Abstract
Y chromosome (ChrY), the male-specific sex chromosome, has been considered as a genetic wasteland. Aging-related mosaic loss of ChrY (LOY) has been known for more than half a century, but it was constantly considered as a neutral karyotype related to normal aging. These views have been challenged with genome-wide association studies identifying mosaic LOY in human somatic cells is the most commonly acquired mutation in male’s genome and is associated with a wide spectrum of human diseases including cancer, Alzheimer’s disease, and cardiovascular disease. These previously undescribed clinical significances deeply modify our perception on ChrY and open up a range of new questions. Here, we review the latest advances in our knowledge of the biological origins and clinical consequences of mosaic LOY. We highlight the association of mosaic LOY to pathogenic conditions and evaluate the cause-and-consequence relationships between mosaic LOY and pathogenesis. The known risk factors of mosaic LOY including age, genetic variants, ChrY structural aberrations and environmental stressors are discussed. In light of evidence from pioneering and more recent studies, we propose the micronucleation hypothesis and centromere-dysfunction and telomere-attrition models to explain how mosaic LOY occurs and why ChrY is prone to lose. We believe it is importantly and timely to extend mosaic LOY research from epidemiological associations to mechanistic studies. In this regard, we outline important gaps and assess several future directions from a biological and clinical perspective. An improved understanding of mosaic LOY will open new pathways to modify and increase healthy aging in males.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human Y chromosome (ChrY) is the sex-determining chromosome found only in males. ChrY is known to undergo genetic degeneration: over the last 300 million years, the ancestral autosome that evolved to the human ChrY lost all (~ 1500 genes) but ~ 78 of its protein-coding genes (Bachtrog 2013). Such was the massive genetic decay assigned to ChrY that some geneticists described it as a “genetic wasteland”. This once was a widely accepted view, which results in the genetic characterization of human ChrY has lagging behind the rest of the chromosomes. Genetic and theoretical studies based on a linear rate of decay have suggested that the functional genes of ChrY would be lost in human spermatozoa within 15 million years (Aitken and Marshall Graves 2002). Although there is an ongoing controversy about this (Blackmon and Demuth 2015), loss of ChrY (LOY) in somatic cells of aging men has been observed for more than 50 years (Jacobs et al. 1963; Pierre and Hoagland 1972).
The mosaic LOY, which refers to the occurrence of LOY in a subset of cells, has long been deemed a physiological age-related phenomenon. However, a large number of recent studies have found associations between mosaic LOY in leukocytes and an increased risk for mortality as well as various diseases [reviewed in (Forsberg 2017)]. Such association has challenged the classical view that ChrY is merely restricted to sex determination and spermatogenesis, indicating ChrY is involved in a wide variety of biological processes that have not been fully explored.
In this review, we first present a brief summary of human ChrY and its emerging roles in men’s development and physiology, and follow this with a description of the known risk factors for mosaic LOY. We then discuss the association of mosaic LOY to pathogenic conditions and address whether mosaic LOY is a cause or consequence of these pathogenic settings. Based on the results of pioneering and more recent studies, we propose the micronucleation model to explain why and how ChrY is prone to loss. Finally, we assess future directions for mosaic LOY studies from a biological and clinical perspective.
The ChrY: when a chromosome makes the specificity
Human ChrY differs markedly from the rest of the chromosomes in size, structure, content, stability and inheritance. The uniparental inheritance leads ChrY to be constitutively haploid, a condition allowing male-specific portion of ChrY (MSY) to escape the genetic recombination during meiotic crossover (Jobling and Tyler-Smith 2017). Escaping the reshuffling effects allow the accumulation of repeated sequences (comprising one-half of ChrY), which in turn promote frequent chromosomal rearrangements and a high degree of structural variation via intra-chromosomal recombination (Jobling and Tyler-Smith 2003) (Fig. 1). Largely escapes from meiotic recombination with X chromosome (ChrX) renders natural selection inefficient on ChrY, resulting in the accumulation of detrimental mutations and, in long term, the chromosome-wide degeneration (Repping et al. 2006; Helgason et al. 2015). These features set ChrY apart from the rest of the chromosomes and drive its unique evolution within and between populations, which lead to the emergence of ethnically distinct lineages or haplogroups (Jobling and Tyler-Smith 2003, 2017; Bachtrog 2013). Furthermore, the lack of recombination prevents ChrY from classical genetic and linkage-mapping studies, and the repeat- and amplicon-rich natures have excluded it from most genome-wide association studies (GWAS) (Xue and Tyler-Smith 2017). Thus, the molecular and genetic characterizations of human ChrY have significantly lagged behind the rest of our chromosomes.
Structure of human Y chromosome (ChrY). (A) In humans, biological sex is defined by the sex chromosomes, typically XX for females and XY for males. The X chromosome (ChrX) and ChrY are thought to have once been identical pairs that were free to recombine. Over the course of evolution, only the pseudoautosomal regions (PAR1 and PAR2) of the ChrY pair and recombine with ChrY during meiosis. The non-recombining region, known as male-specific region of the ChrY (MSY), comprises 95% of the ChrY’s length. The ChrY is heterochromatic and gene-poor, with only ~ 78 protein-coding genes being located. Yp is the short arm of the ChrY and it is composed of euchromatin. The long arm, Yq, is composed of both euchromatin and the genetically inactive heterochromatin. The euchromatin of Yq contains the Azoospermia factors (AZFa, AZFb and AZFc) gene families that are essential for sperm production
Currently, it is known that the total number of annotated genes present on human ChrY is 568, of which only ~ 78 of them are encoding proteins (Maan et al. 2017). Most protein-coding genes are unique to testis determination and spermatogenesis. About 109 genes produce non-coding RNA, but their regulatory potential are largely unknown (Maan et al. 2017). The rest of ChrY are repetitive sequences, non-coding regions and pseudogenes (Bachtrog 2013; Maan et al. 2017). Although mammalian ChrY genes decay very rapidly, the survived genes have been stably retained and have broad roles in regulating transcription, translation and protein stability among others (Bellott et al. 2014). In addition, LOY in blood is found to associate with aggregate changes in gene expression, implying ChrY possesses the potential biological functions that needs to be further dissected (Graham et al. 2019).
The ChrY proteome project has found DDX3Y, a MSY gene, may modulate neuronal differentiation (Vakilian et al. 2015) and estimated the contributions of MSY genes to the heart and kidney development (Meyfour et al. 2017a, b). A growing body of evidence supports the associations between ChrY and several polygenic diseases such as prostate cancer (PC), hypertension and cardiovascular diseases (CVDs) (Maan et al. 2017). Particularly, the ChrY haplogroups contribute to the different PC incidence between American and Asian populations (Cannon-Albright et al. 2014). The MSY-located long non-coding RNAs (lncRNAs) TTTY15 is found to promote PC progression (Xiao et al. 2018) and lnc-KDM5D-4 is implicated to key processes of fatty liver and cellular inflammation associated with atherosclerosis and cardiovascular diseases (Molina et al. 2017). The SRY (sex-determining region of the ChrY), as well as several other MSY genes, possess key roles on the state conversion of human pluripotent stem cells (Taleahmad et al. 2019). The SRY possesses oncogenic potential (Murakami et al. 2014) and the abnormal upregulation of SRY may be a potential pathogenic mechanism of Parkinson’s disease (Lee et al. 2019). Moreover, the SRY positively regulates the BMPR2 whose reduction plays crucial roles in pulmonary arterial hypertension (Yan et al. 2018).
Overall, accumulating evidence indicates human ChrY is not only circumscribed to impart male characteristics, but also it serves as a major regulator of gene expression, through which it exerts appreciable impact on cellular phenotypes and disease.
Mosaic LOY: what is it and what causes it?
It was once thought that the cells of a person would share a same genome. However, this supposition has been modified by the acknowledgement that the genomes of every postzygotic somatic cell in the organism are under a constant threat of mutations from both endogenous and external insults. The stochastic nature of mutagenesis implies that most mutations have neutral or deleterious consequences. Occasionally, mutations provide a selective advantage; cells harboring these mutations then undergo positive selection and eventually achieve a stable, detectable and distinct subpopulation characterized by an altered form of the genome, a phenomenon known as somatic mosaicism (Biesecker and Spinner 2013). Large epidemiologic studies have reported that mosaic mutations are prevalent in normal somatic cells from healthy humans, ranging in size from point mutations to gain or loss of entire chromosomes (a condition known as aneuploidy) (Biesecker and Spinner 2013).
For in vivo and free-living human cells, sex chromosome mosaicism is more common than autosomal mosaicism, of which the most frequently observed is mosaic LOY (Xu et al. 2017; Thompson et al. 2019). Although, mosaic LOY in normal hematopoietic cells among elderly males has been known for nearly half a century (Jacobs et al. 1963), it has long been deemed a physiological age-related phenomenon because none of the ChrY-located genes are essential for cell viability and females can survive healthily without ChrY. Mosaic LOY is therefore, grossly neglected by most geneticists for a long time. A large number of studies during the last decade have shown that individuals with mosaic LOY in blood were suffering from an increased risk of disease and death (Table 1). These findings have led to a revival of interest in studying the biological causes and clinical consequences of mosaic LOY. Prior to engaging in a discussion of the pathogenic association of mosaic LOY, it may be informative to first gain a better understanding of what factors cause mosaic LOY.
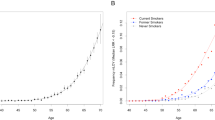
Age is by far the single greatest risk factor of mosaic LOY. Early cytogenetic analyses showed that in male lymphocytes, mosaic LOY is very low up to age 15 years (0.05%) but continuously increased to a frequency of 1.34% in men with the age of 76–80 years (Guttenbach et al. 1995). Large population-based GWAS showed that mosaic LOY in blood samples is < 2% for men under 60 years of age, reaching 15–40% in 70–85 years old males (Forsberg et al. 2014; Dumanski et al. 2015; Zhou et al. 2016; Zink et al. 2017) and 57% of males at 93 years of age (Forsberg et al. 2019). Age-related increase of mosaic LOY is also observed in buccal mucosa cells (Zhou et al. 2016; Forsberg et al. 2019) and dorsolateral prefrontal cortex (Kimura et al. 2018). These data suggest that mosaic LOY trends towards inevitability with aging and increases exponentially with age (Loftfield et al. 2018). In other words, every male will eventually develop mosaic LOY if they live long enough.
Although age is the greatest risk factor of mosaic LOY, it cannot provide any insight into the molecular basis of this biological phenomenon. Thus, the identification of genetic variation influencing mosaic LOY is an important step in understanding the risk of mosaic LOY. The TCL1A is the first susceptibility variation for mosaic LOY (Zhou et al. 2016). Subsequently, 18 additional associated genomic loci implicated in cell cycle regulation, genomic instability (GIN) and cancer susceptibility have been identified to be associated with mosaic LOY (Wright et al. 2017). More recently, 137 novel autosomal genetic determinants of mosaic LOY have been identified (Thompson et al. 2019) and 31 novel mosaic LOY-associated genetic loci have been reported in a Japanese cohort (Terao et al. 2019a). Some of these loci that implicated in the regulation of cell cycle, genome integrity and cell death are shown in Table 2. Interestingly, although there is a strong genetic overlap in association with mosaic LOY across populations/ethnicities (Terao et al. 2019a), the extent of mosaic LOY varies across populations/ethnicities. Mosaic LOY in men of African ancestry is less compared to men of European ancestry (Loftfield et al. 2018, 2019). In line with this, some tested variants for mosaic LOY show higher frequencies in European populations than African populations (Table 2). Overall, these findings suggest mosaic that LOY is a highly polygenic trait. However, we do not know whether these susceptible loci contribute to de novo LOY or they just permit the clonal expansion of LOY cells. Additionally, we do not know whether genetic loci in sex chromosomes would be associated with the risk of mosaic LOY since they are typically excluded from GWAS. Clarification of these causal variants in initiating and/or modulating mosaic LOY is an important field for future studies.
The structural aberrations of ChrY have also found to induce LOY. The long arm of ChrY contains many ampliconic and palindromic sequences, making it predisposed to intra-chromosomal recombination. Since these regions harbor most majorities of the ChrY-located protein-coding genes, intra-chromosomal recombination provides a route for gene conversion (Colaco and Modi 2018). Gene conversion allows functional copies of a given gene within the palindrome to correct a mutant copy, but makes ChrY susceptible to copy number variation (Shi et al. 2018) and intra-chromosomal deletions (Trombetta and Cruciani 2017). ChrY microdeletion has been found to be associated with mosaic LOY in both lymphocytes and sperms (Siffroi et al. 2000; Patsalis et al. 2005). Moreover, if ChrY recombination occurs between sister chromatids, it can give rise to isodicentric ChrY (idicY) (Lange et al. 2009). The inherent instability of idicY causes its loss during chromosome segregation, such that patients with idicY frequently exhibit 46,X,idicY/45,X0 karyotype (Lange et al. 2009; Miyado et al. 2018). Other ChrY anomalies (including rings and derivates) also have been reported in mosaic form (Hsu 1994), indicating ChrY structural aberrations could trigger mosaic LOY. Since no reliable estimates of ChrY structural anomalies frequency in blood exists, to what extent do these aberrations contribute to mosaic LOY remains elusive. However, given the age-dependent increase of large-scale structural mosaicism in human autosomes (Jacobs et al. 2012; Laurie et al. 2012; Machiela et al. 2015; Loh et al. 2018; Terao et al. 2019b), we could predict that ongoing structural mosaicism would occur in ChrY as well. In addition, whether ChrY haplogroups contribute to the different rates of mosaic LOY across populations/ethnicities (Loftfield et al. 2018, 2019) remains to be addressed.
Recent findings indicate that mosaic LOY would be induced by both external and internal environmental stressors. Many independent cohorts observed a strong association between smoking and mosaic LOY and current smokers had a significantly higher degree of mosaic LOY compared with non-smokers and former smokers (Dumanski et al. 2015; Zhou et al. 2016; Loftfield et al. 2018, 2019; Wong et al. 2018). Through the Mendelian randomization analysis, itcases, be extruded, which has been established that smoking may well represent an important causal contribution in the mosaic LOY (Wright et al. 2017). Recently, Liu et al. (2019) found that exposing to polycyclic aromatic hydrocarbons (PAHs) is associated with mosaic LOY in a Chinese cohort. Similarly, increased exposure to outdoor air pollution may lead to leukocyte mosaic LOY even if the pollutant levels are below the regulatory limit (Wong et al. 2018). The widely used insecticides (chlorpyrifos, imidacloprid, and α-cypermethrin) can also induce LOY in the ex vivo lymphocytes at the doses relevant for real exposure scenario (Mužinić et al. 2019). In addition, heavy drinkers display a significantly higher mosaic LOY rate than that of never drinkers and obese men display a significantly higher mosaic LOY frequency than that of normal weight (Loftfield et al. 2018, 2019). It is interesting to see whether mosaic LOY may mediate the link between environmental stressors and its related adverse health outcomes. However, the data on the association of mosaic LOY with alcohol, pollution and obesity are less mature, and further studies are needed to confirm and replicate these findings.
Overall, aging, genetic variants, ChrY structural aberrations and environmental stressors are known risk factors for LOY, indicating that the rise of mosaic LOY is multi-factory. The diverse mechanisms underlying mosaic LOY are not necessarily incompatible with each other. Indeed, Liu et al. demonstrated that age, genetic variant of TCL1A (rs1122138) and environmental stressors (PAHs and smoking) exhibit obvious joint effects on mosaic LOY (Liu et al. 2019). Thus, we anticipate that the cumulative effect of these risk factors is likely to be behind the profound inter-individual variation in mosaic LOY (Danielsson et al. 2019). Assessing the relative contribution of these factors to mosaic LOY is essential to properly predict and modify individuals’ risk of mosaic LOY and to identify targets to modify mosaic LOY-associated pathogenesis (see below).
Mosaic LOY and the diseases susceptibility: viewing the ChrY in a new light
A growing body of recent studies, using various advanced methods, demonstrates that mosaic LOY in blood is associated with the susceptibility to multiple diseases, including four most common aging-related diseases: cancer, Alzheimer’s disease (AD), CVDs, and diabetes (Table 1).
A landmark study has shown that elderly men with mosaic LOY in blood have higher risks for non-hematopoietic cancer diagnosis and mortality (Forsberg et al. 2014). The association between mosaic LOY in blood and increased risk for cancer in other organs has been described in several independent studies (Ganster et al. 2015; Noveski et al. 2016; Zhou et al. 2016; Machiela et al. 2017; Loftfield et al. 2019; Terao et al. 2019a). However, mosaic LOY in leukocytes is not associated with prevalent cancer in a large cohort of men from UK biobank (Loftfield et al. 2018); Mosaic LOY is not associated with risk nor survival of lung cancer as well (Zhou et al.2016); Interestingly, natural mosaic LOY in leukocytes may reduce the risk and ensures a better prognosis of lung cancer in a Chinese cohort (Qin et al. 2019). Overall, these data support that mosaic LOY is associated with the incidence of solid cancers, although such association is more complicated in some cancer subtypes (e.g., lung cancer). Thus, it is highly possible that mosaic LOY in blood could become a predictive biomarker of male carcinogenesis.
AD is a progressive and irreversible neurodegenerative disorder of the central nervous system and accounts for ~ 70% of all dementia cases. AD can be divided into early-onset familial AD (< 65 years; comprising < 5% of all AD cases) and late-onset sporadic AD (> 65 years; comprising > 95% of all AD cases) (Masters et al. 2015). Mutations in PSEN1, PSEN2 and APP are the most cause of familial AD (Masters et al. 2015). Numerous genes have been identified as important risk factors for sporadic AD. Among them the apolipoprotein E4 (ApoE4) allele is the greatest genetic risk factor, with ApoE4 homozygotes which are 14 times more likely to develop AD than non-carriers and subjects heterozygous for ApoE4 have a three-fold increased risk (Yamazaki et al. 2019). Interestingly, mosaic LOY in blood, a fundamentally different genetic variant from ApoE4, has a 6.8-fold greater risk for sporadic AD diagnosis (Dumanski et al. 2016). Similarly, mosaic LOY in the dorsolateral prefrontal cortex is modestly associated with sporadic AD risk and cognitive pathologies (Graham et al. 2019).
In addition, recent studies have reported that mosaic LOY in blood is associated with diagnosis of many other age-related diseases, such as CVDs (Haitjema et al. 2017; Loftfield et al. 2018), diabetes (Loftfield et al. 2018) and age-related macular degeneration (Grassmann et al. 2019a). Importantly, the disease spectrum associated with mosaic LOY has begun to expand beyond age-related diseases. For example, mosaic LOY in blood is associated with the occurrence of autoimmune diseases such as autoimmune thyroiditis (Persani et al. 2012) and primary biliary cirrhosis (Lleo et al. 2013). Mosaic LOY in blood is associated with the course of illness in schizophrenia, despite it is not remarkably associated with the occurrence of schizophrenia (Hirata et al. 2018). Mosaic LOY in blood rather than in dorsolateral prefrontal cortex is found to be associated with suicide completion, suggesting ChrY may participate in the pathophysiology of suicide and provides an explanation to the observation that men have a much higher suicide rate than women (Kimura et al. 2018). It is of note that the studies reported the association between mosaic LOY and clinical outcomes including diabetes, autoimmune diseases, schizophrenia and suicide are limited and such associations should be replicated in other cohorts.
In summary, human ChrY may have a magnified effect on men beyond sex determination despite the small number of genes it harbors in human genome. The clinical significances of mosaic LOY provide a starting point for a new generation of studies into the emerging roles of ChrY in male health and disease. Also, such association rapidly changes our understanding of mosaic mutation in normal human tissues and promotes some to speculate the use of mosaic LOY as a clinical biomarker for assessing the risks of aging-related diseases.
In what ways does mosaic LOY influence male health?
To explain how mosaic LOY in blood cells can be associated with disease processes in other organs, Forsberg (2017) speculated that the normal immune function of leukocytes is compromised by LOY. The “immunosurveillance” hypothesis of mosaic LOY (Forsberg 2017) seems to be highly attractive because the immune cells reside in nearly all tissues and mutations that alter their function could have a variety of phenotypic consequences (Fig. 2a). Indeed, low immune activation or defective immunosurveillance is critical for accelerating the course of many diseases (Senovilla et al. 2013). Despite of this, evidence supporting the “immunosurveillance” hypothesis is rare. Very recently, Dumanski et al. (2019) showed that AD and PC patients display a higher rate of mosaic LOY in natural killer cells and CD4+ T-lymphocytes respectively and many immune-related genes are differentially expressed in LOY natural killer cells and monocytes. These findings provide direct evidence to support the “immunosurveillance” hypothesis, as well as implicate a ChrY-dependent mechanism for sex differences in immunity (Klein and Flanagan 2016).
Potential models for explaining the observed associations of mosaic loss of Y chromosome (LOY) in leukocytes and disease susceptibility. a The “immunosurveillance” hypothesis of mosaic LOY (Forsberg 2017). This hypothesis describes that the mosaic LOY in immune cells would result in immunosurveillance impairment (ISI). Because the immune cells reside in nearly all tissues and mosaic LOY that alter their function could have a variety of phenotypic consequences including the etiology of cancers, Alzheimer’s disease (AD), cardiovascular diseases (CVDs) and diabetes. b The “common soil” hypothesis (Thompson et al. 2019). This hypothesis argues the genetic susceptibility to mosaic LOY represents the “common soil” of the susceptibility to other disease. Because this common genetic architecture is associated with genomic instability (GIN), GIN is highlighted as a biological mechanism underpinning diseases such as cancers, AD, CVDs and diabetes. This hypothesis is not necessarily incompatible with the “immunosurveillance” hypothesis. LOY in leukocytes may also have a direct role in disease of other tissues through ISI
However, genes deregulated in mosaic LOY versus non-mosaic LOY cells are not observed in any enrichment of immune-related ontology in head and neck squamous-cell carcinoma (Hollows et al. 2019). These different results suggest that LOY may alter the expression of immune-related genes in a cell-type specific manner and indicate LOY may contribute to cancer development in some other ways. Mosaic LOY is frequently observed in tumor cells and is more frequent in advanced tumors than low-grade tumors (Bianchi 2009). When human ChrY is incorporated into LOY human PC cell line PC-3, tumor suppression is observed in nearly all the athymic nude mice studied (Vijayakumar et al. 2005). These findings suggest ChrY per se has a tumor-suppressor role. Considering this, it is surprising that no apparent driver tumor-suppressor gene has been found in ChrY. Functional studies have verified several tumor suppressor-like genes in ChrY. For example, the TMSB4Y normally functions through interaction with β-actin to regulate cell morphology and cell proliferation (Wong et al. 2015); Overexpression of KDM5D, which encodes a lysine-specific histone H3 demethylase, reduces viability of renal cancer cells (Arseneault et al. 2017), while knockdown of KDM5D increases the growth rate and reduces apoptosis of PC cells (Jangravi et al. 2015); lncRNA TTTY15 is found to inhibit non-small cell lung cancer proliferation and metastasis via promoting the expression of TBX4, whose knockdown increases lung cancer cell migration and invasion (Lai et al. 2019).
The mild tumor-suppressor effects of ChrY genes suggest that the overall tumor-suppressor activity of ChrY depends on the combined effects of ChrY genes and many other autosomal- or ChrX-linked genes. Indeed, LOY has been found to activate oncogenes located in autosomes. For example, single-cell RNA sequencing reveals that LOY leukocytes upregulate the expression of TCL1A, a known oncogene maps at 14q32.13 whose product (TCL1) mediates intracellular signaling and stimulates cell proliferation and survival (Thompson et al. 2019). LOY tumors overexpress genes involved in redox process, including those implicated in resistance to both radiotherapy and cisplatin-based chemotherapeutics (Hollows et al. 2019). All of these evidence lead to the speculation that concomitant loss of multiple genes due to LOY can create phenotypes that are difficult to predict by analyzing individual genes and the impact of LOY on tumor development would be fundamentally different from loss of individual ChrY-located tumor-suppressor genes. In this sense, LOY should be considered and studied as a distinct somatic mutation.
While we are still debating whether impaired immunosurveillance or dysfunctional tumor suppressor network is the actual player underpins the mosaic LOY-associated cancer risk; a recent study has put an additional level of complexity to this question. Thompson et al. (2019) demonstrated that most of the mosaic LOY-associated genetic variants are frequently located near genes that contribute to cancer susceptibility and encode the drivers of tumor growth and targets of cancer therapies. Thus, these variants increase the susceptibility of males to non-hematological cancers, including both male-specific (PC and testicular germ cell tumor) and non-sex-specific (lung cancer, colorectal cancer, glioma and renal cell carcinoma,) (Thompson et al. 2019). The most unexpected finding of this study was that these mosaic LOY-related genetic loci are also associated with increased risk of female-specific non-hematological cancers (breast, ovarian and endometrial cancers) in women. These findings indicate that LOY does not play direct roles in increasing the cancer risk in males.
Alternatively, mosaic LOY may also be the consequence of carcinogenesis. For example, LOY in cancer cells may be solely attributed to its tiny size, as cancer cells preferentially lose small chromosomes (Duijf et al. 2013). In addition, mosaic LOY in blood fails to connect with the risk of hematological cancers in many large cohorts (Forsberg et al. 2014; Zhou et al. 2016; Loftfield et al. 2019; Terao et al. 2019a). This result is surprising, not only because the detected mosaic LOY occurs in hematological system, but also mosaic LOY occurs more frequently in hematological cancers than solid cancers in males (Bianchi 2009). Considering this, it remains to see whether mosaic LOY is a consequence associated with the development of hematological cancers. Meanwhile, mosaic LOY may be an epiphenomenon associates with cancers. For example, Tetrao et al. (2019) found that mosaic LOY was significantly associated with mortality from lung cancer in a Japanese cohort. However, this association mostly attributes to tobacco smoking because the significance is lost when smoking individuals are extracted, indicating mosaic LOY may just be an epiphenomenon in the process of smoking-associated lung cancer risk. In addition, mosaic LOY displays a highly significant association with clonal hematopoiesis, a novel driver for cancers (Zink et al. 2017).
Mosaic LOY in blood is associated with a remarkably increased risk for many nonmalignant diseases of aging like AD (Dumanski et al. 2016), but the underlying causality of this association remains elusive. A recent study shows that the induced pluripotent stem cells (iPSC)-derived nerve-like cells from fibroblasts carrying PSEN1 E280A mutation that is predisposed to familial AD, display LOY (Mendivil-Perez et al. 2019). Interestingly, one susceptibility variation for mosaic LOY maps in HM13 (Wright et al. 2017), which encodes an integral membrane protein with sequence motif characteristic of PSEN1/2 though its function remains unclear. Mosaic LOY is modestly associated with the ApoE4 genotype (Graham et al. 2019). Additionally, mosaic LOY in a Japanese cohort is significant with a genetic variation nearby the SPON1 (Terao et al. 2019a), a gene that was previously reported to be associated with the dementia severity (Jahanshad et al. 2013). These results indicate that LOY may be induced by mutations of familial and sporadic AD genes.
Similarly, several mosaic LOY risk variants have also implicated in the susceptibility to other aging-related diseases. For example, KCNQ1 variants were found to be significantly associated with mosaic LOY in the Japanese and UK populations (Terao et al. 2019a). Interestingly, variants of this gene have shown to be strongly associated with susceptibility to type 2 diabetes in East Asian and European populations (Unoki et al. 2008; Yasuda et al. 2008). Recently, Thompson et al. (2019) highlighted that another 6 mosaic LOY loci (TP53INP1, SUGP1, CCND2, EIF2S2, PTH1R and BCL2L11) overlapped with previously reported risk variants for type 2 diabetes (Xue et al. 2018). In addition, ANGPTL2 is a mosaic LOY risk gene (Terao et al. 2019a) whose product has been found to be associated with an increased risk of CVDs in diabetic patients (Gellen et al. 2016) and accelerate heart failure by perturbing cardiac function and energy metabolism in cardiac pathologies (Tian et al. 2016).
Based on all of these observations, Thompson et al. (2019) proposed the “common soil” hypothesis, which argues the genetic susceptibility to mosaic LOY represents the “common soil” of the susceptibility to other diseases like non-hematological cancers and diabetes (Fig. 2b). Since genetic susceptibility to mosaic LOY are associated with GIN (Wright et al. 2017; Thompson et al. 2019), the “common soil” hypothesis predicts that GIN may play key roles in the pathogenesis of these diseases. This hypothesis is not necessarily incompatible with the “immunosurveillance” hypothesis and other models. LOY in leukocytes may also have a direct role in disease of other tissues through impaired immunosurveillance and LOY in cancer cells promote cancer progression via dysfunctional tumor suppressor network. Thus, further functional studies are warranted to characterize how and in what ways may mosaic LOY influence male health.
Cancers, AD, CVDs and diabetes are four aging-related diseases that are also prevalent in females with XX sex chromosomes. The prevalence and susceptibility to cancers (Dorak and Karpuzoglu 2012), CVDs (Kander et al. 2017), and diabetes (Kautzky-Willer et al. 2016) are indeed higher in men than in women. In contrast, AD disproportionally affects women more than men in both prevalence and severity (Ferretti et al. 2018). Given that mosaic LOY increases the risk of cancers, CVDs, and diabetes in males, it is interesting to ask whether mosaic LOY contributes to the sex difference of these diseases. The possible mechanisms underlying these differences are complex and whether mosaic LOY is involved remains entirely unknown. Based on the “immunosurveillance” hypothesis, it seems plausible that mosaic LOY would be underlying in these differences because immunosurveillance impairment resulting from mosaic LOY only occurs in men, rather than in women. However, mosaic LOY may not contribute to the arising of these differences if we consider the “common soil” hypothesis. This is because the genetic variants for mosaic LOY present in the genomes of both men and women, and they induce mosaic LOY in male genome while inducing instability in female genome. Therefore, although mosaic LOY in blood may explain why men have shorter lives than women (Forsberg 2017), it is premature to think whether mosaic LOY would contribute to sex difference in the etiology of cancers, CVDs, and diabetes. More data need to be collected before we can address this question.
How and why ChrY prone to loss: a hypothesized micronucleation model
Although the risk genetic variants for mosaic LOY have been widely deciphered, the molecular mechanisms that might be responsible for the development of mosaic LOY are entirely unknown. Considering that most of mosaic LOY-associated variants are cell cycle genes (Zhou et al. 2016; Wright et al. 2017; Grassmann et al. 2019b; Terao et al. 2019a; Thompson et al. 2019) (Table 2), it has been proposed that the development of mosaic LOY is most likely to have a mitotic origin.
During cell division, chromosomes are duplicated and then segregated evenly to each daughter cell. Chromosome segregation is driven by the centromeres and mitotic spindles. Kinetochore, the protein complex assembled at each centromere, serves as the attachment site for spindle microtubules. The incorrectly attached kinetochores activate the spindle assembly checkpoint (SAC) to delay anaphase onset. Defective SAC signaling leads to premature chromosome segregation with mis-attached chromosomes, resulting in chromosome lagging at ana-telophase. If the lagging chromosomes are sufficiently far from the rest of the chromatin mass at mitotic exit, they form into isolated micronuclei [reviewed in (Guo et al. 2019c)]. The centromeres of micronuclei-enclosed chromosomes become damaged and are unable to support a functional kinetochore (Vázquez-Diez et al. 2016). Micronuclei therefore fail to be aligned at the metaphase plate and keep their micronuclei state during the following one or more cell cycles (Soto et al. 2018; He et al. 2019). This confers enough times for the micronucleated chromosomes to be degraded by autophagy or extruded out of the cell [reviewed in (Guo et al. 2019c)]. Based on the results of pioneering studies that ChrY is preferentially micronucleated and in an age-dependent way (Nath et al. 1995; Catalán et al. 1998), we propose the micronucleation hypothesis of LOY (Fig. 3) although the actual processes of LOY are undoubtedly more complex than this model. Importantly, ChrY micronucleation has shown to induce chromothripsis-like rearrangement in ChrY and structural variations in whole genome (Ly et al. 2017, 2019). Our micronucleation model thus, may provide novel insights into why mosaic LOY is associated with the occurrence of large autosomal mosaic events (Zhou et al. 2016). Our micronucleation model can also predict that the ChrY retained in somatic cells of aging persons would harbor high levels of complex rearrangement due to micronuclei-induced chromothripsis.
Hypothesized models of loss of Y chromosome (LOY). (A) The centromere of ChrY is intrinsically defective and the telomere of ChrY is thought to be quickly attrited in an age-related way. These characterizes lead ChrY prone to be incorrectly attached and/or non-disjunctioned during mitosis and be incorporated into isolated micronuclei after mitotic exit. Thus, the karyotypes of the daughter nuclei could be either 45,X0 and/or 46,XY and the micronuclei would harbor one or two ChrY. The non-micronucleated 45,X0 cells generated immediately after mitosis (shown bolded) would be held in check in young cells but may provide selective advantages to old cells. In ChrY-micronucleated 46,XY cells, the micronuclei prone to rupture and trigger the chromothripsis-like rearrangement in ChrY, which usually induces large-scale structural mosaicism in remainder genome. After this, the micronuclei can be degraded by autophagy or, in some cases, be extruded, which results in 45,X0 cells with large-scale structural mosaicism (This cell type is shown underlined to distinguish it from 45,X0 cell). Compared to 45,X0 cells, 45,X0 cells are held in check more tightly in young cells but provide much more selective advantages to old cells due to the high-degree of karyotype diversity
A question of vital importance is how LOY can arise so frequently, or in other words, why human ChrY is micronucleated so frequently. Although all the current known risk genes for mosaic LOY do not locate on ChrY, we think the most possible factors that make the ChrY prone to be micronucleated lies in the ChrY per se. One prime candidate factor is the unique feature of ChrY centromere. Normal human centromeres are enriched with ~ 171-bp tandem repeats (α-satellite array) that contains a 17-bp consensus motif for CENP-B binding (CENP-B box). CENP-B binding is critical for centromere to identity and function, since it stabilizes CENP-A and CENP-C, a key nucleator of kinetochore assembly (Barra and Fachinetti 2018). Thus, CENP-B box seems to be essential for ensuring segregation fidelity. Indeed, a recent study showing that the rate of chromosome missegregation is negatively correlated with the CENP-B box amount within chromosomes (Dumont et al. 2019). However, human ChrY is completely devoid of CENP-B box and contains the shortest α-satellite array of all chromosomes (Barra and Fachinetti 2018). These features result in reduced ChrY-centromere stretch, delayed congression of ChrY, and increased ChrY merotelic attachment, which eventually lead ChrY to be missegregated and micronucleated (Fachinetti et al. 2015). Consistent with this, ChrY-centromere inactivation induces 70% of micronuclei containing ChrY and 20% of cells display LOY in human DLD-1 cells (Ly et al. 2017). Expressing the fused CENP-B with CRISPR/Cas9 on the ChrY centromere could partially but significantly rescue the micronucleation-prone phenotype of ChrY (Dumont et al. 2019).
In addition, centromere dysfunction can induce chromosome nondisjunction, a well-known mechanism of aneuploidy (Vig 1984). This process can hypothetically produce ChrY nullisomic and disomic daughter cells. However, mosaic gain of ChrY (GOY) is highly lower than mosaic LOY in blood cells (Zhou et al. 2016) and cancer cells (Bianchi 2009). Therefore, non-disjunctioned sister ChrY are more likely to be incorporated into micronuclei instead of being included into one daughter nucleus. Consistent with this, 32.4% of micronuclei in DLD-1 cells entrapped two ChrY after ChrY-centromere inactivation (Ly et al. 2017). Fluorescence in situ hybridization (FISH) analysis showed that 26% of ChrY+ micronuclei had two signals in males above 50 years of age and only 5% of ChrY+ micronuclei had two signals in males under 30 years of age, indicating the age-dependent micronucleation of non-disjunctioned ChrY (Catalán et al. 1998). In light of the centromere-dysfunction model, it’s interesting to evaluate whether ChrY-centromere dysfunction (such as breakage, loss and rearrangement) is age-dependent. Recently, the complete assembly and characterization of human ChrY-centromere (Jain et al. 2018) may help to figure out this question.
Although the centromere-dysfunction model is attractive, other possibilities exist. Human chromosomes terminate in variable numbers of TTAGGG nucleotide repeats termed as telomere. Since telomere shortening is age dependent and the chromosomes with short telomeres are more frequently incorporated in micronuclei than chromosomes of normal telomere length (Pampalona et al. 2010), we postulate that the telomere shortening in ChrY may be fast than other chromosomes. Telomere-attrition model is supported by several lines of evidence. First, human cells have large inter-chromosomal variations in telomere length, indicating that chromosome-specific factors regulating the length of individual telomeres (Lansdorp et al. 1996; Martens et al. 1998). Second, given that homologous recombination provides a pathway for telomere protection and acquisition in human cells (Dunham et al. 2000; Tacconi and Tarsounas 2015), ChrY may be particularly susceptible to telomere shortening because of the inability of the haploid ChrY to deploy homologous recombination. Third, a variant maps to TERT, which encodes telomerase to stabilize telomere by adding TTAGGG repeats to the telomeric ends, has found to be a common susceptibility locus for mosaic LOY (Terao et al. 2019a; Thompson et al. 2019). Fourth, inactivated ChrX in female blood is more susceptible to age-related telomere shortening (Surrallés et al. 1999), micronucleation (Hando et al. 1997), and mosaic loss (Machiela et al. 2016). The telomere-attrition model of LOY is thus highly plausible, but merits further investigation.
Overall, our micronucleation model of LOY argues that certain structural features of ChrY which are intrinsic for ChrY (e.g., devoid of CENP-B box in centromere) and/or acquired during aging (e.g., a higher rate of telomere attrition) make the ChrY prone to be missegregated into micronuclei, which ultimately mediates the LOY in the following mitosis. In addition, we think the risk genes of mosaic LOY may coordinate with these structural alterations to increase the rate of LOY. More work is needed to advance our understanding of the molecular pathways that may underpin LOY.
Cellular and molecular processes contribute to the landscape of mosaic LOY
Importantly, in what ways age-dependent mosaic LOY can rise is poorly understood. Given that mosaic LOY is strongly associated with clonal hematopoiesis (Zink et al. 2017), it seems that mosaic LOY may be driven by aging-related alterations in the hematopoietic stem cells (HSCs) niche, as the process of clonal hematopoiesis. Indeed, through heritability enrichment analysis of specifically expressed mosaic LOY risk genes in several cell types in a Japanese cohort; Terao et al. recently demonstrated that the development of mosaic LOY is likely the result of clonal expansion of multipotent progenitor cells and HSCs after LOY (Terao et al. 2019a). More specifically, they used the heritability enrichment analysis in a total of 220 HSC subtypes and found CD34+ is primarily involved in the development of mosaic LOY (Terao et al. 2019a). Moreover, they found that the binding site of transcription factor FLI1 is the most significant region that overlaps with regions positively enriched in mosaic heritability. Since FLI1 drives the HSCs to produce platelets rather than red blood cells, Terao et al. also showed that individuals have a higher frequency of mosaic LOY have higher platelet counts but lower red blood cell (Terao et al. 2019a). Data from Dumanski et al (2019) and Thompson et al. (2019) also support this model. Despite these exciting findings, we cannot exclude the possibility that a subset of mosaic LOY event may be driven simply by progressive acquisition of LOY during the division of terminally differentiated cells and ensuing clonal selection that lead to increased detectability at older ages. This may partially explain the observation that the increase of mosaic LOY over time displays a non-linear model in a subset of studied men (Danielsson et al. 2019).
Another important unanswered question is why mosaic LOY rate keeps relatively low in young males and increases exponentially in aged males? Like clonal hematopoiesis, the successful estimation of a LOY cell clone needs both the initial generation and subsequent survival of the LOY cells. In line with this, mosaic LOY is associated with genes involved in cell cycle and apoptosis signaling (Grassmann et al. 2019b; Thompson et al. 2019) (Table 2). On one hand, we think the LOY may occur more frequently in aged cells. Based on our telomere-attrition model, we could predict that the occurrence of LOY is lower in young cells whose telomeres are long enough to confer telomeric function while being higher in aged cells whose telomeres become critically short. In addition, given that several genomic variations are found to be highly associated with mosaic LOY (Table 2), LOY is particularly sensitive to these somatic mutations. Therefore, very large somatic mutations accumulating with aging (Lodato et al. 2018) may contribute to the high occurrence of LOY in aged males. To obtain more evidence for supporting that the occurrence of LOY is more frequent in aged cells, further studies are necessary to validate the idea that the function of ChrY centromere and telomere undergo an age-dependent decay and the increased rate of stochastic acquisition of somatic mutations in mosaic LOY-associated genes in HSCs.
On the other hand, we think the LOY cells may have a higher possibility to survive in aging males as compared to young males. In primary mammalian cells, aneuploidy suppresses cell proliferation under standard culture condition, but confers selective advantages under suboptimal conditions (Williams et al. 2008; Rutledge et al. 2016), a phenomenon that is termed as aneuploidy paradox (Sheltzer and Amon 2011). Since aging is associated with several stressed cellular microenvironments (López-Otín et al. 2013), we could consider young body as a cumulation of optimal conditions and aging body as a cumulation of suboptimal conditions. According to the aneuploidy paradox model, the optimal conditions in young males are disadvantageous for LOY cells, making them to be almost completely eliminated from the cycling population. However, the frequency of aneuploidy in healthy persons has found to increase with age (Farkas et al. 2016), suggesting the suboptimal conditions in aging males provide advantageous for the expansion of aneuploid cells. Thus, although LOY is incompatible with the viability of young cells, we predict that it would confer selective survival advantages in aged cells. We think LOY cells, as compared to their non-LOY counterparts, may display mitotic advantage and apoptotic resistance in stressed (aging) conditions. This view is supported by the observation that mosaic LOY is associated with genes in cell cycle and apoptosis signaling (Grassmann et al. 2019b; Thompson et al. 2019). So what underlies the growth advantage from LOY? Since the occurrence of mosaic LOY is typically associated with large autosomal mosaic events (Zhou et al. 2016) and clonal hematopoiesis (Zink et al. 2017), such as karyotypic heterogeneity increases the chance that some LOY cells within population to explore adaptive karyotypes to suboptimal environments. Eventually, the genetic drift may result in the clonal expansion of LOY cells and lead to LOY cells colonizing the majority of the blood cells by older age. This model is summarized in Fig. 4.
Cellular and molecular processes contribute to the landscape of mosaic LOY. The development of mosaic LOY is likely the result of clonal expansion of hematopoietic stem cells (HSCs) after LOY. We propose that the baseline of LOY in young HSCs is very low because the telomere and centromere is stable and the mutation rate is low. However, the LOY rate increases exponentially in aged HSCs due to the age-dependent ChrY-telomere attrition, ChrY-centromere dysfunction and accumulation of somatic mutations. On the other hand, the LOY-positive HSCs have different fates in young and aged males. Since aging is associated with several stressed cellular microenvironments, we can consider young body as a cumulation of optimal conditions and aging body as a cumulation of suboptimal condition. The optimal conditions in young males are disadvantageous for LOY-positive HSCs, making them to be almost completely eliminated from the cycling population. In contrast, the LOY-positive HSCs may display mitotic advantage and apoptotic resistance in suboptimal (aging) conditions, as compared to their non-LOY counterparts. Since the occurrence of LOY is typically associated with large autosomal mosaic events, such karyotypic heterogeneity increases the chance that some LOY-positive HSCs within population to explore adaptive karyotypes to suboptimal environments. Eventually, the genetic drift may result in the clonal expansion of LOY-positive HSCs. Such positive selection is very strong in aged males, leading to LOY cells colonizing the majority of the blood cells by older age
It is note here that the LOY may have a mild effect on the balance of gene products required for cell proliferation because ChrY is gene-poor. Alternatively, loss of many other gene-rich autosomes decreases the expression of each of many genes on these chromosome, thereby disrupting the homeostasis of gene products required for normal cell growth and cycling. These may explain why loss of ChrY, but not other chromosomes, can be clonally expanded in aging males. Interestingly, mosaic GOY can also be detected in blood cells of aging males, but in a significantly lower frequency than mosaic LOY (0.96% vs 7.03% in all tested men) (Zhou et al. 2016; Danielsson et al. 2019), suggesting GOY is disadvantageous for cell proliferation and survival. Considering that most cancers exhibit the gains of chromosome (Weaver and Cleveland 2008) but not ChrY (Bianchi 2009), why GOY is incompatible with the cell viability and selected out in cancer cells remain open questions. Since single-chromosome gains can function as a tumor promoter or suppressor dependent on which chromosome is involved (Sheltzer et al. 2017; Vasudevan et al. 2019), it seems that GOY may act as a tumor suppressor by activating and/or strengthening a strong tumor suppressor network.
In aggregate, the development of mosaic LOY cell may be the result of clonal expansion of LOY-positive HSCs. The estimation of a mosaic LOY cell clone not only needs the initial missegregation of ChrY, but also requires the cellular environments that support the proliferation, survival, and clonal expansion of LOY cells. These environments seem rare in young cells but highly prevalent in old cells, explaining why mosaic LOY increases exponentially in aged males.
The biological and clinical studies of mosaic LOY: what’s next?
The past few years have seen substantial developments in research into the mosaic LOY. As is often the cases, research allows us to reveal a little more of the unknown, but raises even more questions. We issue the following five major research paths that, in our view, are promising for future mosaic LOY studies. We hope that some of the questions discussed are of interest to others and will contribute to ongoing research efforts in this new area of ChrY.
Estimating the extent of mosaic LOY in other human tissues and other mammals
As we have shown, mosaic LOY has been found in non-blood cells and tissues, such as brain and buccal mucosa. Recently, Terao et al. (2019a, b) estimated a causal effect of mosaic LOY on the increased aspartate transaminase (an index of liver damage), implying mosaic LOY may also occur in the liver and mediates the susceptibility to liver damage. The mosaic LOY occurs more frequently in blood than in brain (Graham et al. 2019) and is not equally distributed between different subsets of leukocytes in patients with AD or PC (Dumanski et al. 2019), reflecting a non-stochastic inter-tissue and intra-tissue accumulation of LOY. Larger comparative studies are needed to accurately estimate the actual distribution of mosaic LOY in tissues with high (i.e., the lung) and very low (i.e., the heart) cancer rates. The long-term goal should be to generate a genetic atlas of mosaic LOY in tissues of healthy individuals, sampled across the age spectrum and from distinct ancestries. In addition, whether aging-associated mosaic LOY occurs in other mammals, and if so, comparing the situation in humans with other mammals would allow us to rationalize why human ChrY is prone to loss in light of evolution.
Evaluating strategies protecting against LOY and buffering its detrimental consequences
In a large part of the studied men, mosaic LOY levels are relatively low during the entire study period (48 years) (Danielsson et al. 2019). Given that ChrY is highly prone to lose, determining whether surveillance mechanisms exist to prevent LOY will be of significant interest since it may provide valuable insights into the protective mechanisms against cancer. Particularly worthy of consideration is the identification of an age-related hypermethylation signature in human ChrY and this ChrY-specific methylation pattern is associated with reduced risk of death (Lund et al. 2019). This finding raises the question of whether ChrY hypermethylation is a candidate surveillance mechanism against LOY. Moreover, not all mosaic LOY males would develop cancer in 20 years after sampling (Forsberg et al. 2014; Dumanski et al. 2016), indicating second-site mutations and/or environmental factors (e.g., nutrition) may participate in enabling resistance to (or buffer against) disease associated with mosaic LOY in these persons. Thus, it is critical to filter through disease-resistant mosaic LOY individuals to validate their genomic status and environmental factors and ultimately obtain potential factors that enable resistance. Identifying such factors raises an intriguing translational opportunity to develop interventions to eliminate LOY clones from healthy tissues.
Developing LOY cell models
One of the most challenging aspect of LOY research is to extend from epidemiological associations to mechanistic studies on multiple levels of analysis. The exact consequences of LOY have remained unaddressed, partially because of the lack of suitable genetic models. Recently, researchers have developed CRISPR/Cas9 methodologies (Adikusuma et al. 2017; Zuo et al. 2017) and an inducible CENP-A replacement strategy (Ly et al. 2017, 2019) to induce ChrY depletion. These LOY cell models may be used to (1) screen the direct and indirect mechanisms by which the ChrY interacts with autosomes using a multi-omics approach (e.g., genomics, transcriptomics and proteomics). This will help to ascertain the extent to which LOY affect genes and how LOY influence the cells’ physiological properties; (2) access how LOY contributes to genomic remodeling. In free-living human cells, gain of an extra activate ChrX and subsequent one haploid complement of autosomes was found to accompany the LOY (Xu et al. 2017). The finding that LOY is associated with deregulation of DNA replication, repair, and recombination pathways may provide an explanation for LOY-related genomic remodeling (González et al. 2019). Intriguingly, the loss of deubiquitinating enzyme USP9X, the ChrX-linked homologue of USP9Y, reduces the effectiveness of the SAC and elevates chromosomal instability (Skowyra et al. 2018). Since USP9Y (see NCBI, NP_004645.2) shows 91.59% amino acid sequence identity to USP9X (see NCBI, NP_001034680.2) ,and they possess a similar function in protein deubiquitination (Bellott et al. 2014), we speculate that loss of USP9Y after LOY weakens the SAC and contribute to genomic remodeling; (3) explore whether LOY alone could induce oncogenic transformation, and if so, what are the underlying molecular pathways; or if not, which other mutations are needed; (4) address whether LOY in iPSC-derived neuron-like cells induces AD-like phenotypes, such as increased amyloid-β production and tau phosphorylation; (5) evaluate whether LOY cells provide proliferative advantages or apoptosis resistance to various environmental stress conditions. If so, LOY may not only be a result of segregation error, but also a driver of adaptation to stressful environments.
Developing ChrY-tagged cell models
LOY is routinely studied by several strategies after cell harvest (Table 1). Live imaging can be used to noninvasively determine the kinetics and spatio-temporal extent of LOY in individual cells and cell clones. Several living-cell imaging systems have developed to monitor ChrX (Masui et al. 2011) and specific autosomes (Xia et al. 2018). We can take advantages of these systems to develop ChrY-tagged cell models that could carry out living imaging to assess mitotic behavior of ChrY. Mutations of mosaic LOY susceptibility genes (Table 2) could be introduced into these models via genome editing to explore which genetic variants are actually genetic predisposition loci for LOY and which are these mediate LOY tolerance. Such models would also be excellent for studying (1) the exposure to what environmental factors can induce LOY. Interestingly, it seems this cell model has the potential to serve as a sensitive system for genotoxic studies; (2) whether the environmental stressors interact with susceptibility genes to induce mosaic LOY; (3) the rate at which LOY spontaneously occurs, whether the occurrence rate in old cells is higher than that in young cells, in which ways such changes occur, whether the micronucleation model of LOY (Fig. 3) holds true; (4) whether the micronutrients (e.g., folate supplementation) (Guo et al. 2017, 2019a) and phytochemicals (such as geraniin, resveratrol, and epigallocatechin-3-gallate) (Guo and Wang 2016; Guo et al. 2018a, b; Ni et al. 2018; Guo et al. 2019b) that are able to reduce micronuclei and strengthen SAC can prevent the spontaneous and/or induced LOY.
Applying tumor organoid technologies for LOY studies
Organoids are tissue-like structures derived from stem cells growing in substrata and they recapitulate many structural and functional aspects of their in vivo counterpart organs (Tuveson and Clevers 2019). Considering the differences between human and rodents ChrY (Bellott et al. 2014), we could culture patient-derived LOY iPSCs or genetically-engineered LOY tumor stem cells into organoids which mimic the original cancer tissue. Since organoids are amenable to live-cell imaging, we could examine the rates and types of mitotic errors in LOY organoids. By performing periodic single-cell DNA or RNA sequencing, we could also track the change of intra-tumoral heterogeneity induced by LOY. In addition, we can monitor the fate of LOY cells exposing to numerous chemotherapeutic agents, thereby evaluating their responses to therapy.
Conclusions
Despite its tiny size, ChrY is one of the most storied human chromosomes. The isolationist behavior of ChrY not only results in its decay at the level of species, but also turns it prone to be lost at the level of individual cells. Mosaic LOY is the most frequently occurred somatic mutation in aging men and is associated with an increased risk for various diseases. In this sense, mosaic LOY is increasingly recognized as a male-specific risk factor for multiple diseases and, therefore, the interest of mosaic LOY in personalized risk management is likely to increase in the coming years. The goal of this review is to prompt discussion and more intensive study of current findings toward a new area of ChrY research. We summarize the current understanding of the biological causes and clinical consequences of mosaic LOY (Fig. 5) and try to answer four questions at the heart of mosaic LOY: how ChrY is lost? Why ChrY is prone to loss? What factors determine the occurrence and prevalence of mosaic LOY? In what ways does mosaic LOY influence male health? In addition, we outline important gaps in mosaic LOY studies and assess several future directions from a biological and clinical perspective. Precisely answering these questions would undoubtedly yield many fascinating insights to help us more completely understand ChrY’s role in male health and disease and may help slow down the LOY-linked disease pathogenesis and develop strategies aiming to achieve healthy aging in males.
Diagram of the causes and consequences of mosaic loss of Y chromosome (LOY). The intrinsic and extrinsic factors, such as aging, genetic background, ChrY structural aberrations and environmental stressors, could induce mosaic LOY. These risk factors may compromise centromere function or accelerate telomere attrition in ChrY. In addition, the centromere of human ChrY, but not other human chromosomes, is intrinsically deficient for CENP-B box. Besides, the telomere of ChrY may be attrited more quickly than this of other chromosomes. All these factors make the ChrY to be more instable than other chromosomes during mitosis and, therefore, prone to be incorporated into isolated micronuclei after mitotic exit. The micronucleated ChrY tend to be lost because the micronuclei will be degraded or extruded. The process of ChrY micronucleation would cause ChrY to undergo chromothripsis, as well as induce large-scale structural mosaicism in autosomes and ChrX. ChrY is a key regulator of gene expression in males, LOY will result in global alteration in gene expression. These molecular processes would lead to distinct fitness outcomes to LOY cells. The LOY is thought to be neutral to cell’s fitness or can decrease or increase cell’s fitness dependent on the age of the affected individuals. The clinical phenotypes of mosaic LOY are highly variable: When mosaic LOY occurs in sperm cells, it results in male infertility; when mosaic LOY occurs in somatic cells, it increases the risks of several chronic diseases, such as solid cancers, Alzheimer’s disease (AD), cardiovascular diseases (CVDs) and diabetes. Meanwhile, mosaic LOY in aging men is associated with all-caused mortality. The “mLOY” on the tombstone refers to “mosaic loss of Y chromosome”, which was firstly reported by the year of 1963
References
Adikusuma F, Williams N, Grutzner F, Hughes J, Thomas P (2017) Targeted deletion of an entire chromosome using CRISPR/Cas9. Mol Ther 25:1736. https://doi.org/10.1016/j.ymthe.2017.05.021
Aitken RJ, Marshall Graves JA (2002) Human spermatozoa: the future of sex. Nature 415:963. https://doi.org/10.1038/415963a
Arseneault M, Monlong J, Vasudev NS, Laskar RS, Safisamghabadi M, Harnden P, Egevad L, Nourbehesht N, Panichnantakul P, Holcatova I, Brisuda A, Janout V, Kollarova H, Foretova L, Navratilova M et al (2017) Loss of chromosome Y leads to down regulation of KDM5D and KDM6C epigenetic modifiers in clear cell renal cell carcinoma. Sci Rep 7:44876. https://doi.org/10.1038/srep44876
Bachtrog D (2013) Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nat Rev Genet 14:113–124. https://doi.org/10.1038/nrg3366
Barra V, Fachinetti D (2018) The dark side of centromeres: types, causes and consequences of structural abnormalities implicating centromeric DNA. Nat Commun 9:4340. https://doi.org/10.1038/s41467-018-06545-y
Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Cho T-J, Koutseva N, Zaghlul S, Graves T, Rock S, Kremitzki C, Fulton RS, Dugan S, Ding Y, Morton D et al (2014) Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature 508:494–499. https://doi.org/10.1038/nature13206
Bianchi NO (2009) Y chromosome structural and functional changes in human malignant diseases. Mutat Res Rev Mutat Res 682:21–27. https://doi.org/10.1016/j.mrrev.2009.02.001
Biesecker LG, Spinner NB (2013) A genomic view of mosaicism and human disease. Nat Rev Genet 14:307–320. https://doi.org/10.1038/nrg3424
Blackmon H, Demuth JP (2015) The fragile Y hypothesis: Y chromosome aneuploidy as a selective pressure in sex chromosome and meiotic mechanism evolution. BioEssays 37:942–950. https://doi.org/10.1002/bies.201500040
Cannon-Albright LA, Farnham JM, Bailey M, Albright FS, Teerlink CC, Agarwal N, Stephenson RA, Thomas A (2014) Identification of specific Y chromosomes associated with increased prostate cancer risk. Prostate 74:991–998. https://doi.org/10.1002/pros.22821
Catalán J, Autio K, Kuosma E, Norppa H (1998) Age-dependent inclusion of sex chromosomes in lymphocyte micronuclei of man. Am J Hum Genet 63:1464–1472. https://doi.org/10.1086/302092
Colaco S, Modi D (2018) Genetics of the human Y chromosome and its association with male infertility. Reprod Biol Endocrinol 16:14. https://doi.org/10.1186/s12958-018-0330-5
Danielsson M, Halvardson J, Davies H, Moghadam BT, Mattisson J, Rychlicka-Buniowska E, Jaszczyński J, Heintz J, Lannfelt L, Giedraitis V, Ingelsson M, Dumanski JP, Forsberg LA (2019) Longitudinal changes in the frequency of mosaic chromosome Y loss in peripheral blood cells of aging men varies profoundly between individuals. Eur J Hum Genet. https://doi.org/10.1038/s41431-019-0533-z
Dorak MT, Karpuzoglu E (2012) Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet. https://doi.org/10.3389/fgene.2012.00268
Duijf PHG, Schultz N, Benezra R (2013) Cancer cells preferentially lose small chromosomes. Int J Cancer 132:2316–2326. https://doi.org/10.1002/ijc.27924
Dumanski JP, Rasi C, Lönn M, Davies H, Ingelsson M, Giedraitis V, Lannfelt L, Magnusson PKE, Lindgren CM, Morris AP, Cesarini D, Johannesson M, Tiensuu Janson E, Lind L, Pedersen NL et al (2015) Smoking is associated with mosaic loss of chromosome Y. Science 347:81–83. https://doi.org/10.1126/science.1262092
Dumanski JP, Lambert J-C, Rasi C, Giedraitis V, Davies H, Grenier-Boley B, Lindgren CM, Campion D, Dufouil C, Pasquier F, Amouyel P, Lannfelt L, Ingelsson M, Kilander L, Lind L et al (2016) Mosaic loss of chromosome Y in blood is associated with Alzheimer disease. Am J Hum Genet 98:1208–1219. https://doi.org/10.1016/j.ajhg.2016.05.014
Dumanski JP, Halvardson J, Davies H, Rychlicka-Buniowska E, Mattisson J, Moghadam BT, Nagy N, Węglarczyk K, Bukowska-Strakova K, Danielsson M, Olszewski P, Piotrowski A, Ambicka A, Przewoźnik M, Bełch Ł et al (2019) Loss of Y in leukocytes, dysregulation of autosomal immune genes and disease risks. bioRxiv. https://doi.org/10.1101/673459
Dumont M, Gamba R, Gestraud P, Klaasen S, Worrall JT, De Vries SG, Boudreau V, Salinas-Luypaert C, Maddox PS, Lens SM, Kops GJPL, McClelland SE, Miga KH, Fachinetti D (2019) Human chromosome-specific aneuploidy is influenced by DNA-dependent centromeric features. EMBO J 39:e102924. https://doi.org/10.15252/embj.2019102924
Dunham MA, Neumann AA, Fasching CL, Reddel RR (2000) Telomere maintenance by recombination in human cells. Nat Genet 26:447–450. https://doi.org/10.1038/82586
Fachinetti D, Han Joo S, McMahon Moira A, Ly P, Abdullah A, Wong Alex J, Cleveland Don W (2015) DNA sequence-specific binding of CENP-B enhances the fidelity of human centromere function. Dev Cell 33:314–327. https://doi.org/10.1016/j.devcel.2015.03.020
Farkas G, Jurányi Z, Székely G, Kocsis ZS, Gundy S (2016) Relationship between spontaneous frequency of aneuploidy and cancer risk in 2145 healthy Hungarian subjects. Mutagenesis 31:583–588. https://doi.org/10.1093/mutage/gew024
Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A, Baracchi F, Girouard H, Misoch S, Giacobini E, Depypere H, Hampel H, for the Women’s Brain P, the Alzheimer Precision Medicine I (2018) Sex differences in Alzheimer disease—the gateway to precision medicine. Nat Rev Neurol 14:457–469. https://doi.org/10.1038/s41582-018-0032-9
Forsberg LA (2017) Loss of chromosome Y (LOY) in blood cells is associated with increased risk for disease and mortality in aging men. Hum Genet 136:657–663. https://doi.org/10.1007/s00439-017-1799-2
Forsberg LA, Rasi C, Malmqvist N, Davies H, Pasupulati S, Pakalapati G, Sandgren J, de Ståhl TD, Zaghlool A, Giedraitis V, Lannfelt L, Score J, Cross NCP, Absher D, Janson ET et al (2014) Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat Genet 46:624–628. https://doi.org/10.1038/ng.2966
Forsberg LA, Halvardson J, Rychlicka-Buniowska E, Danielsson M, Moghadam BT, Mattisson J, Rasi C, Davies H, Lind L, Giedraitis V, Lannfelt L, Kilander L, Ingelsson M, Dumanski JP (2019) Mosaic loss of chromosome Y in leukocytes matters. Nat Genet 51:4–7. https://doi.org/10.1038/s41588-018-0267-9
Ganster C, Kämpfe D, Jung K, Braulke F, Shirneshan K, Machherndl-Spandl S, Suessner S, Bramlage CP, Legler TJ, Koziolek MJ, Haase D, Schanz J (2015) New data shed light on Y-loss-related pathogenesis in myelodysplastic syndromes. Genes Chromosomes Cancer 54:717–724. https://doi.org/10.1002/gcc.22282
Gellen B, Thorin-Trescases N, Sosner P, Gand E, Saulnier P-J, Ragot S, Fraty M, Laugier S, Ducrocq G, Montaigne D, Llaty P, Rigalleau V, Zaoui P, Halimi J-M, Roussel R et al (2016) ANGPTL2 is associated with an increased risk of cardiovascular events and death in diabetic patients. Diabetologia 59:2321–2330. https://doi.org/10.1007/s00125-016-4066-5
González JR, López-Sánchez M, Cáceres A, Puig P, Esko T, Pérez-Jurado LA (2019) A robust estimation of mosaic loss of chromosome Y from genotype-array-intensity data to improve disease risk associations and transcriptional effects. bioRxiv. https://doi.org/10.1101/764845
Graham EJ, Vermeulen M, Vardarajan B, Bennet D, De Jager P, Pearse RV, Young-Pearse TL, Mostafavi S (2019) Somatic mosaicism of sex chromosomes in the blood and brain. Brain Res. https://doi.org/10.1016/j.brainres.2019.146345
Grassmann F, Kiel C, Hollander AI, Weeks DE, Lotery A, Cipriani V, Weber BHF, on behalf of the International Age-related Macular Degeneration Genomics Consortium (2019a) Y chromosome mosaicism is associated with age-related macular degeneration. Eur J Hum Genet 27:36–41. https://doi.org/10.1038/s41431-018-0238-8
Grassmann F, International AMD Genomics Consortium (IAMDGC), Weber BHF, Veitia RA (2019b) Insights into the loss of the Y chromosome with age in control individuals and in patients with age-related macular degeneration using genotyping microarray data. Hum Genet. https://doi.org/10.1007/s00439-00019-02029-00431
Guo X, Wang X (2016) Phyllanthus emblica fruit extract activates spindle assembly checkpoint, prevents mitotic aberrations and genomic instability in human colon epithelial NCM460 cells. Int J Mol Sci 17:1437. https://doi.org/10.3390/ijms17091437
Guo X, Ni J, Zhu Y, Zhou T, Ma X, Xue J, Wang X (2017) Folate deficiency induces mitotic aberrations and chromosomal instability by compromising the spindle assembly checkpoint in cultured human colon cells. Mutagenesis 32:547–560. https://doi.org/10.1093/mutage/gex030
Guo X, Ni J, Dai X, Zhou T, Yang G, Xue J, Wang X (2018a) Biphasic regulation of spindle assembly checkpoint by low and high concentrations of resveratrol leads to the opposite effect on chromosomal instability. Mutat Res Genet Toxicol Environ Mutagen 825:19–30. https://doi.org/10.1016/j.mrgentox.2017.11.004
Guo X, Wang H, Ni J, Liang Z, Wu X, Xue J, Wang X (2018b) Geraniin selectively promotes cytostasis and apoptosis in human colorectal cancer cells by inducing catastrophic chromosomal instability. Mutagenesis 33:271–281. https://doi.org/10.1093/mutage/gey016
Guo X, Dai X, Ni J, Cao N, Yang G, Xue J, Wang X (2019a) High concentration of sugars is genotoxic to folate-deficient cells. Mutat Res Fundam Mol Mech Mutagen 814:15–22. https://doi.org/10.1016/j.mrfmmm.2019.01.003
Guo X, Dai X, Ni J, Ma X, Xue J, Wang X (2019b) Geraniin differentially modulates chromosome stability of colon cancer and noncancerous cells by oppositely regulating their spindle assembly checkpoint. Environ Mol Mutagen 60:254–268. https://doi.org/10.1002/em.22265
Guo X, Ni J, Liang Z, Xue J, Fenech MF, Wang X (2019c) The molecular origins and pathophysiological consequences of micronuclei: new insights into an age-old problem. Mutat Res Rev Mutat Res 779:1–35. https://doi.org/10.1016/j.mrrev.2018.11.001
Guttenbach M, Koschorz B, Bernthaler U, Grimm T, Schmid M (1995) Sex chromosome loss and aging: in situ hybridization studies on human interphase nuclei. Am J Hum Genet 57:1143–1150
Haitjema S, Kofink D, van Setten J, van der Laan SW, Schoneveld AH, Eales J, Tomaszewski M, de Jager SCA, Pasterkamp G, Asselbergs FW, den Ruijter HM (2017) Loss of Y chromosome in blood is associated with major cardiovascular events during follow-up in men after carotid endarterectomy. Circ Cardiovasc Genet 10:e001544. https://doi.org/10.1161/circgenetics.116.001544
Hando JC, Tucker JD, Davenport M, Tepperberg J, Nath J (1997) X chromosome inactivation and micronuclei in normal and Turner individuals. Hum Genet 100:624–628. https://doi.org/10.1007/s004390050564
He B, Gnawali N, Hinman AW, Mattingly AJ, Osimani A, Cimini D (2019) Chromosomes missegregated into micronuclei contribute to chromosomal instability by missegregating at the next division. Oncotarget 10:2660–2674. https://doi.org/10.18632/oncotarget.26853
Helgason A, Einarsson AW, Guðmundsdóttir VB, Sigurðsson Á, Gunnarsdóttir ED, Jagadeesan A, Ebenesersdóttir SS, Kong A, Stefánsson K (2015) The Y-chromosome point mutation rate in humans. Nat Genet 47:453–457. https://doi.org/10.1038/ng.3171
Hirata T, Hishimoto A, Otsuka I, Okazaki S, Boku S, Kimura A, Horai T, Sora I (2018) Investigation of chromosome Y loss in men with schizophrenia. Neuropsychiatr Dis Treat 14:2115–2122. https://doi.org/10.2147/NDT.S172886
Hollows R, Wei W, Cazier J-B, Mehanna H, Parry G, Halford G, Murray P (2019) Association between loss of Y chromosome and poor prognosis in male head and neck squamous cell carcinoma. Head Neck 41:993–1006. https://doi.org/10.1002/hed.25537
Hsu LY (1994) Phenotype/karyotype correlations of Y chromosome aneuploidy with emphasis on structural aberrations in postnatally diagnosed cases. Am J Med Genet 53:108–140. https://doi.org/10.1002/ajmg.1320530204
Jacobs PA, Brunton M, Court Brown WM, Doll R, Goldstein H (1963) Change of human chromosome count distributions with age: evidence for a sex difference. Nature 197:1080–1081. https://doi.org/10.1038/1971080a0
Jacobs KB, Yeager M, Zhou W, Wacholder S, Wang Z, Rodriguez-Santiago B, Hutchinson A, Deng X, Liu C, Horner M-J, Cullen M, Epstein CG, Burdett L, Dean MC, Chatterjee N et al (2012) Detectable clonal mosaicism and its relationship to aging and cancer. Nat Genet 44:651–658. https://doi.org/10.1038/ng.2270
Jahanshad N, Rajagopalan P, Hua X, Hibar DP, Nir TM, Toga AW, Jack CR, Saykin AJ, Green RC, Weiner MW, Medland SE, Montgomery GW, Hansell NK, McMahon KL, de Zubicaray GI et al (2013) Genome-wide scan of healthy human connectome discovers SPON1 gene variant influencing dementia severity. Proc Natl Acad Sci USA 110:4768. https://doi.org/10.1073/pnas.1216206110
Jain M, Olsen HE, Turner DJ, Stoddart D, Bulazel KV, Paten B, Haussler D, Willard HF, Akeson M, Miga KH (2018) Linear assembly of a human centromere on the Y chromosome. Nat Biotechnol 36:321–323. https://doi.org/10.1038/nbt.4109
Jangravi Z, Tabar MS, Mirzaei M, Parsamatin P, Vakilian H, Alikhani M, Shabani M, Haynes PA, Goodchild AK, Gourabi H, Baharvand H, Salekdeh GH (2015) Two splice variants of Y chromosome-located lysine-specific demethylase 5D have distinct function in prostate cancer cell line (DU-145). J Proteome Res 14:3492–3502. https://doi.org/10.1021/acs.jproteome.5b00333
Jobling MA, Tyler-Smith C (2003) The human Y chromosome: an evolutionary marker comes of age. Nat Rev Genet 4:598–612. https://doi.org/10.1038/nrg1124
Jobling MA, Tyler-Smith C (2017) Human Y-chromosome variation in the genome-sequencing era. Nat Rev Genet 18:485–497. https://doi.org/10.1038/nrg.2017.36
Kander MC, Cui Y, Liu Z (2017) Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J Cell Mol Med 21:1024–1032. https://doi.org/10.1111/jcmm.13038
Kautzky-Willer A, Harreiter J, Pacini G (2016) Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 37:278–316. https://doi.org/10.1210/er.2015-1137
Kimura A, Hishimoto A, Otsuka I, Okazaki S, Boku S, Horai T, Izumi T, Takahashi M, Ueno Y, Shirakawa O (2018) Loss of chromosome Y in blood, but not in brain, of suicide completers. PLoS ONE 13:e0190667. https://doi.org/10.1371/journal.pone.0190667
Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626–638. https://doi.org/10.1038/nri.2016.90
Lai IL, Chang Y-S, Chan W-L, Lee Y-T, Yen J-C, Yang C-A, Hung S-Y, Chang J-G (2019) Male-specific long noncoding RNA TTTY15 inhibits non-small cell lung cancer proliferation and metastasis via TBX4. Int J Mol Sci. https://doi.org/10.3390/ijms20143473
Lange J, Skaletsky H, van Daalen SK, Embry SL, Korver CM, Brown LG, Oates RD, Silber S, Repping S, Page DC (2009) Isodicentric Y chromosomes and sex disorders as byproducts of homologous recombination that maintains palindromes. Cell 138:855–869. https://doi.org/10.1016/j.cell.2009.07.042
Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little MT, Dirks RW, Raap AK, Tanke HJ (1996) Heterogeneity in telomere length of human chromosomes. Hum Mol Genet 5:685–691. https://doi.org/10.1093/hmg/5.5.685
Laurie CC, Laurie CA, Rice K, Doheny KF, Zelnick LR, McHugh CP, Ling H, Hetrick KN, Pugh EW, Amos C, Wei Q, Wang L-E, Lee JE, Barnes KC, Hansel NN et al (2012) Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat Genet 44:642–650. https://doi.org/10.1038/ng.2271
Lee J, Pinares-Garcia P, Loke H, Ham S, Vilain E, Harley VR (2019) Sex-specific neuroprotection by inhibition of the Y-chromosome gene, SRY, in experimental Parkinson’s disease. Proc Natl Acad Sci USA 116:16577. https://doi.org/10.1073/pnas.1900406116
Liu Y, Bai Y, Wu X, Li G, Wei W, Fu W, Wang G, Feng Y, Meng H, Li H, Li M, Guan X, Zhang X, He M, Wu T et al (2019) Polycyclic aromatic hydrocarbons exposure and their joint effects with age, smoking, and TCL1A variants on mosaic loss of chromosome Y among coke-oven workers. Environ Pollut. https://doi.org/10.1016/j.envpol.2019.113655
Lleo A, Oertelt-Prigione S, Bianchi I, Caliari L, Finelli P, Miozzo M, Lazzari R, Floreani A, Donato F, Colombo M, Gershwin ME, Podda M, Invernizzi P (2013) Y chromosome loss in male patients with primary biliary cirrhosis. J Autoimmun 41:87–91. https://doi.org/10.1016/j.jaut.2012.12.008
Lodato MA, Rodin RE, Bohrson CL, Coulter ME, Barton AR, Kwon M, Sherman MA, Vitzthum CM, Luquette LJ, Yandava CN, Yang P, Chittenden TW, Hatem NE, Ryu SC, Woodworth MB et al (2018) Aging and neurodegeneration are associated with increased mutations in single human neurons. Science 359:555–559. https://doi.org/10.1126/science.aao4426
Loftfield E, Zhou W, Graubard BI, Yeager M, Chanock SJ, Freedman ND, Machiela MJ (2018) Predictors of mosaic chromosome Y loss and associations with mortality in the UK Biobank. Sci Rep 8:12316. https://doi.org/10.1038/s41598-018-30759-1
Loftfield E, Zhou W, Yeager M, Chanock SJ, Freedman ND, Machiela MJ (2019) Mosaic Y loss is moderately associated with solid tumor risk. Cancer Res 79:461–466. https://doi.org/10.1158/0008-5472.CAN-18-2566
Loh P-R, Genovese G, Handsaker RE, Finucane HK, Reshef YA, Palamara PF, Birmann BM, Talkowski ME, Bakhoum SF, McCarroll SA, Price AL (2018) Insights into clonal haematopoiesis from 8342 mosaic chromosomal alterations. Nature 559:350–355. https://doi.org/10.1038/s41586-018-0321-x
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Lund JB, Li S, Christensen K, Mengel-From J, Soerensen M, Marioni RE, Starr J, Pattie A, Deary IJ, Baumbach J (2019) Age-dependent DNA methylation patterns on the Y chromosome in elderly males. Aging Cell 18:e12907. https://doi.org/10.1111/acel.12907
Ly P, Teitz LS, Kim DH, Shoshani O, Skaletsky H, Fachinetti D, Page DC, Cleveland DW (2017) Selective Y centromere inactivation triggers chromosome shattering in micronuclei and repair by non-homologous end joining. Nat Cell Biol 19:68–75. https://doi.org/10.1038/ncb3450
Ly P, Brunner SF, Shoshani O, Kim DH, Lan W, Pyntikova T, Flanagan AM, Behjati S, Page DC, Campbell PJ, Cleveland DW (2019) Chromosome segregation errors generate a diverse spectrum of simple and complex genomic rearrangements. Nat Genet 51:705–715. https://doi.org/10.1038/s41588-019-0360-8
Maan AA, Eales J, Akbarov A, Rowland J, Xu X, Jobling MA, Charchar FJ, Tomaszewski M (2017) The Y chromosome: a blueprint for men’s health? Eur J Hum Genet 25:1181–1188. https://doi.org/10.1038/ejhg.2017.128
Machiela MJ, Zhou W, Karlins E, Sampson JN, Freedman ND, Yang Q, Hicks B, Dagnall C, Hautman C, Jacobs KB, Abnet CC, Aldrich MC, Amos C, Amundadottir LT, Arslan AA et al (2016) Female chromosome X mosaicism is age-related and preferentially affects the inactivated X chromosome. Nat Commun 7:11843. https://doi.org/10.1038/ncomms11843
Machiela MJ, Dagnall CL, Pathak A, Loud JT, Chanock SJ, Greene MH, McGlynn KA, Stewart DR (2017) Mosaic chromosome Y loss and testicular germ cell tumor risk. J Hum Genet 62:637–640. https://doi.org/10.1038/jhg.2017.20
Machiela MJ, Zhou W, Sampson JN, Dean MC, Jacobs KB, Black A, Brinton LA, Chang IS, Chen C, Chen C, Chen K, Cook LS, Crous BM, De VI, Doherty J et al (2015) Characterization of large structural genetic mosaicism in human autosomes. Am J Hum Genet 96:487–497. https://doi.org/10.1016/j.ajhg.2015.01.011
Martens UM, Zijlmans JMJ, Poon SS, Dragowska W, Yui J, Chavez EA, Ward RK, Lansdorp PM (1998) Short telomeres on human chromosome 17p. Nat Genet 18:76–80. https://doi.org/10.1038/ng0198-76
Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer's disease. Nat Rev Dis Primers 1:15056. https://doi.org/10.1038/nrdp.2015.56
Masui O, Bonnet I, Le Baccon P, Brito I, Pollex T, Murphy N, Hupé P, Barillot E, Belmont AS, Heard E (2011) Live-cell chromosome dynamics and outcome of X chromosome pairing events during ES cell differentiation. Cell 145:447–458. https://doi.org/10.1016/j.cell.2011.03.032
Mendivil-Perez M, Velez-Pardo C, Kosik KS, Lopera F, Jimenez-Del-Rio M (2019) iPSCs-derived nerve-like cells from familial Alzheimer’s disease PSEN 1 E280A reveal increased amyloid-beta levels and loss of the Y chromosome. Neurosci Lett 703:111–118. https://doi.org/10.1016/j.neulet.2019.03.032
Meyfour A, Ansari H, Pahlavan S, Mirshahvaladi S, Rezaei-Tavirani M, Gourabi H, Baharvand H, Salekdeh GH (2017a) Y chromosome missing protein, TBL1Y, may play an important role in cardiac differentiation. J Proteome Res 16:4391–4402. https://doi.org/10.1021/acs.jproteome.7b00391
Meyfour A, Pooyan P, Pahlavan S, Rezaei-Tavirani M, Gourabi H, Baharvand H, Salekdeh GH (2017b) Chromosome-centric human proteome project allies with developmental biology: a case study of the role of Y chromosome genes in organ development. J Proteome Res 16:4259–4272. https://doi.org/10.1021/acs.jproteome.7b00446
Miyado M, Muroya K, Katsumi M, Saito K, Kon M, Fukami M (2018) Somatically acquired isodicentric Y and mosaic loss of chromosome Y in a boy with hypospadias. Cytogenet Genome Res 154:122–125. https://doi.org/10.1159/000488162
Molina E, Chew GS, Myers SA, Clarence EM, Eales JM, Tomaszewski M, Charchar FJ (2017) A novel Y-specific long non-coding RNA associated with cellular lipid accumulation in HepG2 cells and atherosclerosis-related genes. Sci Rep 7:16710. https://doi.org/10.1038/s41598-017-17165-9
Murakami S, Chishima S, Uemoto H, Sakamoto E, Sato T, Kurabe N, Kawasaki Y, Shibata T, Akiyama H, Tashiro F (2014) The male-specific factor Sry harbors an oncogenic function. Oncogene 33:2978–2986. https://doi.org/10.1038/onc.2013.262
Mužinić V, Ramić S, Želježić D (2019) Chromosome missegregation and aneuploidy induction in human peripheral blood lymphocytes in vitro by low concentrations of chlorpyrifos, imidacloprid and α-cypermethrin. Environ Mol Mutagen 60:72–84. https://doi.org/10.1002/em.22235
Nath J, Tucker JD, Hando JC (1995) Y Chromosome aneuploidy, micronuclei, kinetochores and aging in men. Chromosoma 103:725–731. https://doi.org/10.1007/bf00344234
Ni J, Guo X, Wang H, Zhou T, Wang X (2018) Differences in the effects of EGCG on chromosomal stability and cell growth between normal and colon cancer cells. Molecules. https://doi.org/10.3390/molecules23040788
Noveski P, Madjunkova S, Sukarova Stefanovska E, Matevska Geshkovska N, Kuzmanovska M, Dimovski A, Plaseska-Karanfilska D (2016) Loss of Y chromosome in peripheral blood of colorectal and prostate cancer patients. PLoS ONE 11:e0146264. https://doi.org/10.1371/journal.pone.0146264
Pampalona J, Soler D, Genescà A, Tusell L (2010) Whole chromosome loss is promoted by telomere dysfunction in primary cells. Genes Chromosomes Cancer 49:368–378. https://doi.org/10.1002/gcc.20749
Patsalis PC, Skordis N, Sismani C, Kousoulidou L, Koumbaris G, Eftychi C, Stavrides G, Ioulianos A, Kitsiou-Tzeli S, Galla-Voumvouraki A, Kosmaidou Z, Hadjiathanasiou CG, McElreavey K (2005) Identification of high frequency of Y chromosome deletions in patients with sex chromosome mosaicism and correlation with the clinical phenotype and Y-chromosome instability. Am J Med Genet A 135A:145–149. https://doi.org/10.1002/ajmg.a.30712
Persani L, Bonomi M, Lleo A, Pasini S, Civardi F, Bianchi I, Campi I, Finelli P, Miozzo M, Castronovo C, Sirchia S, Gershwin ME, Invernizzi P (2012) Increased loss of the Y chromosome in peripheral blood cells in male patients with autoimmune thyroiditis. J Autoimmun 38:J193–J196. https://doi.org/10.1016/j.jaut.2011.11.011
Pierre RV, Hoagland HC (1972) Age-associated aneuploidy: loss of Y chromosome from human bone marrow cells with aging. Cancer 30:889–894. https://doi.org/10.1002/1097-0142(197210)30:4%3c889:AID-CNCR2820300405%3e3.0.CO;2-1
Qin N, Li N, Wang C, Pu Z, Ma Z, Jin G, Zhu M, Dai M, Hu Z, Ma H, Shen H (2019) Association of mosaic loss of chromosome Y with lung cancer risk and prognosis in a Chinese population. J Thorac Oncol 14:37–44. https://doi.org/10.1016/j.jtho.2018.09.013
Repping S, van Daalen SKM, Brown LG, Korver CM, Lange J, Marszalek JD, Pyntikova T, van der Veen F, Skaletsky H, Page DC, Rozen S (2006) High mutation rates have driven extensive structural polymorphism among human Y chromosomes. Nat Genet 38:463–467. https://doi.org/10.1038/ng1754
Rutledge SD, Douglas TA, Nicholson JM, Vila-Casadesús M, Kantzler CL, Wangsa D, Barroso-Vilares M, Kale SD, Logarinho E, Cimini D (2016) Selective advantage of trisomic human cells cultured in non-standard conditions. Sci Rep 6:22828. https://doi.org/10.1038/srep22828
Senovilla L, Galluzzi L, Zitvogel L, Kroemer G (2013) Immunosurveillance as a regulator of tissue homeostasis. Trends Immunol 34:471–481. https://doi.org/10.1016/j.it.2013.06.005
Sheltzer JM, Amon A (2011) The aneuploidy paradox: costs and benefits of an incorrect karyotype. Trends Genet 27:446–453. https://doi.org/10.1016/j.tig.2011.07.003
Sheltzer JM, Ko JH, Replogle JM, Habibe Burgos NC, Chung ES, Meehl CM, Sayles NM, Passerini V, Storchova Z, Amon A (2017) Single-chromosome gains common function as tumor suppressors. Cancer Cell 31:240–255. https://doi.org/10.1016/j.ccell.2016.12.004
Shi W, Massaia A, Louzada S, Banerjee R, Hallast P, Chen Y, Bergström A, Gu Y, Leonard S, Quail MA, Ayub Q, Yang F, Tyler-Smith C, Xue Y (2018) Copy number variation arising from gene conversion on the human Y chromosome. Hum Genet 137:73–83. https://doi.org/10.1007/s00439-017-1857-9
Siffroi JP, Le Bourhis C, Krausz C, Barbaux S, Quintana-Murci L, Kanafani S, Rouba H, Bujan L, Bourrouillou G, Seifer I (2000) Sex chromosome mosaicism in males carrying Y chromosome long arm deletions. Hum Reprod 15:2559–2562. https://doi.org/10.1093/humrep/15.12.2559
Skowyra A, Allan LA, Saurin AT, Clarke PR (2018) USP9X limits mitotic checkpoint complex turnover to strengthen the spindle assembly checkpoint and guard against chromosomal instability. Cell Rep 23:852–865. https://doi.org/10.1016/j.celrep.2018.03.100
Soto M, García-Santisteban I, Krenning L, Medema RH, Raaijmakers JA (2018) Chromosomes trapped in micronuclei are liable to segregation errors. J Cell Sci 131:jcs214742. https://doi.org/10.1242/jcs.214742
Surrallés J, Hande MP, Marcos R, Lansdorp PM (1999) Accelerated telomere shortening in the human inactive X chromosome. Am J Hum Genet 65:1617–1622. https://doi.org/10.1086/302665
Tacconi EMC, Tarsounas M (2015) How homologous recombination maintains telomere integrity. Chromosoma 124:119–130. https://doi.org/10.1007/s00412-014-0497-2
Taleahmad S, Alikhani M, Mollamohammadi S, Yousefi M, Taei A, Hassani SN, Baharvand H, Salekdeh GH (2019) Inhibition of human Y chromosome gene, SRY, promotes naïve state of human pluripotent stem cells. J Proteome Res. https://doi.org/10.1021/acs.jproteome.9b00396
Tang D, Han Y, Lun Y, Jiang H, Xin S, Duan Z, Zhang J (2019) Y chromosome loss is associated with age-related male patients with abdominal aortic aneurysms. Clin Interv Aging 14:1227–1241. https://doi.org/10.2147/cia.s202188
Terao C, Momozawa Y, Ishigaki K, Kawakami E, Akiyama M, Loh P-R, Genovese G, Sugishita H, Ohta T, Hirata M, Perry JRB, Matsuda K, Murakami Y, Kubo M, Kamatani Y (2019a) GWAS of mosaic loss of chromosome Y highlights genetic effects on blood cell differentiation. Nat Commun 10:4719. https://doi.org/10.1038/s41467-019-12705-5
Terao C, Suzuki A, Momozawa Y, Akiyama M, Ishigaki K, Yamamoto K, Matsuda K, Murakami Y, McCarroll SA, Kubo M, Loh P-R, Kamatani Y (2019b) The genomic landscape of clonal hematopoiesis in Japan. bioRxiv. https://doi.org/10.1101/653733
Thompson DJ, Genovese G, Halvardson J, Ulirsch JC, Wright DJ, Terao C, Davidsson OB, Day FR, Sulem P, Jiang Y, Danielsson M, Davies H, Dennis J, Dunlop MG, Easton DF et al (2019) Genetic predisposition to mosaic Y chromosome loss in blood. Nature 575:652–657. https://doi.org/10.1038/s41586-019-1765-3
Tian Z, Miyata K, Kadomatsu T, Horiguchi H, Fukushima H, Tohyama S, Ujihara Y, Okumura T, Yamaguchi S, Zhao J, Endo M, Morinaga J, Sato M, Sugizaki T, Zhu S et al (2016) ANGPTL2 activity in cardiac pathologies accelerates heart failure by perturbing cardiac function and energy metabolism. Nat Commun 7:13016. https://doi.org/10.1038/ncomms13016
Trombetta B, Cruciani F (2017) Y chromosome palindromes and gene conversion. Hum Genet 136:605–619. https://doi.org/10.1007/s00439-017-1777-8
Tuveson D, Clevers H (2019) Cancer modeling meets human organoid technology. Science 364:952–955. https://doi.org/10.1126/science.aaw6985
UKCCG (1992) Loss of the Y chromosome from normal and neoplastic bone marrows. Genes Chromosomes Cancer 5:83–88. https://doi.org/10.1002/gcc.2870050112
Unoki H, Takahashi A, Kawaguchi T, Hara K, Horikoshi M, Andersen G, Ng DPK, Holmkvist J, Borch-Johnsen K, Jørgensen T, Sandbæk A, Lauritzen T, Hansen T, Nurbaya S, Tsunoda T et al (2008) SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet 40:1098–1102. https://doi.org/10.1038/ng.208
Vakilian H, Mirzaei M, Sharifi Tabar M, Pooyan P, Habibi Rezaee L, Parker L, Haynes PA, Gourabi H, Baharvand H, Salekdeh GH (2015) DDX3Y, a male-specific region of Y chromosome gene, may modulate neuronal differentiation. J Proteome Res 14:3474–3483. https://doi.org/10.1021/acs.jproteome.5b00512
Vasudevan A, Baruah PS, Smith JC, Wang Z, Sayles NM, Andrews P, Kendall J, Chunduri NK, Levy D, Wigler M, Storchová Z, Sheltzer JM (2019) Single chromosome gains can function as metastasis suppressors and metastasis promoters. bioRxiv. https://doi.org/10.1101/590547
Vázquez-Diez C, Yamagata K, Trivedi S, Haverfield J, FitzHarris G (2016) Micronucleus formation causes perpetual unilateral chromosome inheritance in mouse embryos. Proc Natl Acad Sci USA 113:626–631. https://doi.org/10.1073/pnas.1517628112
Vig BK (1984) Sequence of centromere separation another mechanism for the origin of nondisjunction. Hum Genet 66:239–243. https://doi.org/10.1007/bf00286609
Vijayakumar S, Garcia D, Hensel CH, Banerjee M, Bracht T, Xiang R, Kagan J, Naylor SL (2005) The human Y chromosome suppresses the tumorigenicity of PC-3, a human prostate cancer cell line, in athymic nude mice. Genes Chromosomes Cancer 44:365–372. https://doi.org/10.1002/gcc.20250
Weaver BA, Cleveland DW (2008) The aneuploidy paradox in cell growth and tumorigenesis. Cancer Cell 14:431–433. https://doi.org/10.1016/j.ccr.2008.11.011
Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, Amon A (2008) Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science 322:703–709. https://doi.org/10.1126/science.1160058
Wong HY, Wang GM, Croessmann S, Zabransky DJ, Chu D, Garay JP, Cidado J, Cochran RL, Beaver JA, Aggarwal A, Liu M-L, Argani P, Meeker A, Hurley PJ, Lauring J et al (2015) TMSB4Y is a candidate tumor suppressor on the Y chromosome and is deleted in male breast cancer. Oncotarget 6:44927–44940. https://doi.org/10.18632/oncotarget.6743
Wong JYY, Margolis HG, Machiela M, Zhou W, Odden MC, Psaty BM, Robbins J, Jones RR, Rotter JI, Chanock SJ, Rothman N, Lan Q, Lee JS (2018) Outdoor air pollution and mosaic loss of chromosome Y in older men from the cardiovascular health study. Environ Int 116:239–247. https://doi.org/10.1016/j.envint.2018.04.030
Wright DJ, Day FR, Kerrison ND, Zink F, Cardona A, Sulem P, Thompson DJ, Sigurjonsdottir S, Gudbjartsson DF, Helgason A, Chapman JR, Jackson SP, Langenberg C, Wareham NJ, Scott RA et al (2017) Genetic variants associated with mosaic Y chromosome loss highlight cell cycle genes and overlap with cancer susceptibility. Nat Genet 49:674–679. https://doi.org/10.1038/ng.3821
Xia Y, Zhu K, Irianto J, Andrechak JC, Dooling LJ, Pfeifer CR, Discher DE (2018) Live cell monitoring for factors affecting genome variation. bioRxiv. https://doi.org/10.1101/508150
Xiao G, Yao J, Kong D, Ye C, Chen R, Li L, Zeng T, Wang L, Zhang W, Shi X, Zhou T, Li J, Wang Y, Xu CL, Jiang J et al (2018) The long noncoding RNA TTTY15, which is located on the Y chromosome, promotes prostate cancer progression by sponging let-7. Eur Urol 76:315–326. https://doi.org/10.1016/j.eururo.2018.11.012
Xu J, Peng X, Chen Y, Zhang Y, Ma Q, Liang L, Carter AC, Lu X, Wu C-I (2017) Free-living human cells reconfigure their chromosomes in the evolution back to uni-cellularity. eLife 6:e28070. https://doi.org/10.7554/eLife.28070
Xue Y, Tyler-Smith C (2017) Past successes and future opportunities for the genetics of the human Y chromosome. Hum Genet 136:481–483. https://doi.org/10.1007/s00439-017-1806-7
Xue A, Wu Y, Zhu Z, Zhang F, Kemper KE, Zheng Z, Yengo L, Lloyd-Jones LR, Sidorenko J, Wu Y (2018) Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun 9:2941. https://doi.org/10.1038/s41467-018-04951-w
Yamazaki Y, Zhao N, Caulfield TR, Liu C-C, Bu G (2019) Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol 15:501–518. https://doi.org/10.1038/s41582-019-0228-7
Yan L, Cogan JD, Hedges LK, Nunley B, Hamid R, Austin ED (2018) The Y chromosome regulates BMPR2 expression via SRY: a possible reason “why” fewer males develop pulmonary arterial hypertension. Am J Respir Crit Care Med 198:1581–1583. https://doi.org/10.1164/rccm.201802-0308LE
Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, Hirota Y, Mori H, Jonsson A, Sato Y, Yamagata K, Hinokio Y, Wang H-Y, Tanahashi T, Nakamura N et al (2008) Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet 40:1092–1097. https://doi.org/10.1038/ng.207
Zhou W, Machiela MJ, Freedman ND, Rothman N, Malats N, Dagnall C, Caporaso N, Teras LT, Gaudet MM, Gapstur SM, Stevens VL, Jacobs KB, Sampson J, Albanes D, Weinstein S et al (2016) Mosaic loss of chromosome Y is associated with common variation near TCL1A. Nat Genet 48:563–568. https://doi.org/10.1038/ng.3545
Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, Thorgeirsson TE, Sigurdsson A, Gudjonsson SA, Gudmundsson J, Jonasson JG, Tryggvadottir L, Jonsson T, Helgason A, Gylfason A et al (2017) Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 130:742–752. https://doi.org/10.1182/blood-2017-02-769869
Zuo E, Huo X, Yao X, Hu X, Sun Y, Yin J, He B, Wang X, Shi L, Ping J (2017) CRISPR/Cas9-mediated targeted chromosome elimination. Genome Biol 18:224. https://doi.org/10.1186/s13059-017-1354-4
Acknowledgements
We thank the anonymous reviewers for helpful comments and suggestions. We apologize to our colleagues whose work we were unable to include because of space restrictions. This work was funded by the National Natural Science Foundation of China (Project no. 31860301 to X.W. and 31900410 to X.G.).
Author information
Authors and Affiliations
Contributions
XG contributed towards the design and structure of the manuscript. XG and XD reviewed the literature, designed the figures and drafted the manuscript, TZ, HW and JN contributed to the discussion. JX and XW contributed to editing. All authors read and approve the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, X., Dai, X., Zhou, T. et al. Mosaic loss of human Y chromosome: what, how and why. Hum Genet 139, 421–446 (2020). https://doi.org/10.1007/s00439-020-02114-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-020-02114-w