. Nat Neurosci 21: 1656–1669. https://doi.org/10.1038/s41593-018-0275-1
Wang M, Zhao Y, Zhang B (2015) Efficient test and visualization of multi-set intersections. Sci Rep 5:16923. https://doi.org/10.1038/srep16923
Watanabe K, Taskesen E, van Bochoven A, Posthuma D (2017) Functional mapping and annotation of genetic associations with FUMA. Nat Commun 8:1826. https://doi.org/10.1038/s41467-017-01261-5
Wray NR, Birley AJ, Sullivan PF, Visscher PM, Martin NG (2007) Genetic and phenotypic stability of measures of neuroticism over 22 years. Twin Res Hum Genet 10:695–702. https://doi.org/10.1375/twin.10.5.695
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E, Air TM, Andlauer TMF, Bacanu SA, Baekvad-Hansen M, Beekman AFT, Bigdeli TB, Binder EB, Blackwood DRH, Bryois J, Buttenschon HN, Bybjerg-Grauholm J, Cai N, Castelao E, Christensen JH, Clarke TK, Coleman JIR, Colodro-Conde L, Couvy-Duchesne B, Craddock N, Crawford GE, Crowley CA, Dashti HS, Davies G, Deary IJ, Degenhardt F, Derks EM, Direk N, Dolan CV, Dunn EC, Eley TC, Eriksson N, Escott-Price V, Kiadeh FHF, Finucane HK, Forstner AJ, Frank J, Gaspar HA, Gill M, Giusti-Rodriguez P, Goes FS, Gordon SD, Grove J, Hall LS, Hannon E, Hansen CS, Hansen TF, Herms S, Hickie IB, Hoffmann P, Homuth G, Horn C, Hottenga JJ, Hougaard DM, Hu M, Hyde CL, Ising M, Jansen R, Jin F, Jorgenson E, Knowles JA, Kohane IS, Kraft J, Kretzschmar WW, Krogh J, Kutalik Z, Lane JM, Li Y, Li Y, Lind PA, Liu X, Lu L, MacIntyre DJ, MacKinnon DF, Maier RM, Maier W, Marchini J, Mbarek H, McGrath P, McGuffin P, Medland SE, Mehta D, Middeldorp CM, Mihailov E, Milaneschi Y, Milani L, Mill J, Mondimore FM, Montgomery GW, Mostafavi S, Mullins N, Nauck M, Ng B et al (2018) Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 50:668–681. https://doi.org/10.1038/s41588-018-0090-3
Yu D, Sul JH, Tsetsos F, Nawaz MS, Huang AY, Zelaya I, Illmann C, Osiecki L, Darrow SM, Hirschtritt ME, Greenberg E, Muller-Vahl KR, Stuhrmann M, Dion Y, Rouleau G, Aschauer H, Stamenkovic M, Schlogelhofer M, Sandor P, Barr CL, Grados M, Singer HS, Nothen MM, Hebebrand J, Hinney A, King RA, Fernandez TV, Barta C, Tarnok Z, Nagy P, Depienne C, Worbe Y, Hartmann A, Budman CL, Rizzo R, Lyon GJ, McMahon WM, Batterson JR, Cath DC, Malaty IA, Okun MS, Berlin C, Woods DW, Lee PC, Jankovic J, Robertson MM, Gilbert DL, Brown LW, Coffey BJ, Dietrich A, Hoekstra PJ, Kuperman S, Zinner SH, Luethvigsson P, Saemundsen E, Thorarensen O, Atzmon G, Barzilai N, Wagner M, Moessner R, Ophoff R, Pato CN, Pato MT, Knowles JA, Roffman JL, Smoller JW, Buckner RL, Willsey AJ, Tischfield JA, Heiman GA, Stefansson H, Stefansson K, Posthuma D, Cox NJ, Pauls DL, Freimer NB, Neale BM, Davis LK, Paschou P, Coppola G, Mathews CA, Scharf JM, Tourette Association of America International Consortium for Genetics tGdlTGRItTICGS, the Psychiatric Genomics Consortium Tourette Syndrome Working G (2019) Interrogating the genetic determinants of tourette’s syndrome and other tic disorders through genome-wide association studies. Am J Psychiatry 176:217–227. https://doi.org/10.1176/appi.ajp.2018.18070857
Zaveri HP, Beck TF, Hernandez-Garcia A, Shelly KE, Montgomery T, van Haeringen A, Anderlid BM, Patel C, Goel H, Houge G, Morrow BE, Cheung SW, Lalani SR, Scott DA (2014) Identification of critical regions and candidate genes for cardiovascular malformations and cardiomyopathy associated with deletions of chromosome 1p36. PLoS ONE 9:e85600. https://doi.org/10.1371/journal.pone.0085600
Zhu Z, Zheng Z, Zhang F, Wu Y, Trzaskowski M, Maier R, Robinson MR, McGrath JJ, Visscher PM, Wray NR, Yang J (2018) Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat Commun 9:224. https://doi.org/10.1038/s41467-017-02317-2
Zoltewicz JS, Stewart NJ, Leung R, Peterson AS (2004) Atrophin 2 recruits histone deacetylase and is required for the function of multiple signaling centers during mouse embryogenesis. Development 131:3–14. https://doi.org/10.1242/dev.00908
Acknowledgements
We thank members of the Psychiatric Genomics Consortium, 23andMe, the UKB, and other teams, who generously shared the GWAS data.
Funding
This work was supported by the National Key R&D Program of China (2016YFC1307000, 2020YFC2008002), and the Shanghai Key Laboratory of Psychotic Disorders (13dz2260500, 14-K06).
Author information
Authors and Affiliations
Contributions
FZ and MX conceived and designed the study. FZ performed the data analysis and wrote the manuscript. FZ, AB, and MX revised the manuscript. CZ, XZ, JC, HC, and MX contributed to the data preparation and interpretation of the results. All the authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest for any author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to corrections in the author contribution.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, F., Baranova, A., Zhou, C. et al. Causal influences of neuroticism on mental health and cardiovascular disease. Hum Genet 140, 1267–1281 (2021). https://doi.org/10.1007/s00439-021-02288-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-021-02288-x