Abstract
The presence of a monosomic gametocidal chromosome (GC) in a barley chromosome addition line of common wheat generates structural aberrations in the barley chromosome as well as in the wheat chromosomes of gametes lacking the GC. A collection of structurally aberrant barley chromosomes is analogous to a panel of radiation hybrid (RH) mapping and is valuable for high-throughput physical mapping. We developed 90 common wheat lines (GC lines) containing aberrant barley 7H chromosomes induced by a gametocidal chromosome, 2C. DNAs isolated from these GC lines provided a panel of 7H chromosomal fragments in a wheat genetic background, comparable with RH mapping panels in mammals. We used this 7H GC panel and the methodology for RH mapping to physically map PCR-based barley markers, SSRs and AFLPs, onto chromosome 7H, relying on polymorphism between the 7H chromosome and the wheat genome. We call this method GC mapping. This study describes a novel adaptation and combination of methods of inducing chromosomal rearrangements to produce physical maps of markers. The advantages of the presented method are similar to RH mapping in that non-polymorphic markers can be used and the mapping panels can be relatively easily obtained. In addition, mapping results are cumulative when using the same mapping set with new markers. The GC lines will be available from the National Bioresources Project-KOMUGI (http://www.nbrp.jp/index.jsp).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last 20 years, genetic linkage mapping with molecular markers has been extremely productive and many gene loci have been located in a wide range of species. However, traditional recombination-based linkage mapping has been struggling to locate the tens of thousands of genes that have recently been assembled through genomic-driven EST programs.
In mammalian species, this problem has largely been overcome by the development of radiation hybrid (RH) mapping technology which provides a systematic method of locating genes and genomic sequences along chromosomes (Goss and Harris 1975; Cox et al. 1990). Although RH mapping has greatly advanced studies on the genome organization of mammals including humans, there have been few attempts to apply this technology to plants. The RH-panels of maize (Riera-Lizarazu et al. 2001) and barley (Wardrop et al. 2002, 2004) have been described, but their application in mapping genes has so far been limited.
In wheat, a unique genetic system in which chromosomal structural changes are induced by the so-called gametocidal chromosome (GC) (Endo 1988, 1990) has shown great potential in physical chromosome mapping as an alternative to RH mapping. When a GC is introduced, chromosomal changes occur in the gametes that lack the GC (Endo 1988). This is comparable to chromosomal breakage by radiation in the RH method. Using this approach, a set of wheat deletion stocks were produced (Endo and Gill 1996) and have been extensively utilized to physically map various DNA markers, such as RFLPs, STSs (Gill et al. 1996), ESTs (Qi et al. 2003), and genes (Nomura et al. 2003). The gametocidal system can also effectively induce chromosomal changes in alien chromosomes such as barley and rye chromosomes, added to common wheat (Shi and Endo 1997, 1999, 2000; Endo 2003; Serizawa et al. 2001). Masoudi-Nejad et al. (2002) successfully transferred the Sec-1 gene and a cluster of rust resistance genes on specific regions of the satellite of the rye chromosome 1R onto wheat chromosomes by using this system.
In barley, an enormous volume of DNA markers including ESTs are now available and an unlimited number of structurally aberrant barley chromosomes (GC line) can be generated by the gametocidal system. In this paper, we demonstrate that the method of RH mapping employed in mammals can be applied to a fairly large-scale, high-resolution physical mapping of a barley chromosome.
Materials and methods
Plant materials
The 90 wheat lines carrying structural changes in the barley (Hordeum vulgare cv. Betzes, 2n=2x=14) chromosome 7H used in this study (GC lines) were derivatives of an alien addition line of a common wheat (Triticum aestivum L., 2n=6x=42) cultivar, Chinese Spring, carrying a pair of barley 7H chromosomes and a single GC (2C from Aegilops cylindrica, 2n=4x=28) (Shi and Endo 1997, 1999, 2000). We screened those GC lines carrying different 7H deletions or translocations (between 7H and a wheat chromosome) in either the hemizygous or homozygous condition cytologically by a combination of C-banding and in situ hybridization, fluorescence in situ hybridization (FISH) and genomic in situ hybridization (GISH), as described by Masoudi-Nejad et al. (2002). We used probes of barley-specific subtelomeric repeat sequence HvT01 (Brandes et al. 1995) for FISH and the barley total genomic DNA for GISH. We also used Betzes barley, Chinese Spring wheat, and the barley 7H, 7HS, and 7HL addition lines of Chinese Spring as controls.
DNA extraction and molecular marker assays
Genomic DNAs were extracted from 3 to 6 g of fresh leaves of the GC lines, and the controls by the CTAB method (Saghai-Maroof et al. 1984), and purified by passing over a CsCl gradient. Simple sequence repeat-based markers (SSRs) used in this study had previously been mapped onto chromosome 7H by Ramsay et al. (2000). The PCR reactions using fluorescence-labeled primers were performed in a total volume of 10 μl in a reaction consisting of 100 ng of genomic DNA, one time PCR buffer, 0.3 units Taq polymerase (Roche), 0.1 μM of forward primer, 0.1 μM of reverse primer which had a 5′ end nucleotide labeled with fam, hex or tet, 200 μM dNTPS run on a PE 9600 or PE 9700 PCR machine. The fluorescent SSR primers used in this study is listed in Macaulay et al. (2001). Fragment separation and analysis were conducted with an ABI 377 automated sequencer using Genescan Version 3.4 and Genotyper Version 2.5 Software (Applied Biosystems, CA, USA).
Amplified fragment length polymorphisms (AFLPs) were generated using AFLP Analysis System I with AFLP Starter Primer Kit (Invitrogen, UK) according to the manufacturer’s protocol. Selective amplification reactions were performed using all 64 combinations of primers with three selective nucleotides (EcoRI primers: E-AAC, E-AAG, E-ACA, E-ACT, E-ACC, E-ACG, E-AGC, E-AGG, and MseI primers: M-CAA, M-CAC, M-CAG, M-CAT, M-CTA, M-CTG, and M-CTT). They were performed in a final volume of 10 μl containing 2 μl diluted pre-amplified template DNA (50 pg), 15 ng MseI selective primer, 0.25–0.4 μl labeled EcoRI selective primer, 0.2 U Taq polymerase, 2.5 mM MgCl2, and 200 μM dNTPs. PCR amplification conditions were as follows: 1 cycle of 2 min at 94°Cfollowed by 36 cycles of 30 s at 94°C, a 30 s annealing step, and 1 min at 72°C. The annealing temperature in the first cycle was 65°C, subsequently reduced by 0.7°C for each of the next 12 cycles, and then continued at 56°C for the remaining 23 cycles. Reactions were completed after a final extension of 5 min at 72°C. PCR products were run on a Bio-RAD sequi-gen GT sequencing cell.
Map construction
All SSR and AFLP loci were screened in duplicate and any loci that were amplified in wheat were discarded as unreliable. Loci with abnormally high or low retention rates or too many discrepancies between duplicate experiments were also discarded. The software MultiMap (Matise et al. 1994) was used to order the markers, to compute the distances between them, and to construct a RH reference map. Conflicting loci were placed in the most likely position to show strong pairwise linkages (lod≥3).
Results
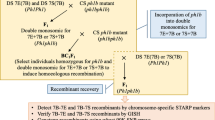
Figure 1 shows the images of sequential C-banding and FISH/GISH of a representative selection of the aberrant chromosomes present individually in the GC lines used in this study. Such aberrant chromosomes lacked or retained a fraction of the barley chromosome 7H. We used FISH analysis with the telomeric probe HvT01 to confirm minor terminal deficiencies in chromosome 7H, and the combination of C-banding and GISH to determine the type of aberration (deletion or translocation) and also to locate the breakpoints in the short arm, long arm or centromeric region (Table 1 see supplementary data). Of the 90 GC lines screened, 43 carried deletions, 46 had translocations between the 7H and wheat chromosomes, and one, 7H-60, had a normal-looking 7H.
Sequential C-banding (on the left of each pair) and combined GISH/FISH (on the right) of the aberrant 7H chromosomes in 20 representative GC lines. Barley chromosomal segments are shown by magenta GISH/FISH signals. Stronger FISH signals at barley chromosome termini indicate the subtelomeric repeat HvT01
The breakpoints of the 43 deletions were distributed along the chromosomes: 18 in the long arm (7HL), 13 in the short arm (7HS), and 12 in the centromeric region: it is noteworthy that the breakage in the centromeric region gave rise to only one 7HL telocentric chromosome. Out of the 46 translocations, 8 were of the whole-arm or Robertsonian type, involving 7HS (2) or 7HL (6), 13 had 7H segments translocated to wheat chromosomes, i.e., barley-to-wheat (b–w) translocations, and 25 had wheat chromosomal segments translocated to 7H, i.e., wheat-to-barley (w–b) translocations whose translocation points were in 7HS (14) and in 7HL (11). In the case of the b–w translocations, we could not cytologically identify the 7H chromosome arm in which the breakage occurred because 7H has no diagnostic C-bands in the distal regions of either chromosome arm. Two of the translocation lines, 7H-77 and 7H-91, had two breakpoints, one in 7HS and the other in the centromere.
In summary, of the 78 breakpoints whose chromosomal locations were cytologically identified, 29 were located in 7HL, 27 in 7HS and 22 in the centromeric region. This shows that the events of chromosomal breakage by the gametocidal system occurred all along the 7H chromosome but does not show that they were evenly distributed along the chromosome. Evidently, the frequency of breakage was higher in the centromeric region, considering it occupies a smaller region relative to that of the short and long arms. These GC lines will be available from the National Bioresources Project-KOMUGI (http://www.nbrp.jp/index.jsp).
To construct a physical chromosome map using the GC lines, we first selected SSRs from a barley genetic map (Ramsay et al. 2000). These markers were previously mapped with a linkage mapping population (Lina X H. spontaneum Canada Park). We tested all markers in barley, common wheat, and common wheat lines carrying 7H, 7HS and 7HL, respectively. Twenty-eight SSR markers amplified 7H-specific PCR products. Of these, 15 were located on 7HS and 13 on 7HL.
To increase the number of 7H specific markers, we conducted AFLP analysis using 64 primer combinations with three selective nucleotides for MseI/EcoRI, which generated a total of ~5,000 amplification products. We obtained 23 clear AFLPs specific to chromosome 7H that showed no corresponding amplification product in wheat. Ten of the AFLP markers were located on 7HS, mostly at centromeric and telomeric regions. Thirteen AFLPs on 7HL were distributed almost uniformly along the chromosome arm. The distribution patterns of the AFLP markers along chromosome 7H were in agreement with those in a previous study (Serizawa et al. 2001). We applied the RH procedure to the mapping of the SSR and AFLP markers by scoring the presence or absence of PCR products in the GC lines (hereafter we will describe this mapping as GC mapping). The underlying assumption is that loci that lie in close proximity to each other will rarely be separated by chromosome breakage by gametocidal action and will frequently be retained in the same chromosome fragment. The retention frequency of the 7H-specific markers in the GC panel ranged from 35 to 71% depending on the locus, with an average of 56% (Fig. 2).
We used MultiMap to obtain a reference map for SSR and AFLP markers, in which their optimal order agreed almost completely between the genetic and GC maps (Fig. 3). We placed the remaining loci at their most likely position in the reference map if they showed strong pair-wise linkage. The final GC map contained 50 loci distributing across chromosome 7H. The total length of the chromosome 7H is ~600 map units, corresponding to ~187 cM in the genetic map. The distance between markers in the GC map is expressed in cG, where 1 cG corresponds to 1% frequency of breakage between two markers by the gametocidal action. Comparison of the cG and cM (genetic linkage) shows that 1 cM is equivalent to approximately 3.5 cG.
The GC map (right) of barley chromosome 7H aligned with the genetic map (left). A reference map (a continuous vertical bar) was constructed in which the optimal order of the 27 markers completely agreed between the linkage and GC maps (the likelihood is ≥1,000-fold higher than the next possible highest likelihood using the same markers in alternate orders). The remaining 23 loci (markers shown by thick, thin and dotted bars on the right side of the reference map) were positioned by the maximum-likelihood method. The thick bars indicate 1,000:1 reference map positions, the thin bars represent positions ordered with <1,000:1 odds and the dotted bars are only for visual connections between separated positions of equal likelihood. Latin characters next to some of the markers shows that those markers produced different bands (PCR products) in this study; for example HVM02 produced five bands, and three of them (HVM02-I, HVM02-II and HVM02-IV) were mapped. Inter-marker distances are expressed in cG (centi-G, Gametocidal-induced-breakage), where 1 cG corresponds to a breakage frequency of 1% between adjacent markers. 7H was ~600 cG, corresponding to a linkage map of 187 cM (Lina X H. spontaneum) and physical map of 750 Mbp
Discussion
Genetic linkage mapping has three major limitations. Firstly, resolution is limited by the frequency of meiotic recombination and can only be manipulated by increasing the population size. Secondly, only polymorphic markers can be mapped, and thirdly, genetic distances do not always reflect true physical distances. In rice 1 cM varies from 120 to 1000 kb (Kurata et al. 1994), in wheat 1 cM equal to 118 kb in regions of high recombination but 22,000 kb in regions of low recombination.
Physical mapping allows genes and molecular marker loci to be located on the barley genome without relying on the detection of polymorphism between parental lines and, as a result, greatly enhances the ability to place a large number of markers on the map. GC mapping has the significant advantage of being cumulative, i.e., once a locus is scored on a panel of GC lines, it does not need to be reanalyzed when subsequent loci are added, and data from all markers can be analyzed simultaneously.
The procedure of GC mapping is based on the same principle as that of RH mapping. The underlying assumption in RH mapping is that irradiation induces chromosomal breaks independent of the chromosomal architecture (Lwrence et al. 1991). In GC mapping we also assumed that the GC system induced breakage evenly all along the chromosome. However, this assumption might be wrong because breakage hot spots by the gametocidal action were reported in various chromosomal regions in common wheat (Endo and Gill 1996; Tsujimoto et al. 2001), and the centromeric region of chromosome 7H has been demonstrated to be vulnerable to the gametocidal action in this study. Although non-random breakage by the GC action would cause distortion in the distances between markers in GC mapping, it would not affect the order of markers.
The frequency of the RH breakpoints and the resolution of the map are controlled by the dosage of irradiation. A high-resolution GC mapping panel could also be produced with production of unlimited number of GC lines in future. In addition, the GC mapping algorithm gives estimates of distances in cG, like cR in RH mapping, which would be replace FL (fraction length) values based on the cytological measurement of deletion chromosomes (e.g. Gill et al. 1996).
Perhaps the most significant contribution of GC mapping to barley genetics is to provide an independent approach to locate the physical positions of genes and markers on chromosomes. GC mapping would complement the clone-based physical mapping projects that are currently underway in barley research. GC mapping will become a powerful tool to anchor and order assembled but non-overlapping BAC contigs to the physical map. Furthermore, GC lines with alien sub-chromosome fragments can be an excellent vehicles for the production and characterization of libraries highly enriched in DNA markers and genes for particular sub-chromosomal segments (Janda et al. 2004; Safar et al. 2004). We believe that chromosome-specific GC panels for each barley chromosome will enhance the value of the extensive barley EST collections available to the global barley research community.
References
Brandes A, Roder MS, Ganal M (1995) Barley telomeres with two different types of satellite DNA sequences. Chromosome Res 3:315–320
Cox DR, Burmeister M, Price ER, Kim S, Myers RM (1990) Radiation hybrid mapping: a somatic cell genetic method for constructing high-resolution maps of mammalian chromosomes. Science 250:245–250
Endo TR (1988) Induction of chromosomal structural changes by a chromosome of Aegilops cylindrica L. in common wheat. J Hered 79:366–370
Endo TR (1990) Gametocidal chromosomes and their induction of chromosome mutations in wheat. Jpn J Genet 65:135–152
Endo TR (2003) Wheat stocks carrying alien chromosomal segments induced by the gametocidal system. In: Proceedings of the 10th international wheat genetics symposium, Paestum, pp 69–72
Endo TR, Gill BS (1996) The deletion stocks of common wheat. J Hered 87:295–307
Gill KS, Gill BS, Endo TR, Boyko EV (1996) Identification and high-density mapping of gene-rich regions in chromosome group 5 of wheat. Genetics 143(2):1001–1012
Goss SJ, Harris H (1975) New method for mapping genes in human chromosomes. Nature 255:680–684
Janda J, Bartos J, Safar J, Kubalakova M, Valarik M, Cihalikova J, Simkova H, Caboche M, Sourdille P, Bernard M, Chalhoub B, Dolezel J (2004) Construction of a subgenomic BAC library specific for chromosomes 1D, 4D and 6D of hexaploid wheat. Theor Appl Genet 109(7):1337–1345
Kurata N, Nagamura Y, Yamamoto K, Harushima Y, Sue N, Wu J, Antonio BA, Shomura A, Shimizu T, Lin SY (1994) A 300 kilobase interval genetic map of rice including 883 expressed sequences. Nat Genet 8(4):365–372
Lawrence S, Morton NE, Cox DR (1991) Radiation hybrid mapping. Proc Natl Acad Sci 88:7477–7480
Macaulay M, Ramsay R, Powell P, Waugh R (2001) A representative highly informative ‘genotyping set’ of barley SSRs. Theor Appl Genet 102:801–809
Masoudi-Nejad A, Nasuda S, McIntosh RA, Endo TR (2002) Transfer of rye chromosome segments to wheat by a gametocidal system. Chromosome Res 10:349–357
Matise TC, Perlin M, Chakravarti A (1994) Automated construction of genetic linkage maps using an expert system (MultiMap): a human genome linkage map. Nat Genet 6(4):384–390
Nomura T, Ishihara A, Imaishi H, Ohkawa H, Endo TR, Iwamura H (2003) Rearrangement of the genes for the biosynthesis of benzoxazinones in the evolution of Triticeae species. Planta 217(5):776–782
Qi L, Echalier B, Friebe B, Gill BS (2003) Molecular characterization of a set of wheat deletion stocks for use in chromosome bin mapping of ESTs. Funct Integr Genomics 3(1–2):39–55
Ramsay L, Macaulay M, Ivanissevich S, MacLean K, Cardle L, Fuller J, Edwards KJ, Tuvesson S, Morgante M, Massari A, Maestri E, Marmiroli N, Sjakste T, Ganal M, Powell W, Waugh R (2000) A simple sequence repeat-based linkage map of barley. Genetics 156:1997–2005
Riera-Lizarazu O, Vales MI, Ananiev EV, Rines HW, Phillips RL (2001) Production characterization of maize chromosome 9 radiation hybrids derived from an oat–maize addition line. Genetics 156:327–339
Safar J, Bartos J, Janda J, Bellec A, Kubalakova M, Valarik M, Pateyron S, Weiserova J, Tuskova R, Cihalikova J, Vrana J, Simkova H, Faivre-Rampant P, Sourdille P, Caboche M, Bernard M, Dolezel J, Chalhoub B (2004) Dissecting large and complex genomes: flow sorting and BAC cloning of individual chromosomes from bread wheat. Plant J 39(6):960–968
Saghai-Maroof MA, Soilman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance chromosomal location and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Serizawa N, Nasuda S, Shi F, Endo TR, Prodanovic S, Schubert I, Künzel G (2001) Deletion-based physical mapping of barley chromosome. Theor Appl Genet 103:827–834
Shi F, Endo TR (1997) Production of wheat-barley disomic addition lines possessing an Aegilops cylindrica gametocidal chromosome. Genes Genet Syst 72:243–248
Shi F, Endo TR (1999) Genetic induction of structural changes in barley chromosomes added to common wheat by a gametocidal chromosome derived from Aegilops cylindrica. Genes Genet Syst 74:49–54
Shi F, Endo TR (2000) Genetic induction of chromosomal rearrangements in barley chromosome 7H added to common wheat. Chromosoma 109(5):358–363
Tsujimoto H, Yamada T, Hasegawa K, Usami N, Kojima T, Endo TR, Ogihara Y, Sasakuma T (2001) Large-scale selection of lines with deletions in chromosome 1B in wheat and applications for fine deletion mapping. Genome 44(4):501–508
Wardrop J, Snape J, Powell W, Machray GC (2002) Constructing plant radiation hybrid panels. Plant J 31(2):223–228
Wardrop J, Fuller J, Powell W, Machray GC, (2004) Exploiting plant somatic radiation hybrids for physical mapping of expressed sequence tags. Theor Appl Genet 108(2):343–348
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research (A) (No. 123066001) from the Ministry of Education, Science, Sports, and Culture, Japan and facilitated by a BBRSC Japan Partnering award to R. Waugh and T.R. Endo. Technical help from Malcolm Macaulay is much appreciated. Contribution No. 582 from the Laboratory of Plant Genetics, Graduate School of Agriculture, Kyoto University, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Hagemann
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Masoudi-Nejad, A., Nasuda, S., Bihoreau, MT. et al. An alternative to radiation hybrid mapping for large-scale genome analysis in barley. Mol Genet Genomics 274, 589–594 (2005). https://doi.org/10.1007/s00438-005-0052-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-005-0052-1