Abstract
SQUAMOSA promoter-binding protein (SBP)-box family genes encode plant-specific transcription factors that play crucial roles in plant development, especially flower and fruit development. However, little information on this gene family is available for Prunus mume, an ornamental and fruit tree widely cultivated in East Asia. To explore the evolution of SBP-box genes in Prunus and explore their functions in flower and fruit development, we performed a genome-wide analysis of the SBP-box gene family in P. mume. Fifteen SBP-box genes were identified, and 11 of them contained an miR156 target site. Phylogenetic and comprehensive bioinformatics analyses revealed that different groups of SBP-box genes have undergone different evolutionary processes and varied in their length, structure, and motif composition. Purifying selection has been the main selective constraint on both paralogous and orthologous SBP-box genes. In addition, the sequences of orthologous SBP-box genes did not diverge widely after the split of P. mume and Prunus persica. Expression analysis of P. mume SBP-box genes revealed their diverse spatiotemporal expression patterns. Three duplicated SBP-box genes may have undergone subfunctionalization in Prunus. Most of the SBP-box genes showed high transcript levels in flower buds and young fruit. The four miR156-nontargeted genes were upregulated during fruit ripening. Together, these results provide information about the evolution of SBP-box genes in Prunus. The expression analysis lays the foundation for further research on the functions of SBP-box genes in P. mume and other Prunus species, especially during flower and fruit development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prunus mume, an important ornamental and fruit tree belonging to the Rosaceae, has been cultivated in East Asia for more than 3000 years. This species has prominent ornamental characteristics such as colorful corollas, a pleasant fragrance, and various types of flowers (Sun et al. 2013). Its fruit is economically valuable, as it can be preserved or processed into wine and juice. The fruit products are considered to be beneficial to human health. Despite its importance, the molecular mechanisms that regulate its flower and fruit development are still largely unknown. SQUAMOSA promoter-binding protein (SBP)-box genes encode plant-specific transcription factors that control the expression of genes involved in floral organ identity determination. Therefore, SBP-box genes affect floral transition and flower development (Klein et al. 1996; Gandikota et al. 2007). These genes also play roles in fruit development and ripening after fertilization (Manning et al. 2006). To provide insights into the molecular mechanisms of flower and fruit development in Prunus genus and other Rosaceae trees, it is essential to conduct a genome-wide analysis of these genes in P. mume and to elucidate their roles in flower and fruit development.
SBP-box genes are defined by a conserved region of approximately 76 amino acids designated as the SBP domain (Cardon et al. 1999; Klein et al. 1996). This domain features two zinc-binding sites (one is C3H or C4, and the other is C2HC), and it is essential for both DNA-binding and nuclear localization (Birkenbihl et al. 2005; Yamasaki et al. 2004). SBP proteins bind to the cis-element sequence TNCGTACAA in the promoter region of SQUAMOSA (Cardon et al. 1999). SBP-box genes were initially identified in Antirrhinum majus and were shown to play roles in regulating the expression of SQUAMOSA in early flower development (Klein et al. 1996). Since then, many SBP-box genes have been identified in other plants. The first functionally characterized Arabidopsis SBP-box gene was the SQUAMOSA PROMOTER BINDING PROTEIN Like-3 gene (SPL3), which is involved in floral transition. SPL3 also plays roles in the development of the inflorescence and floral organs (Gandikota et al. 2007). There are 16 SBP-box genes in the Arabidopsis genome (Birkenbihl et al. 2005), and they have diverse functions in development, especially in reproduction. For example, SPL8 affects pollen sac development and plays a positive role in GA-mediated anther development (Unte et al. 2003; Zhang et al. 2007). In addition, SPL8 and other SPL genes are expressed in the developing gynoecium, and they redundantly control the development of the female reproductive tract (Xing et al. 2013). SPL2, 9, 10, 11, and 15 have been shown to be involved in the shoot maturation and morphological changes during the reproductive phase (Schwarz et al. 2008; Shikata et al. 2009). Moreover, the distribution of trichomes during flowering is temporally regulated by SPL3, 9, 10, and 13 (Yu et al. 2010). SPL7 is a central regulator for cooper homeostasis because it activates the transcription of multiple genes involved in copper homeostasis (Yamasaki et al. 2009).
Several members of the SBP-box family have been functionally characterized in plants other than Arabidopsis and have been shown to have diverse functions in development. For instance, the two SBP-box genes BpSPL1 and CRR1 (Copper Response Regulator 1) were identified from Betula pendula and Chlamydomonas, respectively (Kropat et al. 2005; Lännenpää et al. 2004). The BpSPL1 protein binds to the promoter region of BpMADS5, a homolog of Arabidopsis FRUITFULL in B. pendula. The CRR1 protein recognizes and binds to the GTAC core sequence in the promoters of CYC6 and CPX1. These results indicate that apart from regulating the expression of floral meristem identity genes (SQUAMOSA and AP1), SBP-box genes also regulate the expression of other genes, and thus, have diverse functions in plant development. Recent studies have shown that SBP-box genes play essential roles in fruit development and ripening. For instance, there are 19 SBP-box genes in the rice genome, and more than half of them are predominantly expressed in young panicles (Xie et al. 2006). OsSPL16 has been shown to play roles in determining rice grain size, shape, and quality (Miura et al. 2010; Wang et al. 2012). In tomato, the SBP-box gene Colorless Non-Ripening (CNR) is critical for fruit ripening, and an epigenetic mutation in the promoter of this gene was shown to inhibit fruit ripening (Manning et al. 2006). A recent study showed that some miR156-targeted SBP-box genes regulate ovary and early fruit development in tomato (Silva et al. 2014).
MicroRNAs (miRNAs) are small, non-coding RNAs (22-24nucleotide) that play crucial regulatory roles in development in plants and animals. They bind to the target sites of mRNAs for transcript cleavage or translational repression. About half of the target genes of miRNAs encode transcription factors. SBP-box genes are potential targets of miR156/157 family members (Bartel 2004; Hou et al. 2013; Rhoades et al. 2002). For example, it has been reported that 11 of the 19 SBP-box genes are targets of miR156 in rice (Xie et al. 2006). Similarly, more than half of the putative SBP-box genes in tomato, apple, and grape contain miR156 target sites (Hou et al. 2013; Li et al. 2013; Salinas et al. 2012). The target sites are located both in the coding region and in the 3′-untranslated region (3′ UTR) (Hou et al. 2013; Li et al. 2013).
Although genome-wide analyses have been performed on SBP-box genes in Arabidopsis, rice, and other species (Hou et al. 2013; Li and Lu 2014; Li et al. 2013; Riese et al. 2007; Yang et al. 2008), a systematic analysis of this gene family has not been conducted for the genus Prunus, which includes many important ornamental and fruit trees. Also, little is known about the functions and expression patterns of SBP-box genes in Prunus flower and fruit development or about the evolution of SBP-box genes in related species, especially in two species belonging to the same genus. In this study, we first identified 15 SBP-box genes in the P. mume genome and then conducted comprehensive bioinformatics analyses of gene structure, phylogeny, conserved motifs, miRNA target sites, and chromosomal location. The driving forces for the evolution of orthologous genes in two Prunus species were also determined. Finally, we investigated the expression patterns of P. mume SBP-box genes in various organs, and in flowers and fruit at different developmental stages. The results of this study provide an overview of the organization of the SBP-box family in P. mume and other Prunus species. These results lay the foundation for further functional analyses of SBP-box genes in P. mume and further our understanding of the roles of SBP-box genes in regulating flower and fruit development.
Materials and methods
Identification and annotation of SBP-box genes in P. mume
The genome database of P. mume was downloaded from the P. mume genome project (http://prunusmumegenome.bjfu.edu.cn/). A Hidden Markov Model was constructed from the SBP-domain profile (PF03110) downloaded from the Pfam database (http://pfam.janelia.org/) and was used to search the P. mume genome database using HMMER software. The e-value threshold was 0.1 as recommended in the HMMER user’s guide (Finn et al. 2011). All of the obtained protein sequences were submitted to Interpro (http://www.ebi.ac.uk/interpro/) to confirm the presence of the SBP domain. Sequences without the SBP domain were discarded from further analyses. The nomenclature of the putative SBP-box genes was according to their gene IDs. Arabidopsis SBP-box genes were downloaded from TAIR using the accession numbers reported by Yang (Yang et al. 2008). P. persica SBP-box genes were downloaded from the Plant Transcription Factor Database (http://planttfdb.cbi.pku.edu.cn/index.php, Jin et al. 2014).
Phylogenetic and sequence analyses
Multiple sequence alignment of SBP-box protein sequences from Arabidopsis, P. mume and P. persica was performed using ClustalX 2.0 with default parameters (Larkin et al. 2007), and then refined manually. Phylogenetic trees were constructed using MEGA 5.1 software using the neighbor-joining (NJ) method with 1000 bootstrap replications. The full-length protein sequences of P. mume SBP-box genes were analyzed using the MEME online tool (http://meme.nbcr.net/meme/intro.html) to identify conserved motifs. The parameters were as follows: number of repetitions, any; maximum number of motifs, 20; minimum motif width, 6 and maximum motif width, 80. The identified motifs were annotated by SMART (http://smart.embl-heidelberg.de/). Sequence logos were created using the Weblogo online application (http://weblogo.threeplusone.com/). Structural information for SBP-box genes was obtained from the P. mume and P. persica genome project. Diagrams of exon–intron structures were produced using Gene Structure Display Server 2.0 (http://gsds.cbi.pku.edu.cn/index.php).
Chromosomal location and miR156 target site prediction
The chromosomal locations of SBP-box genes were assessed according to P. mume and P. persica genome data. MapDraw V2.1 was used to construct gene location diagrams (Liu and Meng 2003). To predict miR156 target sites, full-length PmSBPs nucleic acid sequences (including introns and UTRs) were analyzed using the psRNATarget online tool (http://plantgrn.noble.org/psRNATarget/?function).
Calculation of synonymous (Ks) and nonsynonymous (Ka) substitutions per site
Orthologous genes in P. mume and P. persica were identified from the phylogenetic tree and sequence similarity. Paralogous genes were inferred from the phylogenetic tree and duplicated blocks. Pairwise alignments of homologous nucleotide sequences were performed using the RevTrans 1.4 Server, which aligned the nucleotide sequences with the corresponding protein sequences as guides (Wernersson and Pedersen 2003). Ks and Ka values were estimated using K-estimator 6.1 V (Comeron 1999).
Plant materials
Plant materials for expression analyses were collected from P. mume ‘Sanlun Yudie’ at the Beijing Jiufeng International Plum Blossom Garden, Beijing, China (40°07′N, 116°11′E). The samples included various tissues (root, stem, leaf, sepal, petal, stamen, pistil, exocarp and mesocarp, endocarp, and seed), flower buds at different developmental stages (from flower primordium formation to anther and ovule development stages) and fruit at different developmental stages (from fruit set to ripening). The development stages of flower buds were identified from paraffin sections. Fruit at various developmental stages (FrS1–FrS6) was sampled at 10, 30, 60, 100, 125, and 140 days after blooming. All samples were collected, frozen in liquid nitrogen, and stored at −80 °C until RNA extraction.
Expression analyses
Total RNA was extracted from samples using TRIzol reagent (Invitrogen, USA) following the manufacturer’s instructions. RNA was treated with RNase-free DNase (Promega, USA) to remove residual genomic DNA. First-strand cDNA was synthesized from 2 µg total RNA using a TIANScript First Strand cDNA Synthesis Kit (Tiangen, China) according to manufacturer’s instructions. Real-time RT-PCR was conducted using the PikoReal real-time PCR system (Thermo Fisher Scientific, Germany). Reactions were carried out in a 20-μl volume containing 0.5 μl cDNA, 400 nM each primer (Online resource 1), and 10 μl SYBR Premix Ex Taq II (Takara, China) with the following conditions: 30 s at 95 °C, 40 cycles of 5 s at 95 °C, and 30 s at 60 °C. The specificity of the amplicon for each primer pair was verified by melting curve analysis. All real-time RT-PCR experiments were performed with three biological replicates, and each replicate was analyzed in triplicate. The relative expression levels were calculated using the 2−ΔΔCt method, with the protein phosphatase 2A (PP2A) gene of P. mume as the reference gene.
Results
Identification and chromosomal location of SBP-box genes in P. mume
To identify the SBP-box genes in P. mume, we conducted HMMER searches against the P. mume protein database using the SBP-domain profile PF03110 as the search query. Initially, 16 protein sequences were obtained. However, two of them were located at the same genome locus (Pm008474 and Pm008475) and were the results of alternative splicing of one gene. They were named PmSBP5a and 5b, and only one form (PmSBP5b) was included in the following analyses (Table 1). The Interpro analysis revealed that all 15 sequences contained an SBP-domain. Therefore, the P. mume genome contained at least 15 SBP-box genes. These genes were designated as PmSBP1 to PmSBP15, with PmSBP5 represented by two forms (Table 1, Online resource 2). As shown in Table 1, the PmSBP proteins varied greatly in their length and molecular weight. The protein length ranged from 161 to 1070 amino acids and the molecular weight from 18.45 to 118.28 kDa.
The conserved domains of PmSBP proteins were identified by Interpro software and aligned by ClustalX 2.0. All 15 SBP-box proteins contained the complete SBP domain; the domain consisted of 76 amino acids and had two zinc-binding sites and one bipartite nuclear localization signal (Fig. 1). All SBP domains contain two zinc fingers. The first zinc finger was C3H for all of the SBPs except for PmSBP4; the first zinc finger of PmSBP4 was C4. The second finger of all SBPs was CCHC.
Multiple sequence alignment and sequence logo of the P. mume SBP-box domain. Multiple sequence alignment was performed using DNAMAN. The two conserved zinc finger and NLS are indicated. The sequence logo was obtained from Weblogo online software. The overall height of the stack indicates the sequence conservation at that position
Based on the P. mume genome data, we located 14 SBP-box genes on chromosomes. One gene was located on an anchored scaffold. The SBP-box genes were unevenly distributed across the P. mume genome (Fig. 2). There were four SBP-box genes located on the longest chromosome, Chr 2, and none on Chr 6 and Chr 8. The gene distribution pattern was similar in P. persica (Online resource 3). Five P. persica SBP-box genes were located on Chr 1, the longest chromosome in P. persica, and none was located on Chr 8. Three P. mume SBP-box genes, PmSBP 3, 9, and 13, were located in duplicated blocks. Their orthologous genes in P. persica (ppa023657 m, ppa007202 m, and ppa006611 m; Fig. 3) were also located in duplicated regions. None of the P. mume SBP-box genes was tandemly duplicated, but the P. persica genome contained one tandemly duplicated pair of SBP-box genes (ppa000690 m and ppa000792 m).
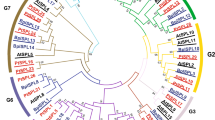
Phylogenetic analysis
To examine the phylogenetic relationships of SBP-box genes between Prunus and Arabidopsis, we constructed a phylogenetic tree based on multiple sequence alignments of P. mume, P. persica, and Arabidopsis SBP-box proteins (Fig. 3). The 48 SBP-box genes formed nine groups in the unrooted phylogenetic tree. The Prunus SBP-box genes were distributed in all nine groups and grouped with their Arabidopsis counterparts in eight groups (Fig. 3). Group 5 contained genes only from P. mume and P. persica, indicating that this Prunus-specific subfamily had emerged after the divergence of Prunus and Arabidopsis. Every P. mume SBP-box gene except for PmSBP1 and 5 had an orthologous gene in P. persica; both PmSBP1 and 5 had two orthologous genes in P. persica. This phenomenon suggested that most SBP-box genes existed before the divergence of P. mume and P. persica and the orthologous genes of PmSBP1 and 5 duplicated after their divergence to give rise to two new paralogs. Some Arabidopsis SBP-box genes in groups 1, 2, and 4 had two or more Prunus paralogs, indicating that Prunus SBP-box genes duplicated and diversified after the divergence of Arabidopsis and Prunus. Each P. mume SBP-box gene in groups 8 and 9 had one orthologous gene in P. persica and one in Arabidopsis. The dendrogram suggested that different subfamilies may have undergone different evolutionary processes, and some subfamilies underwent species-specific evolutionary processes after speciation.
Gene structure analysis and miR156 target site prediction
The exon–intron structures of the Prunus SBP-box genes were generated based on their corresponding coding sequences and genome sequences (Fig. 4). To assess the structural diversity among different groups, we constructed an unrooted phylogenetic tree using P. mume and P. persica SBP proteins. The classification of the Prunus SBP-box genes corresponded to the phylogenetic groups described above. The number of introns differed among the different groups. Group 7 genes contained only one intron while genes in groups 6 and 9 had nine introns. Genes in the other groups had two to four introns. Most of the Prunus SBP-box genes within a group shared a similar exon–intron composition. For example, all genes in group 4 had two introns, all those in group 7 had only one intron, and all those in group 3 had three introns. However, the exon–intron structure of some genes differed from that of other members of the same group. For instance, all group 1 members had two introns except for ppa007202 m, which had another short intron next to the last exon, resulting in three introns in total.
Exon-intron structures of P. mume and P. persica SBP-box genes. Phylogenetic tree was constructed based on the P. mume and P. persica SBP-box proteins. Exons of P. mume, exons of P. persica, and introns are indicated by blue rounded rectangles, green rounded rectangles, and black lines, respectively. The scale represents the lengths of the genes, exons, and introns (color figure online)
Eleven miR156 family members (Pmu-miR156a-k) have been identified in P. mume (Wang et al. 2014). Multiple sequence alignments of the P. mume SBP-box genes and reverse complement sequences of Pmu-miR156 showed that 11 PmSBPs contained sequences complementary to the Pmu-miR156 mature sequences, with a maximum of one to three mismatches (Fig. 5). The miR156 target sites were located in the coding regions in eight genes (PmSBP1, 2, 3, 9, 11, 13, 14, and 15), but in the 3′ UTR in group 4 genes (PmSBP6, 7, and 8), like in their orthologous genes (SPL3, 4 and 5) in Arabidopsis (Guo et al. 2008). All genes belonging to a group (including Prunus and Arabidopsis genes) contained the miR156 target site, and the target site locations of group members were similar. This result suggested that the miR156 target sites existed before the divergence of Prunus and Arabidopsis and were retained during the gene duplication and diversification process.
Multiple sequence alignment of Pmu-miR156 complementary sequences with P. mume SBP-box genes. Multiple sequence alignment was performed using DNAMAN software. Numbers indicate the position of the nucleotide. Yellow block highlights the start codons while red blocks highlight the stop codons. Genes bearing miR156 target site in the 3′UTR are marked with asterisks (color figure online)
Conserved motif analysis
To examine conserved sequences other than the SBP-domain and to assess the composition and distribution of conserved sequences in P. mume SBP-box proteins, we used the online MEME tool to identify motifs in the 15 P. mume SBP-box proteins. The number of motifs varied greatly among the P. mume SBP-box genes (Fig. 6); there were 12 in PmSBP5 but only 1 in each of PmSBP6, 7, and 8.
As shown in Fig. 6, only motif 1, which specifies the SBP-domain, was present in all sequences, indicating that no common domain except the SBP existed in the 15 P. mume SBP-box genes. Members of the same group usually had similar motif composition. Some groups had unique motifs; for example, both PmSBP14 and 15 (members of group 2) contained motifs 16, 5, 1, 8, 3, and 6 and the distribution of the motifs was similar. Motifs 4, 12, and 20 were restricted to group 6 while motif 16 was unique to group 2. Some motifs were shared by different groups. For instance, motif 5 was present in some members of groups 1, 2, 3, 4, 5, and 6. Motif 3 was encoded by a conserved sequence that contained the miR156 target site. This sequence was shared by all of the miR156-targeted genes with the target site located in the coding region (PmSBP1, 2, 3, 9, 11, 14, and 15).
Driving forces for divergence of SBP-box genes in Prunus
To explore the degree of sequence diversification and determine which selection pressures drove the evolution of SBP-box genes after the split of P. mume and P. persica, we calculated the Ka, Ks, and Ka/Ks for each of the 15 orthologous gene pairs and 8 paralogous gene pairs. As a general rule, a Ka/Ks ratio of >1 implies positive selection, a ratio of 1 may indicate neutral evolution, and a ratio of <1 implies purifying selection. All the Ka/Ks ratios of orthologous genes were <1 (Table 2), suggesting that SBP-box genes have evolved mainly under purifying selection after the divergence of P. mume and P. persica. The Ka/Ks ratios of the eight paralogous gene pairs were also <1. This result indicated that purifying selection was also involved in driving gene diversification after duplication events. In general, the values of Ka and Ks were smaller for orthologous gene pairs than for paralogous gene pairs; therefore, the degree of divergence was smaller for orthologous sequences than for paralogous sequences. We also calculated the Ka, Ks, and Ka/Ks ratios for sequences inside and outside the SBP domain. As shown in Table 2, the Ka values of most orthologous SBP domains were zero or near zero, suggesting that the amino acid sequences of orthologous SBP domains were less diverged. For most gene pairs, the Ka/Ks ratios were smaller for the SBP domain than for the regions outside the SBP domain. These results implied that the SBP domain was under stronger purifying selection and evolved more slowly than did the region outside the SBP domains.
Expression profiles of P. mume SBP-box genes in different organs
To elucidate the potential roles of P. mume SBP-box genes in organ development, the expression profiles of the 15 SBP-box genes in ten organs (root, stem, leaf, four whorls of flower, and three parts of fruit) were determined by quantitative RT-PCR. There were diverse transcription patterns of the PmSBPs among the different organs (Fig. 7). Out of the ten organs analyzed, more than four organs contained PmSBPs transcripts, indicating that some P. mume SBP-box genes play roles in the development of different organs. Nine genes (SBP1, 2, 3, 4, 5, 8, 12, 13, and 14) were expressed in all organs analyzed, while six genes (SBP6, 7, 9, 10, 11, and 15) showed organ-specific expression patterns.
Expression profiles of P. mume SBP-box genes in different organs. Total RNA was extracted from the root (R), stem (Ste), leaf (L), sepal (Sep), petal (Pe), stamen (Sta), pistil (Pi), exocarps and mesocarps (EM), endocarps (En), and seed (Se). The expression levels of the SBP-box genes were assessed by quantitative real-time RT-PCR. The Protein Phosphatase 2A (PP2A) gene of P. mume was used as reference gene. Three independent biological replicates were performed, and each replicate was measured in triplicate. The error bars show the standard deviation of the results of three technical replicates
Further analysis of the expression patterns of the PmSBPs in each group showed that some genes in a group exhibited similar expression patterns or had specific predominant-expression organ. For example, transcripts of the two genes in group 6, PmSBP5 and 12, were detected in all organs at relatively stable levels. Although three genes in group 7 (PmSBP6, 7, and 8) showed different expression patterns, all of them were strongly expressed in reproductive organs (flower and fruit) but weakly expressed in vegetative organs (root, stem, and leaf). In contrast, the expression patterns of three duplicated genes (PmSBP3, 9, and 13) differed in the flower. PmSBP3 and 13 transcripts were detected in all floral organs, with the highest transcript levels in the sepal and pistil, respectively. However, no PmSBP9 transcripts were detected in the petal. Some genes in different groups had the same predominantly expressed organs. For example, PmSBP1, PmSBP7, PmSBP10, and PmSBP15, which belonged to four different groups, were predominantly expressed in the pistil. PmSBP2, PmSBP6, PmSBP8, and PmSBP9 were predominately expressed in the exocarp and mesocarp, indicating they play roles in fruit development.
Expression patterns of P. mume SBP-box genes during flower bud differentiation and fruit development
Previous studies have revealed that SBP-box genes play key roles in flower and fruit development (Cardon et al. 1997; Manning et al. 2006). To investigate their roles in P. mume flower and fruit development, we conducted quantitative real-time RT-PCR analyses to determine the relative expression levels of the 15 PmSBPs during flower bud differentiation and fruit development. Based on analyses of paraffin sections (Online resource 4), we divided the flower bud differentiation of P. mume into six stages: flower primordium formation (FlS1), sepal initiation (FlS2), petal initiation (FlS3), stamen initiation (FlS4), pistil initiation (FlS5), and anther and ovule development (FlS6). Samples were collected at each of the six stages. The fruit development process was also divided into six stages (FrS1–FrS6, online resource 5), corresponding to 10, 30, 60, 100, 125, and 140 days after blooming. FrS1–FrS3 represented the fruit development stage and FrS4–FrS6 the fruit ripening stage.
All of the genes except for PmSBP11 showed high expression levels in the flower bud (Fig. 8), indicating that most PmSBPs played important roles in flower bud differentiation. The PmSBPs could be classified into four expression clusters according to their transcriptional patterns. Cluster one included five genes (PmSBP6, 11, 12, 14, and 15) that showed steady expression levels during flower bud differentiation. Cluster two comprised three genes (PmSBP1, 9, and 13) that were up-regulated during flower differentiation. The gene in cluster three (PmSBP3) showed decreased transcript levels after the second stage. The genes in cluster four (PmSBP2, 4, 5, 7, 8, and 10) showed high transcript levels at some stages and low transcript levels at other stages. This “wave” pattern of transcript accumulation during flower bud differentiation may indicate that they had roles in the development of specific organs.
Expression patterns of P. mume SBP-box genes during flower bud and fruit development. S1–S6 indicate development stages (stage1–stage6) of flower bud and fruit. White triangles and navy circles represent the expression levels of SBP-box genes in flower bud and fruit, respectively. The error bars show the standard deviation of the results of three technical replicates. All expression levels of genes were normalized against PmPP2A expression
Generally, the expression levels of PmSBPs were higher in the fruit development stages (FrS1–FrS3) than in the fruit ripening stages (FrS4–FrS5). As shown in Fig. 8, 11 genes (PmSBP1, 2, 3, 6, 7, 8, 9, 11, 13, 14, and 15) showed high transcript levels in the fruit development stages and then decreased transcript levels during fruit ripening. Among them, six genes (PmSBP2, 3, 7, 8, 9 and 13) showed the highest transcript levels at the FrS2 stage while five genes (PmSBP1, 6, 11, 14, and 15) showed the highest transcript levels at the FrS1 stage. PmSBP7 transcripts could not be detected after the FrS3 stage, and PmSBP6 and 11 transcripts could not be detected after the FrS4 stage, indicating that they did not play a role in fruit ripening. In contrast, four genes (PmSBP4, 5, 10, and 12) were down-regulated during the fruit development stages but up-regulated during the fruit ripening stages.
Discussion
SBP-box genes in P. mume and their evolution
SBP-box genes are plant-specific transcription factors that evolved before the divergence of green algae and the ancestor of land plants (Cardon et al. 1999; Guo et al. 2008). The number of SBP-box genes in the genome varies among land plants. For instance, there are 27 SBP-box genes in apple, 28 in Populus trichocarpa, and 15 in Castor Bean (Li and Lu 2014; Li et al. 2013; Zhang and Ling 2014). In this study, we identified 15 SBP-box genes in the P. mume genome. In the phylogenetic tree of SBP-box genes from two Prunus species and Arabidopsis, the genes formed nine groups, one of which was specific to Prunus. This classification was consistent with gene structure and motif composition. Genes within a group shared a similar length, structure, motif distribution, and miR156 target site location. However, there were variations in gene length, structure, and motif composition among the different groups. For example, group 6 genes contained nine introns and were longer than 4500 bp, while group 7 genes contained only one intron and were shorter than 2000 bp (Fig. 4). Therefore, the classification and evolution of SBP-box genes might be closely related to their structural divergence and diversification.
The phylogenetic analysis revealed that each of the two P. mume SBP-box genes, PmSBP1 and PmSBP5, had two orthologous genes in P. persica (Fig. 3). This result indicated that the orthologous genes of PmSBP1 and 5 in P. persica duplicated after the divergence of P. mume and P. persica, whereas PmSBP1 and 5 did not. In fact, the PmSBP5 orthologs ppa000690 m and ppa000792 m arose from tandem duplication. Different gene duplication rates resulted in different numbers of SBP-box genes in the genomes of these two Prunus species. As indicated in Fig. 3, group 1 contained one Arabidopsis SBP-box gene, three P. mume genes, and three P. persica genes. Both the P. mume genes and the P. persica genes were derived from segmental duplication, while the Arabidopsis gene in this group had not undergone a duplication event. It has been reported that SPL10 and 11 were tandemly duplicated genes and that SPL4/5, SPL1/12, and SPL10/11 were located in segmental duplication regions (Hou et al. 2013; Yang et al. 2008). However, their homologs in Prunus have not undergone a duplication event. These results indicate that genes in different groups showed different duplication histories, and some showed a species-specific expansion pattern and duplication rate. The present SBP-box genes in Prunus and Arabidopsis may have originated from a few ancestral genes that existed before the divergence of Prunus and Arabidopsis, via several different duplication events.
A previous study reported that the SBP-domain of paralogous genes has been under purifying selection while some regions outside the SBP domain have been under positive selection or relaxed purifying selection (Yang et al. 2008). However, the selection pressures during the evolution of orthologous SBP-box genes are still unclear. In this study, we calculated Ka, Ks, and Ka/Ks values for 15 orthologous gene pairs from P. mume and P. persica. The results indicated that purifying selection has played an important role in the evolution of orthologous SBP-box genes. Both the SBP-domain and the outside region have been under purifying selection. The Ka and Ks values implied that there was less sequence divergence between two Prunus orthologous genes than between two paralagous genes, because the Ka and Ks values of orthologous gene pairs were nearly zero, substantially lower than those for paralogous gene pairs (Table 2). Purifying selection is the selective removal of harmful variants and is largely responsible for maintenance of functions (Du et al. 2013; Peng et al. 2012). Thus, based on these results, we concluded that the functions of the SBP-box genes did not change substantially after the split of P. mume and P. persica. These results suggest that the functions of orthologous SBP-box genes have been maintained during the evolution of the Prunus genus.
Expression patterns of SBP-box genes in P. mume
The expression pattern of a gene is often correlated with its function (Li and Lu 2014). We determined the expression profiles of the 15 P. mume SBP-box genes in different organs, during flower bud differentiation and fruit development by quantitative real-time RT-PCR. The results indicated that P. mume SBP-box genes may be involved in the development of diverse organs, because the 15 genes showed distinct expression patterns among different organs. The expression profiles of P. mume SBP-box genes were similar to those of their orthologs in Arabidopsis, suggesting that their function has been conserved. For example, SPL2, 10, and 11, the PmSBP2 orthologs in Arabidopsis, were expressed in all tissues including roots, juvenile leaves, adult leaves, cauline leaves, inflorescence stems, flowers, and fruits, with low expression levels in vegetative organs (Shikata et al. 2009). Similarly, PmSBP2 transcripts were detected in all organs analyzed, with low transcript levels in the root, leaf, and stem. It was reported that SPL2, 10, and 11 control morphological changes and shoot maturation in the reproductive phase (Shikata et al. 2009). Therefore, PmSBP2 may play roles in morphogenesis or shoot development in P. mume. A recent study showed that SPL8 together with other miR156-targeted SPL genes controlled gynoecium patterning and that SPL8 was highly expressed in the inflorescence and silique (Xing et al. 2013). Likewise, there were high transcript levels of PmSBP10, the ortholog of SPL8, in the pistil and fruit, implying that it may play a role in gynoecium development.
The duplicated genes in P. mume showed different expression patterns. As shown in Fig. 7, the expression patterns of three group 1 members (PmSBP3, 9, and 13) that arose from segmental duplication events were different from each other. Also, their expression profiles varied considerably during flower bud differentiation (Fig. 8). Previous studies have shown that duplicated genes can be expressed differently. We also observed a divergence in expression profiles between duplicated MADS-box genes (Li et al. 2005; Xu et al. 2014). The different expression patterns of PmSBP3, 9, and 13 indicated that there is also divergence in expression patterns between duplicated SBP-box genes. The chromosome location and phylogenetic analyses indicated that the ancestor of PmSBP3, 9, and 13 underwent a segmental duplication event to give rise to these three genes, while its ortholog in Arabidopsis (SPL13) did not. According to the AtGenExpress Visualization Tool (http://www.weigelworld.org/resources/microarray/AtGenExpress/) (Schmid et al. 2005), the transcript levels of SPL13 were high in the flower and leaf, moderate in the root, and low in the seed. Although it was highly expressed in the flower, the transcript levels varied widely among the different floral organs. There were high transcript levels in the sepal, petal, and pistil, but very low transcript levels in the stamen and mature pollen. Therefore, the ancestral gene of PmSBP3, 9, and 13 should have a similar expression pattern to that of SPL13. It appears that the three duplicated genes had partial functions of their ancestral gene. As shown in Fig. 7, their expression patterns were similar to those of their ancestral gene in the vegetative organs and seed. However, in the floral organs, the combination of their expressions was equal to that of their ancestral gene. Previous studies on shifts in expression patterns have suggested that duplicated regulatory genes may undergo subfunctionalization (where both copies retain partial function of their ancestral gene and their total functions represent that of their ancestral gene) and/or neofunctionalization (one copy gains a new function while the other copy retains the origin function). In either case, the surviving duplicates will be under strong purifying selection pressure (Duarte et al. 2006; Lynch and Conery 2000). The expression profiles and Ka/Ks ratios of PmSBP 3, 9, and 13 indicate that they might have undergone subfunctionalization after the duplication events and that purifying selection has driven the evolution and expression divergence of the three duplicates.
The PmSBPs were expressed during flower bud differentiation, implying that most of these genes play roles in flower development. All of them showed relatively high transcript levels in the flower bud, except for PmSBP11 (Fig. 8). PmSBP11 belongs to group 5, a Prunus-specific group, and has no orthologous gene in Arabidopsis. It might play roles in vegetative organs and in the development of young fruit, because its transcripts were detected in the root, leaf, stem, and fruit at early developmental stages (Figs. 7, 8). Previous studies have shown that an SBP-box gene CNR control fruit ripening in tomato (Karlova et al. 2013; Manning et al. 2006). A recent study showed that miR156-targeted SBP-box genes also regulate early fruit development in tomato (Silva et al. 2014). The expression profiles of the P. mume SBP-box genes suggested that they play roles in fruit development in both the early and ripening stages. All 15 genes showed high transcript levels in fruit at stages 1 and 2 and were down-regulated continuously during the early development process. Only four genes were up-regulated after stage 3 (Fig. 8). These findings indicate that all SBP-box genes are involved in fruit development; most play roles in early fruit development, while four play roles in fruit ripening.
Different expression patterns of miR156-targeted and -nontargeted SBP-box genes in P. mume
Previous studies confirmed that more than half of the miRNA targets are transcription factors such as SBP, MYB, ARF, HD-zip, TCP, and AP2 (Aukerman and Sakai 2003; Martín-Trillo and Cubas 2010; Rhoades et al. 2002). Consistent with previous studies, 11 of the 15 P. mume SBP-box genes contained putative miR156 target sites located in the coding region or the 3′ UTR. These two locations of miR156 target sites have also been observed in SBP-box genes in other plants (Arazi et al. 2005; Guo et al. 2008; Riese et al. 2007; Sun et al. 2013), suggesting that they were present in the ancestors of SBP-box genes. The interaction between miR156 and SBP-box genes is a conserved and ancient regulatory mechanism that has been retained during evolution.
The miR156-targeted genes showed organ-specific or somewhat organ-specific expression patterns, whereas all of the miR156-nontargeted genes except for PmSBP10 were expressed ubiquitously and constitutively in all organs examined (Fig. 7). Similar expression patterns were reported for grape (Hou et al. 2013). This phenomenon suggests that miR156 may regulate the organ-specific expression patterns of SBP-box genes. In addition, there were different expression patterns among the SBP-box genes regulated by miR156. For example, there were high transcript levels of PmSBP6 in the pistil and fruit, while PmSBP7 showed high transcript levels in the pistil but was not detected in fruit. This indicates that different miR156-targeted genes are regulated differently or that a specific miR156-targeted gene is regulated by specific miR156 member. Thus, in further research, it will be important to investigate how specific miR156 members regulate specific SBP-box genes. The results of such studies will further our understanding of the regulatory mechanisms of miRNAs and their target genes.
As mentioned above, four SBP-box genes were up-regulated during fruit ripening (Fig. 8). All of them were miR156-nontargeted genes, and all of the miR156-targeted genes were down-regulated during fruit ripening. These findings indicated that the miR156-nontargeted genes play more important roles than miR156-targeted genes in fruit ripening. In tomato, the mRNA of CNR is degraded by miR156 during fruit ripening (Karlova et al. 2013). Thus, the down-regulated expression patterns of miR156-targeted SBP-box genes during P. mume fruit ripening may result from miR156-guided mRNA cleavage. This indicates that miR156 plays crucial roles in fruit development and ripening. In further research, it will be of great importance to investigate the mechanisms of miR156 and SBP-box genes in regulating fruit ripening.
In conclusion, this study is the first genome-wide analysis of the SBP-box gene family in Prunus. Our results showed that some SBP-box genes have shown species-specific expansion patterns and that their expression patterns have diverged after duplication events. Purifying selection has been the main driving force for the evolution of Prunus SBP-box genes, not only for paralogous genes, but also for orthologous genes. Based on the Ka and Ks values and the expression patterns of SBP-box genes, we conclude that the sequences of orthologous SBP-box genes have not diverged widely after the split of P. mume and P. persica and that the functions of orthologous genes may be conserved among different species. Three P. mume SBP-box genes may have undergone subfunctionalization after duplication. These findings lay the foundation for future research on the evolution of SBP-box genes in plants. Analyses of the different expression patterns of the miR156-targeted SBP-box genes may help to elucidate the regulatory mechanism of miRNAs and their target genes. The different expression patterns of miR156-targeted and -nontargeted genes during the fruit ripening process indicate that miR156-nontargeted genes play more important roles during fruit ripening than miR156-targeted genes and that miR156 may have an important regulatory function during fruit development. Thus, it is essential to perform functional analyses of miR156-nontargeted genes in fruit ripening in the future. The expression analysis of SBP-box genes in flowers and fruit at different developmental stages enables us to investigate the molecular mechanisms that regulate flower and fruit development in P. mume and other species.
References
Arazi T, Talmor-Neiman M, Stav R, Riese M, Huijser P, Baulcombe DC (2005) Cloning and characterization of micro-RNAs from moss. Plant J 43:837–848
Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-Like target genes. Plant Cell 15:2730–2741
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Birkenbihl RP, Jach G, Saedler H, Huijser P (2005) Functional dissection of the plant-specific SBP-domain: overlap of the DNA-binding and nuclear localization domains. J Mol Biol 352:585–596
Cardon G, Höhmann S, Nettesheim K, Saedler H, Huijser P (1997) Functional analysis of the Arabidopsis thaliana SBP-box gene SPL3: a novel gene involved in the floral transition. Plant J 12:367–377
Cardon G, Höhmann S, Klein J, Nettesheim K, Saedler H, Huijser P (1999) Molecular characterisation of the Arabidopsis SBP-box genes. Gene 237:91–104
Comeron JM (1999) K-Estimator: calculation of the number of nucleotide substitutions per site and the confidence intervals. Bioinformatics 15:763–764
Du D, Cheng T, Pan H, Yang W, Wang J, Zhang Q (2013) Genome-wide identification, molecular evolution and expression analyses of the phospholipase D gene family in three Rosaceae species. Sci Hortic 153:13–21
Duarte JM, Cui L, Wall PK, Zhang Q, Zhang X, Leebens-Mack J, Ma H, Altman N, DePamphilis CW (2006) Expression pattern shifts following duplication indicative of subfunctionalization and neofunctionalization in regulatory genes of Arabidopsis. Mol Biol Evol 23:469–478
Finn RD, Clements J, Eddy SR (2011) HMMER web server: interactive sequence similarity searching. Nucleic Acids Res 39:W29–W37
Gandikota M, Birkenbihl RP, Höhmann S, Cardon GH, Saedler H, Huijser P (2007) The miRNA156/157 recognition element in the 3′UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J 49:683–693
Guo A, Zhu Q, Gu X, Ge S, Yang J, Luo J (2008) Genome-wide identification and evolutionary analysis of the plant specific SBP-box transcription factor family. Gene 418:1–8
Hou H, Li J, Gao M, Singer SD, Wang H, Mao L, Fei Z, Wang X (2013) Genomic organization, phylogenetic comparison and differential expression of the SBP-box family genes in grape. PLoS One 8:e59358
Jin J, Zhang H, Kong L, Gao G, Luo J (2014) PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res 42:D1182–D1187
Karlova R, van Haarst JC, Maliepaard C, van de Geest H, Bovy AG, Lammers M, Angenent GC, de Maagd RA (2013) Identification of microRNA targets in tomato fruit development using high-throughput sequencing and degradome analysis. J Exp Bot 64:1863–1878
Klein J, Saedler H, Huijser P (1996) A new family of DNA binding proteins includes putative transcriptional regulators of the Antirrhinum majus floral meristem identity gene SQUAMOSA. Mol Gen Genet 250:7–16
Kropat J, Tottey S, Birkenbihl RP, Depege N, Huijser P, Merchant S (2005) A regulator of nutritional copper signaling in Chlamydomonas is an SBP domain protein that recognizes the GTAC core of copper response element. P Natl Acad Sci Usa 102:18730–18735
Lännenpää M, Jänönen I, Hölttä Vuori M, Gardemeister M, Porali I, Sopanen T (2004) A new SBP-box gene BpSPL1 in silver birch (Betula pendula). Physiol Plantarum 120:491–500
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Li C, Lu S (2014) Molecular characterization of the SPL gene family in Populus trichocarpa. BMC Plant Biol 14:131
Li W, Yang J, Gu X (2005) Expression divergence between duplicate genes. Trends Genet 21:602–607
Li J, Hou H, Li X, Xiang J, Yin X, Gao H, Zheng Y, Bassett CL, Wang X (2013) Genome-wide identification and analysis of the SBP-box family genes in apple (Malus domestica Borkh.). Plant Physiol Bioch 70:100–114
Liu RH, Meng JL (2003) MapDraw: a microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data. Yi chuan 25:317–321
Lynch M, Conery JS (2000) The evolutionary fate and consequences of duplicate genes. Science 290:1151–1155
Manning K, Tör M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB (2006) A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38:948–952
Martín-Trillo M, Cubas P (2010) TCP genes: a family snapshot ten years later. Trends Plant Sci 15:31–39
Miura K, Ikeda M, Matsubara A, Song X, Ito M, Asano K, Matsuoka M, Kitano H, Ashikari M (2010) OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet 42:545–549
Peng X, Zhao Y, Cao J, Zhang W, Jiang H, Li X, Ma Q, Zhu S, Cheng B (2012) CCCH-Type Zinc finger family in maize: genome-wide identification, classification and expression profiling under abscisic acid and drought treatments. PLoS ONE 7:e40120
Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP (2002) Prediction of plant microRNA targets. Cell 110:513–520
Riese M, Höhmann S, Saedler H, Münster T, Huijser P (2007) Comparative analysis of the SBP-box gene families in P. patens and seed plants. Gene 401:28–37
Salinas M, Xing S, Höhmann S, Berndtgen R, Huijser P (2012) Genomic organization, phylogenetic comparison and differential expression of the SBP-box family of transcription factors in tomato. Planta 235:1171–1184
Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37:501–506
Schwarz S, Grande AV, Bujdoso N, Saedler H, Huijser P (2008) The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis. Plant Mol Biol 67:183–195
Shikata M, Koyama T, Mitsuda N, Ohme-Takagi M (2009) Arabidopsis SBP-box genes SPL10, SPL11 and SPL2 control morphological change in association with shoot maturation in the reproductive phase. Plant Cell Physiol 50:2133–2145
Silva GFFE, Silva EM, Da Silva Azevedo M, Guivin MAC, Ramiro DA, Figueiredo CR, Carrer H, Peres LEP, Nogueira FTS (2014) microRNA156-targeted SPL/SBP box transcription factors regulate tomato ovary and fruit development. Plant J 78:604–618
Sun L, Yang W, Zhang Q, Cheng T, Pan H, Xu Z, Zhang J, Chen C (2013) Genome-wide characterization and linkage mapping of simple sequence repeats in mei (Prunus mume Sieb. et Zucc.). PLoS One 8:e59562
Unte US, Sorensen A, Pesaresi P, Gandikota M, Leister D, Saedler H, Huijser P (2003) SPL8, an SBP-box gene that affects pollen sac development in Arabidopsis. Plant Cell 15:1009–1019
Wang S, Wu K, Yuan Q, Liu X, Liu Z, Lin X, Zeng R, Zhu H, Dong G, Qian Q (2012) Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet 44:950–954
Wang T, Pan H, Wang J, Yang W, Cheng T, Zhang Q (2014) Identification and profiling of novel and conserved microRNAs during the flower opening process in Prunus mume via deep sequencing. Mol Genet Genomics 289:169–183
Wernersson R, Pedersen AG (2003) RevTrans: multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Res 31:3537–3539
Xie K, Wu C, Xiong L (2006) Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol 142:280–293
Xing S, Salinas M, Garcia-Molina A, Höhmann S, Berndtgen R, Huijser P (2013) SPL8 and miR156-targeted SPL genes redundantly regulate Arabidopsis gynoecium differential patterning. Plant J 75:566–577
Xu Z, Zhang Q, Sun L, Du D, Cheng T, Pan H, Yang W, Wang J (2014) Genome-wide identification, characterisation and expression analysis of the MADS-box gene family in Prunus mume. Mol Genet Genomics 289:1–18
Yamasaki K, Kigawa T, Inoue M, Tateno M, Yamasaki T, Yabuki T, Aoki M, Seki E, Matsuda T, Nunokawa E (2004) A novel zinc-binding motif revealed by solution structures of DNA-binding domains of arabidopsis SBP-family transcription factors. J Mol Biol 337:49–63
Yamasaki H, Hayashi M, Fukazawa M, Kobayashi Y, Shikanai T (2009) SQUAMOSA promoter binding protein–like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell 21:347–361
Yang Z, Wang X, Gu S, Hu Z, Xu H, Xu C (2008) Comparative study of SBP-box gene family in Arabidopsis and rice. Gene 407:1–11
Yu N, Cai W, Wang S, Shan C, Wang L, Chen X (2010) Temporal control of trichome distribution by microRNA156-targeted SPL genes in Arabidopsis thaliana. Plant Cell 22:2322–2335
Zhang S, Ling L (2014) Genome-wide identification and evolutionary analysis of the SBP-Box gene family in Castor Bean. PLoS One 9:e86688
Zhang Y, Schwarz S, Saedler H, Huijser P (2007) SPL8, a local regulator in a subset of gibberellin-mediated developmental processes in Arabidopsis. Plant Mol Biol 63:429–439
Acknowledgments
This work was supported by the Fundamental Research Funds for the Ministry of Science and Technology (Grant No.2013AA102607) and Special Fund for Beijing Common Construction Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, Z., Sun, L., Zhou, Y. et al. Identification and expression analysis of the SQUAMOSA promoter-binding protein (SBP)-box gene family in Prunus mume . Mol Genet Genomics 290, 1701–1715 (2015). https://doi.org/10.1007/s00438-015-1029-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-015-1029-3