Abstract
Spinal muscular atrophy (SMA) is a recessive neuromuscular disorder caused by loss of the SMN1 gene. The clinical distinction between SMA type I to IV reflects different age of onset and disease severity. SMN2, a nearly identical copy gene of SMN1, produces only 10% of full-length SMN RNA/protein and is an excellent target for a potential therapy. Several clinical trials with drugs that increase the SMN2 expression such as valproic acid and phenylbutyrate are in progress. Solid natural history data for SMA are crucial to enable a correlation between genotype and phenotype as well as the outcome of therapy. We provide genotypic and phenotypic data from 115 SMA patients with type IIIa (age of onset <3 years), type IIIb (age of onset >3 years) and rare type IV (onset >30 years). While 62% of type IIIa patients carry two or three SMN2 copies, 65% of type IIIb patients carry four or five SMN2 copies. Three type IV SMA patients had four and one had six SMN2 copies. Our data support the disease-modifying role of SMN2 leading to later onset and a better prognosis. A statistically significant correlation for ≥4 SMN2 copies with SMA type IIIb or a milder phenotype suggests that SMN2 copy number can be used as a clinical prognostic indicator in SMA patients. The additional case of a foetus with homozygous SMN1 deletion and postnatal measurement of five SMN2 copies illustrates the role of genotypic information in making informed decisions on the management and therapy of such patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive disease characterised by the degeneration of α-motor neurons in the anterior horns of the spinal cord, leading to progressive symmetrical muscle weakness and atrophy. The incidence of SMA is 1 in 6,000–10,000 newborns (Pearn 1978) and the carrier frequency is 1 in 35 among Europeans (Cusin et al. 2003; Feldkotter et al. 2002). SMA is classified into four types (type I–IV) according to the age of onset and disease severity (Munsat and Davies 1992; Zerres and Rudnik-Schoneborn 1995). Type I SMA patients (Werdnig-Hoffmann disease, OMIM #253300) show onset within 6 months after birth and usually die before 2 years of age. They are never able to sit or walk. Type II SMA patients (intermediate form, OMIM #253550) have an onset after 6 months of age. They are able to sit but cannot stand. Type III SMA patients (Kugelberg-Welander disease, OMIM #253400) show their first symptoms after 18 months of age. They are able to sit and walk but often become wheel-chair bound in the course of disease progression. Patients with an age of onset before 3 years are subclassified as IIIa, those with an age of onset after 3 years as IIIb (Zerres and Rudnik-Schoneborn 1995). Type IV SMA patients (adult form of SMA, OMIM #271150) are characterised by an age of onset >30 years and only very mild signs of muscle weakness.
A correlation of phenotype with gentoype is important not only for individual genetic counselling but also for therapeutic trials. In 97% of type I–III SMA patients, homozygous mutations (deletions, gene conversions or point mutations) of the survival motor neuron gene (SMN1; OMIM 600354) were detected (Lefebvre et al. 1995; Wirth 2000). So far, homozygous absence of SMN1 was found only in very few patients with type IV SMA (age of onset >30 years) (Brahe et al. 1995; Clermont et al. 1995) while most of these individuals do not carry any detectable deletions in this gene (Zerres et al. 1995).
An increased number of SMN2 copies is the result of gene conversion of SMN1 into SMN2 (Burghes 1997; Hahnen et al. 1996; van der Steege et al. 1996; Wirth et al. 1997). The severity of SMA has been proven to be influenced by the number of SMN2 (OMIM 601627) copies: about 70% of type I SMA patients carry two SMN2 copies, 82% of type II SMA patients have three SMN2 copies, whereas type III patients have with very few exceptions a minimum of three to four SMN2 copies (Feldkotter et al. 2002; Mailman et al. 2002; Ogino et al. 2003). SMN1 produces almost 100% of full-length (FL) mRNA whereas SMN2 primarily produces transcripts lacking exon 7 (Gennarelli et al. 1995; Helmken et al. 2003; Lefebvre et al. 1995). Only about 10% of SMN2 transcripts are correctly spliced and encode a protein identical to SMN1.
Since each SMA patient retains at least one SMN2 copy and since the number correlates with the phenotype, SMN2 is considered as an interesting gene target for therapy (Wirth 2002).
A large number of various drugs and molecules have been reported to increase the SMN2 protein level in vitro via transcriptional activation and/or by restoring correct SMN2 pre-mRNA splicing. This makes SMA one of the few genetic disorders in humans, in which activation of a copy gene opens a therapeutic approach. The most promising drugs to date are the histone deacetylase inhibitors valproic acid (VPA) (Brichta et al. 2004, 2003; Sumner et al. 2003) and phenylbutyrate (Andreassi et al. 2004; Brahe et al. 2005). Both compounds are currently investigated in phase II and III clinical trials in Europe and US (http://www.fsma.org and www.sma-initiative.de).
In some families, different SMA phenotypes of siblings who carry identical homozygous absence of SMN1 and identical SMN2 copy numbers have been reported (Brahe et al. 1993; Cobben et al. 1995; DiDonato et al. 1997; Hahnen et al. 1995; Prior et al. 2004; Wang et al. 1996). A so far unknown modifying factor has been shown to up-regulate the SMN protein level and to protect some individuals from developing SMA (Helmken et al. 2003). In conclusion, there are two major factors that influence the severity of SMA: the number of SMN2 copies and the SMA modifying gene(s).
In this study, we analysed the phenotype–genotype correlation especially in SMA patients affected by milder forms of the disease. In none of the molecular genetic studies carried out so far, SMA patients were subdivided into type IIIa and IIIb according to their age of onset, before or after 3 years, respectively. However, there is a marked difference in the preservation of ambulatory capacity between type IIIa and IIIb. Whereas only 44% of the type IIIa individuals are still able to walk by the age of 20 years, 90% of type IIIb patients of the same age have retained this ability (Zerres et al. 1995). The aim of this study was to provide solid data concerning the correlation between the number of SMN2 copies and the age of onset especially in mildly affected SMA patients as an important parameter for future clinical trials.
Materials and methods
One hundred and fifteen patients with SMA IIIa, IIIb or IV and confirmed homozygous absence of SMN1 were analysed for their SMN2 copy numbers by real-time quantitative PCR using a SYBR Green assay on a LightCycler® machine as previously described (Feldkotter et al. 2002). Direct screening of SMA patients for homozygous absence of SMN1 was carried out by PCR followed by restriction digestion with HinfI and agarose gel electrophoresis to distinguish SMN1 from SMN2 as described elsewhere (Wirth et al. 1999). Haplotype analysis with the multicopy polymorphic marker C212 localised at the 5′ end of the SMN genes was performed as described (Wirth et al. 1995). Blood samples had been obtained after informed and written consent from each participant or the legal guardian according to a protocol reviewed and approved by the local Ethics Committee. Phenotypic data had largely been collected together with the original blood samples and were completed by individual telephone interviews of the physicians in charge where necessary.
Results and discussion
Previous studies by others and us have analysed type III SMA patients only as a whole group based on the main motor ability: to walk without support. Here, we analysed 111 patients with homozygous absence of SMN1 and type III SMA. Sixty of these patients showed disease onset before 3 years of age and were defined as type IIIa. The remaining 51 patients are characterised by an onset after 3 years of age and consequently were classified as type IIIb (Table 1).
Of note, 4 additional patients with adult (type IV) SMA and homozygous absence of SMN1 were identified amongst a total of 21 patients with an SMA type IV phenotype tested in our routine molecular genetic laboratory. The highest age of onset observed among these patients was 40 years (Table 2). Remarkably, all four patients showed a homogeneous clinical picture with nearly stationary or very slowly progressive weakness and atrophy of hip flexor and knee extensor muscle starting in the fourth decade of life. Slowly progressive segmental anterior horn cell disease of the proximal lower extremities thus forms part of the clinical spectrum of chromosome 5q13-linked spinal muscular atrophy and should be considered in the differential diagnosis even in sporadic cases. However, in 17 out of these 21 patients presenting with an adult SMA phenotype, homozygous absence of SMN1 was not detected. Quantitative genetic analysis revealed that these patients carry two SMN1 copies, thus excluding a compound heterozygosity with a deletion of SMN1 on one chromosome 5 and a subtle SMN1 mutation on the other chromosome 5, a genotype found in about 3% of SMA patients (Wirth et al. 1999). These data confirm association of some adult SMA cases with mutations in SMN1 (Brahe et al. 1995; Clermont et al. 1995; Zerres et al. 1995).
As demonstrated in Table 1, 50% of type IIIa, 31.4% of type IIIb and none of the type IV SMA patients, respectively, carry three SMN2 copies. Conversely, only 38.3% of type IIIa, but 60.8% of type IIIb and 75% of type IV SMA patients, respectively, carry four SMN2 copies (Fig. 1). Two additional patients affected by very mild type IIIb SMA (onset with 19 and 25 years, respectively) carry five SMN2 copies (Table 2). These results suggest that the genotype of type IIIa SMA patients rather resembles the genotype of type II patients [82% with three SMN2 copies, 7% with four SMN2 copies according to Feldkotter et al. (2002)] than the one of type IIIb patients. These data provide statistical significance for a correlation of ≥4 SMN2 copies with type IIIb SMA versus ≤3 SMN2 copies with type IIIa SMA (in the Fisher’s exact test, the two-tailed P value equals 0.008; odds ratio=2.87; 95% CI 1.326–6.209). Moreover, our analysis suggests a strong protective effect of four or more SMN2 copies in SMA patients, which goes along with an age of onset after 3 years up to as late as 40 years. This is the first genotypic data set of statistical significance supporting the empirical subdivision of SMA type III patients into phenotypes IIIa and IIIb.
An even higher number of ≥5 SMN2 copies, an event found only in very few SMA patients (Table 2), clearly indicates a very mild course of SMA. Importantly, even in the presence of six SMN2 copies (estimated amount of FL-SMN RNA: 60%), mild SMA symptoms may still occur. In contrast, SMA carriers with one SMN1 and one SMN2 who should also produce about 60% of FL-SMN are asymptomatic. The idea that Δ7-SMN2 might have some negative impact on the disease phenotype is in line with data suggesting a pro-apoptotic effect of the Δ7-SMN2 protein (Kerr et al. 2000; Vyas et al. 2002). However, the fact that eight SMN2 copies in mice and humans completely rescue the phenotype (Monani et al. 2000; Vitali et al. 1999) and recent data on phenotypic improvements in SMA via increased Δ7-SMN2 protein levels in double transgenic SMA-like mice with the genotype Smn−/−;SMN2+;Δ7SMN2 strongly argue against a negative effect of the Δ7-SMN2 protein (Le et al. 2005).
Despite the crucial influence of the SMN2 copy number on the phenotype in mild SMA, this does not fully explain variable expressivity. In each SMA category, there is still a substantial overlap of SMN2 copy numbers, suggesting that there are other modifying factors such as size of the deletion and molecular rearrangements resulting in incomplete SMN2 genes. The observation of rare phenotypically discordant SMA families, in which some of the siblings with homozygous mutation of SMN1 do not show any symptoms, also points towards additional SMA modifiers (Brahe et al. 1993; Cobben et al. 1995; Hahnen et al. 1995; Helmken et al. 2003; Wang et al. 1996).
Long-term studies investigating the age of losing ambulatory capacities and the survival in SMA patients are important to further define genotype–phenotype correlations. Our previous study in type I SMA patients in whom the age of survival was correlated with the SMN2 copy number revealed a significant difference between the type I SMA patients carrying three SMN2 copies, who presented a mean age of survival of 36 months, and the majority with only two SMN2 copies who showed a mean age of survival of only 11 months (Feldkotter et al. 2002). Thus, it can be assumed that patients with different numbers of SMN2 copies can have a similar age of onset, but the individuals with an increased SMN2 copy number may show slower disease progression and increased probability of staying ambulatory. Long-term observations in milder SMA phenotypes could further support this hypothesis and would be extremely helpful for clinical studies, particularly may help answering the question:at which time point a potential drug treatment should be started. We speculate that a person with four SMN2 copies could afford to start a potential therapy later and may need lower drug doses in order to reach a certain SMN protein level sufficient to protect from developing the disease as compared to individuals with only one to three SMN2 copies.
Our study has refined genotype–phenotype correlations in mild SMA. Enhanced knowledge on how the age of disease onset and that of disease progression are genetically determined is essential to design potential therapeutical trials. An important question in this context will be when to start treatment with the drug on trial. We still have to learn much more about the presymptomatic phase of SMA in mild and adult SMA. Nevertheless, if the currently ongoing clinical trials show clear benefits and improvements in the SMA patients, a neonatal screening for SMN1 deletions could be the next crucial step. In neonates carrying homozygous absence of SMN1, the SMN2 copy number would be an important predictor of the most likely phenotypic outcome. Although others and we have shown that this correlation is not absolute, there is a very strong correlation of one to two SMN2 copies with a severe SMA phenotype, and four or more SMN2 copies with a mild SMA phenotype. Especially for VPA, it is well known that treatment of children <2 years holds a comparatively higher risk of side effects. Therefore, in neonates with four or more SMN2 copies, one should carefully balance between the start of a drug therapy and the higher risk of side effects due to drug administration in the first 2 years of life.
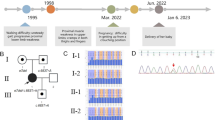
We conclude with an example illustrating the impact that genotypic information can have on clinical management decisions in families with SMA. The child of a father with mild SMA type IIIb (phenotype: discrete symptoms from 19 years of age, only slight problems with climbing stairs at 38 years of age; genotype: homozygous loss of SMN1, five SMN2 copies) and a heterozygous mother was prenatally diagnosed to have a homozygous deletion of SMN1 (Fig. 2). The couple decided to continue the pregnancy. Immediately after birth, the physician started an individual experimental therapeutic approach of the healthy child with valproic acid according to Section 41 German Drug Act (AMG). At the age of 3 weeks, we measured five SMN2 copies in an EDTA blood sample from this child. With this result strongly in favour of a very mild/adult SMA, it was decided together with the parents to suspend the VPA therapy. As expected, at the age of 14 months the child is still healthy. The physician may consider restarting the therapy in 1–2 years based on newer data to be expected from clinical trials.
References
Andreassi C, Angelozzi C, Tiziano FD, Vitali T, De Vincenzi E, Boninsegna A, Villanova M, Bertini E, Pini A, Neri G, Brahe C (2004) Phenylbutyrate increases SMN expression in vitro: relevance for treatment of spinal muscular atrophy. Eur J Hum Genet 12:59–65
Brahe C, Zappata S, Velona I, Bertini E, Servidei S, Tonali P, Neri G (1993) Presymptomatic diagnosis of SMA III by genotype analysis. Am J Med Genet 45:408–411
Brahe C, Servidei S, Zappata S, Ricci E, Tonali P, Neri G (1995) Genetic homogeneity between childhood-onset and adult-onset autosomal recessive spinal muscular atrophy. Lancet 346:741–742
Brahe C, Vitali T, Tiziano FD, Angelozzi C, Pinto AM, Borgo F, Moscato U, Bertini E, Mercuri E, Neri G (2005) Phenylbutyrate increases SMN gene expression in spinal muscular atrophy patients. Eur J Hum Genet 13:256–259
Brichta L, Hofmann Y, Hahnen E, Siebzehnrubl FA, Raschke H, Blumcke I, Eyupoglu IY, Wirth B (2003) Valproic acid increases the SMN2 protein level: a well-known drug as a potential therapy for spinal muscular atrophy. Hum Mol Genet 12:2481–2489
Brichta L, Haug K, Sun Y, Stier S, Klockgether T, Wirth B (2004) Pilot study of in-vivo effects of valproic acid on SMN gene expression in SMA carriers. Eur J Hum Genet 1(Suppl 1):5
Burghes AH (1997) When is a deletion not a deletion? When it is converted. Am J Hum Genet 61:9–15
Clermont O, Burlet P, Lefebvre S, Burglen L, Munnich A, Melki J (1995) SMN gene deletions in adult-onset spinal muscular atrophy. Lancet 346:1712–1713
Cobben JM, van der Steege G, Grootscholten P, de Visser M, Scheffer H, Buys CH (1995) Deletions of the survival motor neuron gene in unaffected siblings of patients with spinal muscular atrophy. Am J Hum Genet 57:805–808
Cusin V, Clermont O, Gerard B, Chantereau D, Elion J (2003) Prevalence of SMN1 deletion and duplication in carrier and normal populations: implication for genetic counselling. J Med Genet 40:E39
DiDonato CJ, Ingraham SE, Mendell JR, Prior TW, Lenard S, Moxley RT III, Florence J, Burghes AH (1997) Deletion and conversion in spinal muscular atrophy patients: is there a relationship to severity? Ann Neurol 41:230–237
Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B (2002) Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet 70:358–368
Gennarelli M, Lucarelli M, Capon F, Pizzuti A, Merlini L, Angelini C, Novelli G, Dallapiccola B (1995) Survival motor neuron gene transcript analysis in muscles from spinal muscular atrophy patients. Biochem Biophys Res Commun 213:342–348
Hahnen E, Forkert R, Marke C, Rudnik-Schoneborn S, Schonling J, Zerres K, Wirth B (1995) Molecular analysis of candidate genes on chromosome 5q13 in autosomal recessive spinal muscular atrophy: evidence of homozygous deletions of the SMN gene in unaffected individuals. Hum Mol Genet 4:1927–1933
Hahnen E, Schonling J, Rudnik-Schoneborn S, Zerres K, Wirth B (1996) Hybrid survival motor neuron genes in patients with autosomal recessive spinal muscular atrophy: new insights into molecular mechanisms responsible for the disease. Am J Hum Genet 59:1057–1065
Helmken C, Hofmann Y, Schoenen F, Oprea G, Raschke H, Rudnik-Schoneborn S, Zerres K, Wirth B (2003) Evidence for a modifying pathway in SMA discordant families: reduced SMN level decreases the amount of its interacting partners and Htra2-beta1. Hum Genet 114:11–21
Kerr DA, Nery JP, Traystman RJ, Chau BN, Hardwick JM (2000) Survival motor neuron protein modulates neuron-specific apoptosis. Proc Natl Acad Sci USA 97:13312–13317
Le TT, Pham LT, Butchbach ME, Zhang HL, Monani UR, Coovert DD, Gavrilina TO, Xing L, Bassell GJ, Burghes AH (2005) SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum Mol Genet 14:845–857
Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, et al. (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80:155–165
Mailman MD, Heinz JW, Papp AC, Snyder PJ, Sedra MS, Wirth B, Burghes AH, Prior TW (2002) Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet Med 4:20–26
Monani UR, Sendtner M, Coovert DD, Parsons DW, Andreassi C, Le TT, Jablonka S, Schrank B, Rossol W, Prior TW, Morris GE, Burghes AH (2000) The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(−/−) mice and results in a mouse with spinal muscular atrophy. Hum Mol Genet 9:333–339
Munsat TL, Davies KE (1992) International SMA consortium meeting. (26–28 June 1992, Bonn, Germany). Neuromuscul Disord 2:423–428
Ogino S, Gao S, Leonard DG, Paessler M, Wilson RB (2003) Inverse correlation between SMN1 and SMN2 copy numbers: evidence for gene conversion from SMN2 to SMN1. Eur J Hum Genet 11:275–277
Pearn J (1978) Incidence, prevalence, and gene frequency studies of chronic childhood spinal muscular atrophy. J Med Genet 15:409–413
Prior TW, Swoboda KJ, Scott HD, Hejmanowski AQ (2004) Homozygous SMN1 deletions in unaffected family members and modification of the phenotype by SMN2. Am J Med Genet 130A:307–310
Sumner CJ, Huynh TN, Markowitz JA, Perhac JS, Hill B, Coovert DD, Schussler K, Chen X, Jarecki J, Burghes AH, Taylor JP, Fischbeck KH (2003) Valproic acid increases SMN levels in spinal muscular atrophy patient cells. Ann Neurol 54:647–654
van der Steege G, Grootscholten PM, Cobben JM, Zappata S, Scheffer H, den Dunnen JT, van Ommen GJ, Brahe C, Buys CH (1996) Apparent gene conversions involving the SMN gene in the region of the spinal muscular atrophy locus on chromosome 5. Am J Hum Genet 59:834–838
Vitali T, Sossi V, Tiziano F, Zappata S, Giuli A, Paravatou-Petsotas M, Neri G, Brahe C (1999) Detection of the survival motor neuron (SMN) genes by FISH: further evidence for a role for SMN2 in the modulation of disease severity in SMA patients. Hum Mol Genet 8:2525–2532
Vyas S, Bechade C, Riveau B, Downward J, Triller A (2002) Involvement of survival motor neuron (SMN) protein in cell death. Hum Mol Genet 11:2751–2764
Wang CH, Xu J, Carter TA, Ross BM, Dominski MK, Bellcross CA, Penchaszadeh GK, Munsat TL, Gilliam TC (1996) Characterization of survival motor neuron (SMNT) gene deletions in asymptomatic carriers of spinal muscular atrophy. Hum Mol Genet 5:359–365
Wirth B (2000) An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum Mutat 15:228–237
Wirth B (2002) Spinal muscular atrophy: state-of-the-art and therapeutic perspectives. Amyotroph Lateral Scler Other Motor Neuron Disord 3:87–95
Wirth B, Hahnen E, Morgan K, DiDonato CJ, Dadze A, Rudnik-Schoneborn S, Simard LR, Zerres K, Burghes AH (1995) Allelic association and deletions in autosomal recessive proximal spinal muscular atrophy: association of marker genotype with disease severity and candidate cDNAs. Hum Mol Genet 4:1273–1284
Wirth B, Schmidt T, Hahnen E, Rudnik-Schoneborn S, Krawczak M, Muller-Myhsok B, Schonling J, Zerres K (1997) De novo rearrangements found in 2% of index patients with spinal muscular atrophy: mutational mechanisms, parental origin, mutation rate, and implications for genetic counseling. Am J Hum Genet 61:1102–1111
Wirth B, Herz M, Wetter A, Moskau S, Hahnen E, Rudnik-Schoneborn S, Wienker T, Zerres K (1999) Quantitative analysis of survival motor neuron copies: identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype–phenotype correlation, and implications for genetic counseling. Am J Hum Genet 64:1340–1356
Zerres K, Rudnik-Schoneborn S (1995) Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol 52:518–523
Zerres K, Rudnik-Schoneborn S, Forkert R, Wirth B (1995) Genetic basis of adult-onset spinal muscular atrophy. Lancet 346:1162
Acknowledgement
We are grateful to all SMA families and clinicians who contributed to this work. We are grateful to Yun Li for statistical advice. This study has been supported by grants from the Deutsche Forschungsgemeinschaft, Families of SMA and Center for Molecular Medicine Cologne. HL is member of the German network on muscular dystrophies (MD-NET; 01GM0302), funded by the German Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Database: SMN1—OMIM: 600354; GeneBank: U18423, SMN2—OMIM: 601627: GeneBank: NM_022875
Rights and permissions
About this article
Cite this article
Wirth, B., Brichta, L., Schrank, B. et al. Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum Genet 119, 422–428 (2006). https://doi.org/10.1007/s00439-006-0156-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-006-0156-7