Abstract
The role of the mitochondria in disease, general health and aging has drawn much attention over the years. Several attempts have been made to describe how the numbers of mitochondria correlate with age, although with inconclusive results. In this study, the relative quantity of mitochondrial DNA compared to nuclear DNA, i.e. the mitochondrial DNA copy number, was measured by PCR technology and used as a proxy for the content of mitochondria copies. In 1,067 Danish twins and singletons (18–93 years of age), with the majority being elderly individuals, the estimated mean mitochondrial DNA copy number in peripheral blood cells was similar for those 18–48 years of age [mean relative mtDNA content: 61.0; 95 % CI (52.1; 69.9)], but declined by −0.54 mtDNA 95 % CI (−0.63; −0.45) every year for those older than approximately 50 years of age. However, the longitudinal, yearly decline within an individual was more than twice as steep as observed in the cross-sectional analysis [decline of mtDNA content: −1.27; 95 % CI (−1.71; −0.82)]. Subjects with low mitochondrial DNA copy number had poorer outcomes in terms of cognitive performance, physical strength, self-rated health, and higher all-cause mortality than subjects with high mitochondrial DNA copy number, also when age was controlled for. The copy number mortality association can contribute to the smaller decline in a cross-sectional sample of the population compared to the individual, longitudinal decline. This study suggests that high mitochondrial DNA copy number in blood is associated with better health and survival among elderly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mitochondria have multiple biological functions, but are primarily the center of energy resource in the cell, and nearly all adenosine triphosphate (ATP) is produced in the mitochondria through oxidative phosphorylation (OXPHOS). The disadvantage of the OXPHOS process is that it is a major source of the toxic by-products reactive oxygen species (ROS). The mitochondrion contains a 16,569-base pair double-stranded circular DNA molecule that is more susceptible to oxidative damage than nuclear DNA due to lack of introns and protective histones, a limited DNA repair capacity and the close proximity to the electron transport chain (Bonner et al. 2009). The mitochondria genome encodes 37 genes: 22 tRNAs, 2 rRNAs and 13 genes encoding subunits of the electron transport chain including ATP synthase. Each mitochondrion contains 2–10 mitochondrial DNA copies, and hundreds or up to several thousands of copies of mitochondria are located in each cell (Hosgood et al. 2010). However, technical issues make it difficult to determine the exact mitochondrial DNA copy number in each mitochondrion, so these numbers may vary even more. The mitochondria are located in all cell types except in red blood cells (Stier et al. 2013), and the number varies both between individuals and between tissues from the same individual. There is evidence that mutations in nuclear genes, e.g. the POLG gene, that encodes one of two subunits in the Human DNA POLymerase Gamma complex, can cause mitochondrial DNA (mtDNA) depletion, and that nuclear factors are involved in the regulation of mtDNA content (Pohjoismaki et al. 2010). Moreover, twin studies have indicated contributions from nuclear factors, as the mtDNA content in white blood cells and in cells from buccal swaps is partially heritable (Curran et al. 2007; Xing et al. 2008; Reiling et al. 2010).
The mtDNA copy number has been associated with various health outcomes. It has been suggested that high mtDNA copy number in the blood is associated with higher cognition in elderly women (Lee et al. 2010) and a lower level of depression (Kim et al. 2011). It has additionally been suggested that mtDNA copy number plays a role in Type II diabetes (Lee et al. 1998), although others have failed to confirm this finding (Reiling et al. 2010). Also, mtDNA copy number has been suggested to be a contributing factor in various cancers, e.g. renal cell carcinoma (Xing et al. 2008), and lung cancer (Hosgood et al. 2010; Bonner et al. 2009). The mtDNA copy number may even provide a link between smoking and lung cancer as smoking increases oxidative stress (Xing et al. 2008) and enhances somatic mtDNA mutations, thus potentially contributing damage to the mitochondrial function (Tan et al. 2008).
Aging is related to increased oxidative stress as well as to various health sequelae. Since the mitochondrion itself and the mitochondrial genome is exposed to damage from, e.g. reactive oxygen species, and since there is evidence to suggest that mtDNA copy number may be a biomarker of various health outcomes, it can be speculated that the copy number of mtDNA may be associated also with chronological age, but results are inconclusive. Some studies have failed to find a significant correlation between age and mtDNA content in blood cells (Miller et al. 2003; Frahm et al. 2005; Xing et al. 2008). However, both positive and negative associations have been found by others (Hosgood et al. 2010; Bai et al. 2004; Bonner et al. 2009), and even a biphasic association has been suggested (Liu et al. 2003). Furthermore, also gender differences have been observed. In a large study including approximately 1,000 subjects, copy numbers of mtDNA pooled from measures in buccal swaps or blood cells showed a significant reduction with age, but only among males (Reiling et al. 2010). The discrepancy between studies may in part be explained by limited sample sizes and the inclusion of only few elderly participants, and also by the possibility that associations may be distinct between the types of biological material that are investigated. In addition, differences in techniques used to determine mtDNA copy number, and the recovery efficiency of mtDNA during the DNA extraction process, could add to the varying correlations with age. Finally, the observed association with age at the group level may be different from the individual changes with increasing age due to, e.g. selection of participants, selection by mortality or varying environmental conditions throughout the lifespan in between cohorts.
The aim of the present study was to examine whether mtDNA copy number serves as a biomarker of aging, mortality and health. We examined the association between mtDNA copy number in the peripheral blood and age in a Danish study population including both twins and oldest old singletons (age 18–93 years) using a cross-sectional design. In a subset of twins older than 73 years, individual decline was estimated in a longitudinal analysis after 10 years of follow-up. Also, we investigated the differences in mtDNA copy number between sex and twin zygosity. Finally, we investigated the association between mtDNA copy number and a number of health parameters.
Results
Age-related decline of mtDNA copy number
mtDNA copy number was measured in blood cells from 1,067 subjects aged 18–93 years. The participants were recruited from the following Danish cohort surveys: the GEMINAKAR cohort, the MADT cohort, the LSADT cohort, and the 1905 birth cohort. All cohorts except the 1905 birth cohort included twins.
A tendency of lower mtDNA copy number with advancing age was observed (Table 1). As illustrated in Fig. 1, a quadratic-fitted model and the best linear spline model fitted the decline in mtDNA copy number with advancing age better than did a linear model. The optimal knot in the linear spline model was obtained at 48 years of age. The average mtDNA copy number was 61.0 [95 % CI (52.1; 69.9)] at age 18 years with no decline with age before the age of 48 years [β −0.01; 95 % CI (−0.21; 0.23)]. However, from the age of 48, an age-related decline was observed [β −0.54; 95 % CI (−0.63; −0.45)], corresponding to 5.4 less mtDNA copies every 10 years (Table 2).
Post-hoc analyses within the largest sub-sample, the LSADT participants, showed a similar age-related decline [β: −0.61 95 % CI (−0.74; −0.48)] as that observed from the age of 48 for the entire study population.
mtDNA copy number by sex, zygosity and body mass index
We found no sex differences in mtDNA copy number either in the crude or the age-adjusted analyses (Table 2).
Within the twin pairs, we observed that among dizygotic twins, the mtDNA copy number was on average 6.05 [95 % CI (3.80; 8.30)] higher than among monozygotic twins. However, the difference between dizygotic and monozygotic twins was considerably larger before the age of 48 years than after the age of 48 years (Table 2).
In overweight individuals [Body mass index (BMI) ≥ 25 kg/m2], the mtDNA copy number was borderline higher than in normal-weight individuals [β: 0.22; 95 % CI (0.00; 0.44)]. Similar tendencies for weight were observed when stratifying by age before or after age 48 years, although none of the sub-analyses reached significance (Table 2). Contrary to this, there was no significant difference in mtDNA copy number among underweight individuals (BMI < 18.5) over the entire age range, although underweight individuals had significantly lower mtDNA copy number than normal-weight individuals [β: −0.87; 95 % CI (−1.45; −0.30)] before the age of 48 years (Table 2).
Longitudinal change in mtDNA copy number
To further examine the hypothesis of an intra-individual decline in mtDNA copy number with age, samples drawn 10 years apart were analyzed in a longitudinal design for 108 of the LSADT twins. Results showed an average individual decline of −12.7 [95 % CI (−17.1; −8.2)] mtDNA copy numbers in 10 years, which is approximately twice as steep a yearly decline as that observed from the cross-sectional data. For the twins with initial mtDNA copy numbers higher than the mean, the 10-year decline in mtDNA copy numbers was steeper than for those with intake mtDNA copy numbers lower than the mean [difference in decline 24.9; 95 % CI (−17.4; 32.3)]. We observed no difference in decline by sex [difference in decline 2.2; 95 % CI (−7.9; 12.3)], zygosity [difference in decline 1.3; 95 % CI (−7.9; 10.5)] or age [difference in decline −6.2; 95 % CI (−15.2; 2.8)] (Table 3).
mtDNA copy number and health status
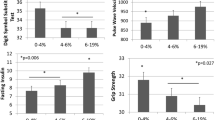
Since mtDNA copy number was shown to decline with age, and age is related to various health outcomes, we evaluated the relationship between mtDNA copy number and physical and mental health status in the LSADT, the MADT and the 1905 birth cohorts. Consistent associations between higher mtDNA copy number and better health outcome were observed, both in terms of better self-rated health [β: 0.05; 95 % CI (0.01; 0.09)] and higher physical performance, represented by a borderline better grip strength [β: 0.28; 95 % CI (0.00; 0.57)]. Both associations appear to be of similar intensity with increasing age from 58 to 93 years of age (Table 4).
Also, higher mtDNA copy number was consistently associated with higher cognitive composite score and MMSE, which seems to become more pronounced with increasing ages for both cognitive measures. The strongest association with cognition was thus observed for those older than 80 years, where an increase of 10 mtDNA copies associated with a 0.35 [95 % CI (0.10; 0.60)] higher cognitive composite score (Table 4).
A comparable result was found when analyzing the association between mtDNA copy number and mortality. An increase of 10 mtDNA copies was borderline associated with a 5 % lower risk of dying [HR: 0.95, 95 % CI (0.90; 1.00)] for participants 58 years of age and older. However, stratification of mtDNA content in quartiles revealed that those who had a high mtDNA copy number (2nd–4th quartiles) had a 17 % lower risk of dying [HR: 0.83, 95 % CI (0.71; 0.98)] compared to those in the 1st quartile.
Post-hoc analyses of the association within each of the cohorts in the LSADT, the 1905 birth cohort study, and the MADT study showed a similar direction of association between high mtDNA copy number and better health, except for the Activity of Daily Living within the LSADT cohort. However, the level of significance was, as would be expected, lower in the analyses within each cohort due to smaller samples sizes (data not shown).
Since smoking has previously been correlated with an increase in mtDNA, copy number analyses were subsequently adjusted for smoking as a potential confounder in the LSADT sub-sample, but this only changed the observed associations marginally (data not shown).
Discussion
The current study firmly supports previous suggestions that mtDNA copy number decreases with age in late life. However, we extend these findings by demonstrating that the decrease in mtDNA copy number seems to initiate in middle age, approximately at age 50. The observed decline indicates a half-life of the mtDNA copy number of approximately 60 years at the population level. In consistency with our cross-sectional data, our longitudinal data also demonstrate that mtDNA copy number declines at the individual level, as assessed in blood samples drawn 10 years apart from the same participants. To our knowledge, this is the first time a longitudinal change in mtDNA copy number has been reported in blood cells among healthy humans. The half-life of the mtDNA copy number was approximately 26 years, thus illustrating a more than doubling of the longitudinal decline compared to the decline estimated cross-sectionally. Another key finding, among the large group of elderly (58+ years of age), was the consistent direction of association between low mtDNA copy number and higher mortality, poorer condition in terms of self-rated health, cognitive as well as physical performance (Table 4).
The onset of decrease in mtDNA copy number found in this study, and the magnitude of the decline, may in part explain why it has been difficult to find consistency between mtDNA copy number and age in previous cross-sectional studies, as most of these studies include mainly younger subjects (Miller et al. 2003; Frahm et al. 2005; Xing et al. 2008; Hosgood et al. 2010; Bai et al. 2004; Bonner et al. 2009; Liu et al. 2003; Reiling et al. 2010). In accordance with our results, mtDNA copy number was recently reported to be lower in parents than in their children (Chu et al. 2012). In addition, an age-related decline in mtDNA copy number has also been observed in the human pancreatic islet among pancreatic islet donors (Cree et al. 2008) and in human muscle tissue from muscle biopsy donors (Short et al. 2005; Welle et al. 2003), as well as in liver and muscle cells from rats and several tissues from a short-lived fish (Barazzoni et al. 2000; Hartmann et al. 2011). Thus, the general age-related decline in mtDNA copy number in the later part of the life span is probably not unique for human blood cells, suggesting that a uniform mtDNA copy number decline with age may take place across tissues and even species in the late life. The demonstrated association of low mtDNA copy number with poorer health is also in line with previous studies (Lee et al. 1998, 2010; Kim et al. 2011; Reiling et al. 2010; Xing et al. 2008; Bonner et al. 2009; Hosgood et al. 2010). Of particular interest, low mtDNA copy number has been associated with low cognitive function in populations of Asian descent (Lee et al. 2010; Kim et al. 2011), and thus our finding of similar tendencies in a population of European descent shows a novel, consistent direction of association across two different populations. The implication of mtDNA copy number as a biomarker of general health is supported by the high consistency across all general health parameters investigated, except for activity of daily living (ADL). The reason for this could be that ADL is also more dependent on the environment (e.g. housing conditions and aids). Using a longitudinal design, we also present the novel and supportive observation that low mtDNA copy number in the general population is associated with increased mortality. This observation can, at least partly, explain the larger longitudinal decline in mtDNA copy number with age compared with that estimated in the widely used cross-sectional design. Thus, the latter study design includes individuals surviving to the older ages and who are also more likely to have a higher mtDNA copy number compared with individuals born within the same year, but who had died.We have previously in a longitudinal study of nonagenarians demonstrated similar patterns with steeper decline in functioning for individuals than for the overall population (Christensen et al. 2008).
The precise mechanisms that regulate mtDNA copy number are unclear. Since associations were observed uniquely between low mtDNA copy number and poor health, we speculate whether low mtDNA copy number among the elderly could indicate a low ATP production and mitochondria gene-expression (Short et al. 2005; Welle et al. 2003) or a shortage of oxidative stress-response (Liu et al. 2003). A combination is, however, likely since the mtDNA copy number shows a steeper decline with age than do mitochondria ATP production and mitochondria gene-expression (Short et al. 2005; Welle et al. 2003). Such disturbances in the cellular homeostasis may even enhance tumor genesis and apoptotic resistance (Venegas et al. 2011). The precise biological mechanisms could to some extent be deduced by the estimated number of mtDNA copies relative to the mitochondrial mass (Navarro-Sastre et al. 2012).
The main strength of the study is our large collection of samples from the elderly and oldest old, which enabled detection of the decline with age. Also, the inclusion of longitudinal data and multiple health parameters provided novel insight into how mtDNA copy number could be considered a biomarker associated with health. A weakness of the study is that by including samples mainly from the elderly the estimated onset of decline at 50 years of age is only approximated. Particularly, since we did not include children and young teenagers, our data could not be extrapolated to reflect mtDNA copy number changes (or lack of changes) in the early phase of life, which is particularly relevant for patients with rare mitochondria disorders. It is also worth noting, that the cohort study participants retrieved for this sub-study naturally included individuals who did not only agree to donate blood but also to participate in the interview, and these participants were slightly better functioning than the cohort members in general. As an example, the cognitive performance was found to be superior among participants donating blood samples compared to the entire group of interviewed participants (Mengel-From et al. 2011). However, in the potential situation that mtDNA copy number is likewise slightly higher among blood contributors, the associations between mtDNA copy numbers and, e.g. cognitive composite score would in fact be under estimated. Thus, we believe our findings would be even more explicit in the entire study population.
It is possible that our observation of mtDNA copy number decline with age, and the potential role of mtDNA copy number as a biomarker of general health could be subject to confounding effects that vary among cohorts, e.g. environmental exposures, such as the varying trends regarding smoking or alcohol consumption over the years. However, adjusting for smoking was of minor importance, at least among the LSADT participants. Also overweight was found to be positively associated with mtDNA copy number. However, this apparent association should be interpreted with caution, since BMI was mostly self-reported and thus highly likely to be underestimated.
As our study sample consisted primarily of twins, we found a difference in mtDNA copy number between MZ and DZ twins. Since this is the first time such findings have been reported, we went through the technical issues and examined the literature for biological reasons that could clarify this phenomenon, but no obvious explanations have come to light. However, due to this observed difference in mtDNA copy number between monozygotic and dizygotic twins, the decline in mtDNA copy number seen in this study may be slightly distinct from that of the general Danish population, although general health and survival after infancy are similar in twins and singletons (Christensen and McGue 2012).
In conclusion, our results emphasize the relevance of considering mtDNA copy number of blood cells or other tissues in future studies, as it may be a key component that links mitochondrial biology to environmental exposures as well as to various diseases and health issues in late life. The mtDNA copy number is already being used as a clinical biomarker of disease development or a possible mean of diagnosis for patients with, e.g. mitochondrial DNA depletion syndrome. In addition to this, our findings suggest that mtDNA copy number may serve as a biomarker for general health in combination with other markers of health and disease among the elderly. Such a biomarker could be useful as an endophenotype of mitochondrial biology in aging and age-related morbidity.
Materials and methods
Surveys
The study population includes subjects from four population-based nationwide surveys conducted at the University of Southern Denmark: The GEMINAKAR (the importance of genes, familiar and common environment for the development of insulin resistance, abdominal adiposity and cardiovascular risk factors) twin study, The study of Middle Aged Danish Twins (MADT), The Longitudinal Study of Aging Danish Twins (LSADT), and The Danish 1905 birth cohort Study.
The GEMINAKAR study is a longitudinal study of healthy Danish twins between 18 and 67 years of age at study baseline in 1997–2000 (Schousboe et al. 2003). The study focuses on genetic and environmental risk factors for developing insulin resistance, diabetes, obesity and cardiovascular risk factors and disease. The twins were recruited from the national, population-based Danish twin registry and were initially free of diabetes mellitus, cardiovascular disease or conditions that might limit participation in the clinical examination. Only complete twin pairs were included in the study. Seven-hundred and fifty-six complete twin pairs participated. From this cohort, a sample of 176 twins, 18–57 years of age, was randomly chosen and included in the present study.
The MADT study is a longitudinal study of twins who were 46–67 years of age when the study was initiated in 1998 (Gaist et al. 2000). A total of 40 monozygotic twin pairs, 40 dizygotic twin pairs and 40 twin pairs of opposite sex for each birth year between 1931 and 1952 were included in the cohort. The participants were re-visited from 2008 to 2011 and blood was donated during the re-assessment 10–14 years later (Skytthe et al. 2013). Samples of acceptable DNA quality from 58 twins, 58–72 years of age, were included in the present study after the re-visit in 2008. In the study, the 2008–2011 assessment was considered baseline.
The LSADT study is a longitudinal study of Danish twins aged 70 years and older (Christensen et al. 2002; Skytthe et al. 2002). The study was initiated in 1995, and the survey was repeated in 1997, 1999, 2001, 2003, 2005, and 2007. In 1997, mainly twin pairs of the same sex were included in the study; also, both twins were to be alive and born before January 1924. Participants were thus between 73 and 95 year of age. Full blood samples were drawn in 1997 from 689 members of intact twin pairs and again in 2007 from the surviving 120 intact twin pairs. The assessment in 1997 was considered baseline.
The Danish 1905 birth cohort study is a prospective investigation of an entire birth cohort (Nybo et al. 2003). The survey was initiated in 1998, when the participants were 92–93 years old, and comprises follow-up studies in 2000, 2003 and 2005. A total of 181 participants provided full blood samples in 1998 and were included in the current study.
All four studies were approved by the relevant Danish Scientific-Ethical Committees, as well as by the Danish Data Protection Board.
DNA extraction
DNA was extracted from total blood cells in peripheral full blood samples packed by centrifugation at 1,000g for 15 min followed by withdrawal of the plasma phase. The DNA was extracted by a standard manual salting-out protocol including a protease digestion step (Das et al. 1988). For samples from the 58 MADT, DNA was extracted using a virtually identical salting-out purification protocol adapted to an Autopure instrument (Qiagen). A comparable mtDNA copy number was observed for both purification procedures. A total of 142 samples were dropped as they had an extremely low average mitochondria DNA yield, probably reflecting poor DNA extraction quality.
Quantification of mitochondrial DNA copy number variations
An assay based on real-time polymerase chain reaction (qPCR) and SYBR® Green technology was used for measuring the amount of mitochondrial DNA relative to the nuclear DNA using two independent PCRs. A 153-bp target PCR product covering the mitochondrially encoded NADH dehydrogenase 1(MT-ND1) gene was generated using the forward primer sequence 5′-AACATACCCATGGCCAACCT-3′ and the reverse primer sequence 5′-AGCGAAGGGTTGTAGTAGCCC-3′. A reference 268-bp PCR product covering the nuclear hemoglobin beta (HBB) gene was generated using the forward primer sequence 5′-GAAGAGCCAAGGACAGGTAC-3′ and the reverse primer sequence 5′-CAACTTCATCCACGTTCACC-3′. All primer designs were originally published by Liu et al. (2003) and were applied in the later study (Lee et al. 2010). The assay was adapted to a high-throughput 96-plate format using a StepOne instrument and SYBR® Green technology (Applied Biosystems). The reactions were performed in a total volume of 10 µl including 1× Fast SYBR® GreenMaster Mix, 5 µM of each of the primers and 2 ng of DNA. The amplification was preheated at 95 °C at 30 s followed by a 40-cycle program of 0.3 s at 95 °C, 15 s at 58 °C and 30 s at 72 °C. The assay was calibrated using a serial dilution of 10, 5, 2.5, 1.25 and 0.625 ng DNA and a straight linear correlation was observed with an R 2 of 0.996 and was used to set the threshold cycle number (Ct) of both the nuclear and the mitochondrial genes. Each DNA sample was assayed in triplicates using either the MT-ND1 primers or HBB primers in parallel reactions. For each 96-plate, a DNA control sample from the same individual and a ‘no template control’ were added in triplicates. Samples from 176 out of 181 participants from the GEMINAKAR study, 58 out of 200 participants (for 142 samples, the mDNA yield was too poor) from the MADT study, 671 out of 689 participants from the LSADT study, and 162 out of 181 participants from the 1905 birth cohort study, and 108 samples out of 120 from the LSADT longitudinal study met our quality criteria after we had removed samples with low or no amplification (Ct > 31) in any of the six amplifications. The six amplifications were made up by three real-time PCR amplifications of each sample with the primer sets for MT-ND1 gene and HBB gene amplification, respectively. The median of the values were used to reduce variance from outliers.
BMI
Body mass index (kg/m2) was calculated using self-reported data on height and weight from all participants except for the participants in the GEMINAKAR study who were measured and weighted by a nurse. For participants in the 1905 birth cohort, approximately half of the persons did not answer the questions themselves; an estimate was then made by the interviewer or reported by the proxy. Participants were categorized into three groups according to their BMI (underweight <18.5 kg/m2; normal weight 18.5–24.9 kg/m2; overweight ≥25 kg/m2) (Herskind et al. 1996; Nybo et al. 2003).
Smoking
In LSADT, smoking status was self-reported as non-smoker, current smoker or former smoker.
Cognitive functions
Cognitive functioning was assessed using the Mini Mental State Examination (MMSE) and a 5-component cognitive composite score in the LSADT cohort and the Danish 1905 birth cohort participants at baseline (McGue and Christensen 2001). However, MADT participants were assessed by the cognitive composite score and not MMSE at baseline. The widely used MMSE ranges from 0 to 30 and can be graded as severely impaired for scores between 0 and 17, mildly impaired for scores between 18 and 23, and non-impaired for scores between 24 and 30. The five-component cognitive composite measures were originally selected to represent tasks that are sensitive to normative age changes, which can be reliably and briefly assessed by lay interviewers. The specific tasks included a fluency task, which involved the number of animals an individual could name in a 1-min interval, forward and backward digit span, and immediate and delayed recall of a 12-item list. The cognitive composite score was computed by taking the sum of the five standardized measures, separately from each cohort (using mean and SD from the initial MADT assessment in 1998). This score has shown its validity in numerous studies (McGue and Christensen 2002).
Grip strength
Grip strength was assessed according to standardized procedures as previously described (Frederiksen et al. 2002) using a Smedley dynamometer (TTM, Tokyo, Japan) in the LSADT cohort in 1999 and the Danish 1905 birth cohort and MADT cohort participants at baseline. To measure maximal strength, the width of the handle was adjusted to fit the hand size with the second phalanx resting against the inner stirrup. The elbow was to be held at 90° and the upper arm to be tight against the trunk in a series of three measurements, with brief pauses between each; the maximum value was used as the estimate. Furthermore, the maximum value of three measurements was identified with each hand. Grip strength discriminates functioning in all adult age groups, predicts incident disability, and is highly correlated with muscular power in other muscular groups. It is easily and reliably measured and correlates with function in activities of daily living and survival among the oldest old (Christensen et al. 2003, 2009).
Self-rated health
Participants from the MADT, the LSADT cohort, and the Danish 1905 birth cohort were at baseline presented with a list of 31 ailments and diseases and asked whether a physician had ever told them that they suffered from any of them. The number of present diseases was divided into three groups (0, 1–2, and ≥3). Major diseases such as cardiovascular disease (CVD), respiratory diseases, cancer, and diabetes mellitus were studied separately. Furthermore, subjective health was assessed using the question: “How do you consider your health in general?” with five response categories (excellent, good, acceptable, poor, and very poor) (Nybo et al. 2003).
Activity of daily living
Physical performance was assessed in the MADT, the LSADT cohort, and the Danish 1905 birth cohort participants using self-reported measures as part of the home-based 2-h interview at baseline. The 11-item measurement contains questions from relatively simple physical tasks to more demanding activities: (1) walking around in the house, (2) walking up and down the stairs, (3) walking up the stairs to the second floor, (4) getting outdoors, (5) walking 400 m without resting, (6) doing any kind of light exercise, (7) doing any kind of hard exercise, (8) outdoor walking in fine weather, (9) outdoor walking in bad weather, (10) running 100 m and (11) carrying 5 kg. At first all items were rated on a scale of 1–4 where 1 = cannot do, 2 = can do with aid or major difficulties, 3 = can do with fatigue or minor difficulties, and 4 = can do without fatigue. Then, the scale score (ADL strength score 1997) was calculated using a scale of 1–4, with higher scale scores indicating the higher levels of physical performance. The calculations of the scores had previously been described in details (Christensen et al. 2000, 2002). This scale showed high internal consistency and stability and has been shown to provide a sensitive quantitative measure of physical ability in other studies of elderly Danes (Tiainen et al. 2012).
Mortality analysis
Vital status was obtained by linkage with the Danish central registries via the unique identification number assigned at birth to all Danish citizens (Pedersen et al. 2006). The observation time for each participant from the MADT cohort, the LSADT cohort and Danish 1905 birth cohort was the period from enrollment into the cohort until death or December 31, 2012, whichever came first. There was no loss to follow-up as none of the participants emigrated.
Technical variation of real-time PCR
In addition to triplicate measures of all samples, a subset of 30 samples were analysed twice on 2 different days and with more than a month between the two analyses. The interplate variation of the mean from the HBB gene was comparable on the first (SD 0.07 Ct) and the second day (SD 0.09 Ct). Likewise, for the MT-ND1 gene the interplate variations were comparable on the first (SD 0.06 Ct) and second day (SD 0.10 Ct). Similar results were observed with only a mean difference of 0.02 Ct (SD 0.12) and the differences between the 30 samples indicated an acceptable technical variation with the mean of 5.56 Ct (SD 0.40). Each plate included a positive control sample, which was also analysed in triplicates, and after repeated analyses of the same positive control sample 36 times, the mean was 3.81 Ct (SD 0.29). Both tests showed acceptable technical variation with a coefficient of variance of 1 and 9 %, respectively. A subset of ten samples was extracted on 2 different days and assayed on the same real-time PCR plate. The coefficient of variance for these technical replications was estimated at 4 %, thus indicating that only a minor part of the variation is due to the DNA extraction method. Calculations were done prior to the transformation to the power of two of the mtDNA copy number.
Statistical analyses
The mtDNA copy number was calculated as the relative number of mtDNA to the nucleus DNA by the formula 2(CtHBBmedian – CtMT-ND1 median) as described elsewhere (Lee et al. 2010) except we did not conduct log transformation, thus we measured the n-fold difference in relation to a nuclear diploid gene. The cross-sectional age-related mtDNA copy number decline trend was evaluated by a quadratic function, which had a better fit than a linear function since the quadratic term reached the 5 % level of significance. Due to a simpler interpretation, a linear spline model was performed with one knot, which assumes a linear association between mtDNA copy number and age with different slopes before and after the knot. The best knot in the spline model was evaluated using the R 2 value and this spline model had a R 2 value better than that of the quadratic model. The best fitted linear spline model was used to evaluate the associations between mtDNA copy number and age, sex, zygosity and BMI. Post-hoc analysis was done subsequently using a knot at 73 years of age to compare the longitudinal change in the mtDNA copy number with that of the cross-sectional age. The individual change in mtDNA copy number of the 108 twins who donated blood twice with 10 years of follow-up was estimated using 95 % CI from a paired Student’s t test. Also, the 10-year differences were evaluated stratified by sex, zygosity, ages in two halves with equal number of participants (73–74 vs. 75–84 years of age at intake) and mtDNA copy number in two halves (49 mtDNA copy number or higher vs. <49 mtDNA copy number) at intake in 1997. Linear regression analysis was performed on continuous health outcomes and adjusted for age and sex in a combined analysis of the cohorts. Cox’s proportional hazards models were performed to study the association between mortality and mtDNA copy number, which was categorized into quartiles and as a continuous variable. Since the mortality was similar in the second, third and fourth quartile, we also analyzed the association between mortality and mtDNA copynumber divided into the first quartile versus the second, third and fourth quartiles. All Cox models were adjusted for gender and age by allowing age to be the underlying timescale and risk was assessed from the age at blood sampling to age at death or end of follow-up on December 31, 2012, whichever came first.
As the data partly pertain to twin pairs, and because observation within twin pairs might be correlated, the Cox’s proportional hazards models were performed using the robust estimator of variance, assuming independence between pairs. The proportional hazard assumption underlying the Cox models was tested using the Schoenfeld residual test and fulfilled the requirements for the Cox model.
An adjustment for a potential plate effect did not have significant influence on the results and was therefore not included in any of the final analyses. Post-hoc adjustment for smoking was done using smoking as a categorical covariate. The statistical calculations were performed using Stata 11.2 (StataCorp).
References
Bai RK, Perng CL, Hsu CH, Wong LJ (2004) Quantitative PCR analysis of mitochondrial DNA content in patients with mitochondrial disease. Ann N Y Acad Sci 1011:304–309
Barazzoni R, Short KR, Nair KS (2000) Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem 275(5):3343–3347
Bonner MR, Shen M, Liu CS, Divita M, He X, Lan Q (2009) Mitochondrial DNA content and lung cancer risk in Xuan Wei, China. Lung Cancer 63(3):331–334
Christensen K, McGue M (2012) Commentary: twins, worms and life course epidemiology. Int J Epidemiol 41(4):1010–1011
Christensen K, McGue M, Yashin A, Iachine I, Holm NV, Vaupel JW (2000) Genetic and environmental influences on functional abilities in Danish twins aged 75 years and older. J Gerontol A Biol Sci Med Sci 55(8):M446–M452
Christensen K, Gaist D, Vaupel JW, McGue M (2002) Genetic contribution to rate of change in functional abilities among Danish twins aged 75 years or more. Am J Epidemiol 155(2):132–139
Christensen K, Frederiksen H, Vaupel JW, McGue M (2003) Age trajectories of genetic variance in physical functioning: a longitudinal study of Danish twins aged 70 years and older. Behav Genet 33(2):125–136
Christensen K, McGue M, Petersen I, Jeune B, Vaupel JW (2008) Exceptional longevity does not result in excessive levels of disability. Proc Natl Acad Sci USA 105(36):13274–13279
Christensen K, Thinggaard M, McGue M, Rexbye H, Hjelmborg JV, Aviv A, Gunn D, van der Ouderaa F, Vaupel JW (2009) Perceived age as clinically useful biomarker of ageing: cohort study. BMJ 339:b5262
Chu HT, Hsiao WW, Tsao TT, Chang CM, Liu YW, Fan CC, Lin H, Chang HH, Yeh TJ, Chen JC, Huang DM, Chen CC, Kao CY (2012) Quantitative assessment of mitochondrial DNA copies from whole genome sequencing. BMC Genom 13(Suppl 7):S5
Cree LM, Patel SK, Pyle A, Lynn S, Turnbull DM, Chinnery PF, Walker M (2008) Age-related decline in mitochondrial DNA copy number in isolated human pancreatic islets. Diabetologia 51(8):1440–1443
Curran JE, Johnson MP, Dyer TD, Goring HH, Kent JW, Charlesworth JC, Borg AJ, Jowett JB, Cole SA, MacCluer JW, Kissebah AH, Moses EK, Blangero J (2007) Genetic determinants of mitochondrial content. Hum Mol Genet 16(12):1504–1514
Das G, Hickey DR, Principio L, Conklin KT, Short J, Miller JR, McLendon G, Sherman F (1988) Replacements of lysine 32 in yeast cytochrome c. Effects on the binding and reactivity with physiological partners. J Biol Chem 263(34):18290–18297
Frahm T, Mohamed SA, Bruse P, Gemund C, Oehmichen M, Meissner C (2005) Lack of age-related increase of mitochondrial DNA amount in brain, skeletal muscle and human heart. Mech Ageing Dev 126(11):1192–1200
Frederiksen H, Gaist D, Petersen HC, Hjelmborg J, McGue M, Vaupel JW, Christensen K (2002) Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. Genet Epidemiol 23(2):110–122
Gaist D, Bathum L, Skytthe A, Jensen TK, McGue M, Vaupel JW, Christensen K (2000) Strength and anthropometric measures in identical and fraternal twins: no evidence of masculinization of females with male co-twins. Epidemiology 11(3):340–343
Hartmann N, Reichwald K, Wittig I, Drose S, Schmeisser S, Luck C, Hahn C, Graf M, Gausmann U, Terzibasi E, Cellerino A, Ristow M, Brandt U, Platzer M, Englert C (2011) Mitochondrial DNA copy number and function decrease with age in the short-lived fish Nothobranchius furzeri. Aging Cell 10(5):824–831
Herskind AM, McGue M, Iachine IA, Holm N, Sorensen TI, Harvald B, Vaupel JW (1996) Untangling genetic influences on smoking, body mass index and longevity: a multivariate study of 2464 Danish twins followed for 28 years. Hum Genet 98(4):467–475
Hosgood HD 3rd, Liu CS, Rothman N, Weinstein SJ, Bonner MR, Shen M, Lim U, Virtamo J, Cheng WL, Albanes D, Lan Q (2010) Mitochondrial DNA copy number and lung cancer risk in a prospective cohort study. Carcinogenesis 31(5):847–849
Kim MY, Lee JW, Kang HC, Kim E, Lee DC (2011) Leukocyte mitochondrial DNA (mtDNA) content is associated with depression in old women. Arch Gerontol Geriatr 53(2):e218–e221
Lee HK, Song JH, Shin CS, Park DJ, Park KS, Lee KU, Koh CS (1998) Decreased mitochondrial DNA content in peripheral blood precedes the development of non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 42(3):161–167
Lee JW, Park KD, Im JA, Kim MY, Lee DC (2010) Mitochondrial DNA copy number in peripheral blood is associated with cognitive function in apparently healthy elderly women. Clin Chim Acta 411(7–8):592–596
Liu CS, Tsai CS, Kuo CL, Chen HW, Lii CK, Ma YS, Wei YH (2003) Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic Res 37(12):1307–1317
McGue M, Christensen K (2001) The heritability of cognitive functioning in very old adults: evidence from Danish twins aged 75 years and older. Psychol Aging 16(2):272–280
McGue M, Christensen K (2002) The heritability of level and rate-of-change in cognitive functioning in Danish twins aged 70 years and older. Exp Aging Res 28(4):435–451
Mengel-From J, Christensen K, McGue M, Christiansen L (2011) Genetic variations in the CLU and PICALM genes are associated with cognitive function in the oldest old. Neurobiol Aging 32(3):554 e557–511 e557
Miller FJ, Rosenfeldt FL, Zhang C, Linnane AW, Nagley P (2003) Precise determination of mitochondrial DNA copy number in human skeletal and cardiac muscle by a PCR-based assay: lack of change of copy number with age. Nucleic Acids Res 31(11):e61
Navarro-Sastre A, Tort F, Garcia-Villoria J, Pons MR, Nascimento A, Colomer J, Campistol J, Yoldi ME, Lopez-Gallardo E, Montoya J, Unceta M, Martinez MJ, Briones P, Ribes A (2012) Mitochondrial DNA depletion syndrome: new descriptions and the use of citrate synthase as a helpful tool to better characterise the patients. Mol Genet Metab 107(3):409–415
Nybo H, Petersen HC, Gaist D, Jeune B, Andersen K, McGue M, Vaupel JW, Christensen K (2003) Predictors of mortality in 2,249 nonagenarians—the Danish 1905-Cohort Survey. J Am Geriatr Soc 51(10):1365–1373
Pedersen CB, Gotzsche H, Moller JO, Mortensen PB (2006) The Danish civil registration system. A cohort of eight million persons. Dan Med Bull 53(4):441–449
Pohjoismaki JL, Goffart S, Taylor RW, Turnbull DM, Suomalainen A, Jacobs HT, Karhunen PJ (2010) Developmental and pathological changes in the human cardiac muscle mitochondrial DNA organization, replication and copy number. PLoS One 5(5):e10426
Reiling E, Ling C, Uitterlinden AG, Van’t Riet E, Welschen LM, Ladenvall C, Almgren P, Lyssenko V, Nijpels G, van Hove EC, Maassen JA, de Geus EJ, Boomsma DI, Dekker JM, Groop L, Willemsen G, 't Hart LM (2010) The association of mitochondrial content with prevalent and incident type 2 diabetes. J Clin Endocrinol Metab 95(4):1909–1915
Schousboe K, Visscher PM, Henriksen JE, Hopper JL, Sorensen TI, Kyvik KO (2003) Twin study of genetic and environmental influences on glucose tolerance and indices of insulin sensitivity and secretion. Diabetologia 46(9):1276–1283
Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102(15):5618–5623
Skytthe A, Kyvik K, Holm NV, Vaupel JW, Christensen K (2002) The Danish twin registry: 127 birth cohorts of twins. Twin Res 5(5):352–357
Skytthe A, Christiansen L, Kyvik KO, Bodker FL, Hvidberg L, Petersen I, Nielsen MM, Bingley P, Hjelmborg J, Tan Q, Holm NV, Vaupel JW, McGue M, Christensen K (2013) The Danish twin registry: linking surveys, national registers, and biological information. Twin Res Hum Genet: Off J Int Soc Twin Stud 16(1):104–111
Stier A, Bize P, Schull Q, Zoll J, Singh F, Geny B, Gros F, Royer C, Massemin S, Criscuolo F (2013) Avian erythrocytes have functional mitochondria, opening novel perspectives for birds as animal models in the study of ageing. Front Zool 10(1):33
Tan D, Goerlitz DS, Dumitrescu RG, Han D, Seillier-Moiseiwitsch F, Spernak SM, Orden RA, Chen J, Goldman R, Shields PG (2008) Associations between cigarette smoking and mitochondrial DNA abnormalities in buccal cells. Carcinogenesis 29(6):1170–1177
Tiainen K, Thinggaard M, Jylha M, Bladbjerg E, Christensen K, Christiansen L (2012) Associations between inflammatory markers, candidate polymorphisms and physical performance in older Danish twins. Exp Gerontol 47(1):109–115
Venegas V, Wang J, Dimmock D, Wong LJ (2011) Real-time quantitative PCR analysis of mitochondrial DNA content. Curr Protoc Hum Genet Chapter 19:Unit 19 17
Welle S, Bhatt K, Shah B, Needler N, Delehanty JM, Thornton CA (2003) Reduced amount of mitochondrial DNA in aged human muscle. J Appl Physiol 94(4):1479–1484
Xing J, Chen M, Wood CG, Lin J, Spitz MR, Ma J, Amos CI, Shields PG, Benowitz NL, Gu J, de Andrade M, Swan GE, Wu X (2008) Mitochondrial DNA content: its genetic heritability and association with renal cell carcinoma. J Natl Cancer Inst 100(15):1104–1112
Acknowledgments
We would like to thank Tinna Stevnsner for commenting on and discussing the paper and Steen Gregersen, Ulla Munk and Susanne Knudsen for technical assistance, colleagues at the epidemiology unit for collecting materials and participants for their contributions. The study was supported by a grant from the US National Institutes of Health/National Institute on Aging, Grant No. P01 AG08761; by a grant from The Danish Agency for Science, Technology and Innovation, Grant No. 09–070081, the European Union’s Seventh Framework Programme (FP7/2007-2011) under grant agreement n° 259679 and by grants from the Oda and Hans Svenningsens Foundation and Dagmar Marshalls Foundation. The Danish Aging Research Center is supported by a grant from the VELUX Foundation. The GEMINAKAR project was supported by grants from the Danish Medical Research Council, the Danish Diabetes Association, the NOVO Foundation and the Danish Heart Foundation. JMF initiated this study. MT contributed with statistical analysis. All co-authors contributed to the work by supplying materials or planning and writing this paper. All authors approved the final version. The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mengel-From, J., Thinggaard, M., Dalgård, C. et al. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet 133, 1149–1159 (2014). https://doi.org/10.1007/s00439-014-1458-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-014-1458-9