Abstract
Eimeria are ubiquitous parasites and eimeriosis treatment is based on coccidiostats or coccidicides used prophylactically, metaphylactically, or therapeutically. The long-term efficacy of toltrazuril (TZR, 15 mg/kg) against experimentally infected naïve calves was investigated. Seven groups (six treated and one control) of six animals each were formed. Animals of each group received a single TZR prophylactic oral treatment on days − 42, − 35, − 28, − 21, − 14, and − 7 before the challenge with infectious sporulated oocysts of Eimeria spp. (100,000 oocysts: 59.5% E. zuernii, 38.1% E. bovis, 1.2%, E. alabamensis, and 1.2% E. ellipsoidalis). The long-term efficacy was assessed based on Eimeria spp. oocyst excretion by fecal oocyst counts. Three calves from the control group presented diarrhea with blood, which was not observed in animals belonging to the treatment groups. The TZR achieved efficacy greater than 95.0% up to 14 and 7 days. This formulation showed efficacy above 95% for 7 to 14 days, between 82 and 84% for 21 to 28 days and between 50 and 64% for 35 to 42 days.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Parasitic infections caused by Eimeria, mainly the species E. zuernii and E. bovis, represent a serious health problem in calves in the world triggering economic losses (Taubert et al. 2008; Bruhn et al. 2011; Cruvinel et al. 2018a, b). In practice, chemical treatment is the main tool used to control eimeriosis. The drugs can be used in a prophylactic, metaphylactic, or therapeutic way, and are classified as coccidiostats (decoquinate, salinomycin, lasalocide, and monenzin) that interrupt the parasite cycle without destroying it, or coccidicides (sulfa, amprolium, diclazuril, and toltrazuril) killing the parasites (Vieira et al. 2005; Lopez and Ayensa 1996; Mundt et al. 2009; Diaferia et al. 2013).
Among these assets mentioned above, the coccidicide toltrazuril (TZR) is a triazine compound that acts against all intracellular stages of Eimeria spp. (Le Suer et al. 2009). In the literature, the works carried out with this molecule on cattle is concentrated on the effectiveness of the metaphylactic therapy, with the treatment in the incubation period of the disease (Epe et al. 2005; Mundt et al. 2005; Veronesi et al. 2013; Enemark et al. 2015), and in the therapeutic efficacy, that is, when infected animals receive a curative treatment (Foreyt et al. 1981; Parker et al. 1986; Mckenna 1988; Hoblet et al. 1989; Mundt et al. 2003; Jonsson et al. 2011; Philippe et al. 2014). However, in general, therapeutic treatment of coccidiosis is ineffective due to the mucosal lesions present in the intestine. So, to prevent losses, the animals should be treated pro- or metaphylactically rather than therapeutically (Daugschies and Najdrowski 2005).
In the medicine leaflet, the TZR withdrawal period can reach 70 days after the administration of the product, demonstrating that toltrazuril and its metabolites remain in the bovine organism for a certain period. This fact demonstrates the importance of conducting studies that assess the long-term efficacy of TZR against Eimeria spp. in cattle, like those conducted by Mundt et al. (2005) and Philippe et al. (2014). Therefore, the present study aimed to evaluate the long-term efficacy up to 42 days of TZR (15 mg/kg) administered prophylactically in calves experimentally infected with different Eimeria species.
Materials and methods
Location, animals, and feed
The experiment was conducted between November 2019 and March 2020 at the Vale da Pedra Farm located in the municipality of Jataí, Goiás state, midwest Brazil (latitude: −17.8759, longitude: −51.7214 17° 52′ 33″ South, 51° 43′ 17″ West). According to the Köppen-Geiger climate classification, the climate of this region is subtropical with annual rainfall of approximately 1534 mm per year and concentrated from October to March (spring–summer).
Forty-two naïve Holstein calves were used in the study, with an initial age between 5 and 15 days, and which had not been treated with anticoccidial formulations since birth. During the entire experimental period, the animals remained in collective pens, with wood shavings. They received 8 L of substitute milk daily (Nattimilk® — Auster) up to 30 days of age. After this period, the quantity of the substitute milk was gradually withdrawn to 6, 4, and 2 L/day, until weaning which occurred at approximately 100 days of life. During the study, each animal was offered 1.5 kg of concentrate and grass ad libitum. Water was provided ad libitum from an artesian source, and water and feed troughs were cleaned daily, in addition located at a height where contamination by the feces of these animals was not possible.
Feces collection for Eimeria spp. infection evaluation before the start of the study and groups allocation/treatment
On days − 56, − 46, − 45, and − 44 of the study, when the animals were between 5 and 15 days old, feces were collected individually directly from each rectum animal, and, after individual identification (using the animal’s ID number), were stored in isothermal containers filled with ice. They were kept in a refrigerator at 4 °C and processed within 48 h. An aliquot was taken for the quantification of Eimeria spp. oocysts per gram (OPG) of feces (Gordon and Whitlock, 1939, as modified by Ueno and Gonçalves, 1998) using McMaster’s slide, in saturated salt solution (density = 1.2 g/mL). Each oocyst found corresponded to 50 OPG, respectively.
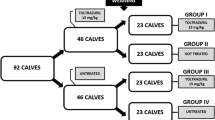
After that, on day − 43, the animals were weighed and distributed into seven groups of six calves each. The randomization and distribution of the animals was based on the weight of each animal on day − 43. The calves were divided into seven blocks of six animals each and, within each block, were then randomly allocated to the different treatment groups (Table 1). The animals were arranged in collective pens; each group was kept in a separate pen. The number of experimental animals per group (T01–T07) was estimated in accordance with Joachim et al. (2018).
Treatments with 15 mg/kg of TZR (Baycox®, suspension — Elanco Animal Health, Brazil) were administered; each group (T01 to T06) received an oral treatment on day − 42, − 35, − 28, − 21, − 14, or − 7 before the challenge with infectious sporulated oocysts of Eimeria spp. Animals in the control group (T07) received saline solution on day − 42 before the challenge (Table 1). To proceed each treatment, the animals were weighed again in the day before.
Inoculum preparation and calves’ infection
The inoculum was performed with fecal samples obtained from cattle naturally infected with Eimeria spp. (positive OPG counts for Eimeria spp.), from the farm school of the Federal University of Goiás, Goiânia, Goiás, Brazil. A pool of feces of these animals was then processed using the method of centrifugal flotation in sugar solution. Later, the samples were filtered using sieves with a folded gauze. A 2% potassium dichromate (K2Cr2O7) solution was added and kept at 24 °C for 14 days under oxygenation (using aquarium oxygenator pumps) to stimulate oocyst sporulation under laboratory conditions (Daugschies and Najdrowski 2005).
Oocysts were recovered by centrifugation in a 60% saturated sugar solution, and approximately 100 oocysts per pool were identified under an optical microscope coupled to a computerized system (LAS, Leica®). This process of recovering 100 oocysts per pool for the identification of Eimeria species was performed in triplicate for each treatment. Differentiation among species was performed according to the phenotypic characteristics of the oocysts, such as color, presence, or absence of micropyle, length, and width (Daugschies and Najdrowski 2005).
On day 0 of the study, all animals (T01 to T07) received, orally, 200 mL of a mixed isolate with approximately 100,000 sporulated oocysts of Eimeria spp. (Mundt et al. 2005), with the following proportion: 59.5% E. zuernii, 38.1% E. bovis, 1.2%, E. alabamensis, and 1.2% E. ellipsoidalis.
Feces collection during the study and assessments of clinical signs suggestive of eimeriosis
On days − 4, − 3, − 2, − 1, and 0 of the study (before experimental infection with Eimeria spp.), to verify if animals were not infected with Eimeria spp. before receiving the inoculum on days + 7, + 14, + 21, + 28, and + 35, fecal samples were collected from the animals for Eimeria spp. quantitative analysis as described previously in the “Inoculum preparation and calves’ infection” section.
To determine the protective potential of TZR (15 mg/kg), between days 0 and + 35 post-inoculation, all animals were subjected to daily clinical examinations for signs suggestive of eimeriosis such as diarrhea, hemorrhagic feces, fever, abdominal pain, tenesmus and anemia, dehydration, weakness, anorexia, and emaciation.
Efficacy
The long-term efficacy of TZR was determined considering the values of the arithmetic means of the dates of fecal collection after Eimeria spp. orally infection, by comparing the mean number of oocyst counts of Eimeria spp. quantified in the different groups (Lopes et al. 2014). To discuss the results, we considered only the mean effectiveness obtained between the days + 21, + 28, and + 35.
where CG is the control group; TG is the treated group; X is the day after Eimeria spp. experimental infection.
In addition, the efficacy per species of Eimeria spp. during that same period was calculated. The mean number of OPG for each species was considered, considering the general prevalence by species/group, throughout the period, according to the formula described below:
1Mean OPG of the treated or control group during the entire period*
*Entire period comprehend the OPG results obtained for each group (treated or control) between days + 7 and + 35.
After performing these calculations, the efficacy formula described above was applied to each of the Eimeria spp. species diagnosed during the study.
Statistical analysis
The experimental data (OPG — Eimeria spp.), after transformed in log (count + 1), met the prerogatives of normality, homogeneity of variances, residual analysis, and randomness of the observations. They were analyzed in a completely randomized design within each counting date and the means of treatments were compared by the Tukey test (p < 0.05), using the GLM procedure of the SAS software, version 9.4 (SAS 2016).
Results
No adverse events related to the administered toltrazuril treatment were observed.
Eimeriosis clinical signs
The main clinical sign observed among the animals was diarrhea. Three animals in the control group (T07) showed diarrhea at some point in the study: #2827 with bloody diarrhea on days + 7, + 8, and + 9; animal #2854 with diarrhea with streaked blood on days + 8 and + 9; and #2876 with diarrhea with streaks of blood on days + 9 and + 10.
In none of these cases was necessary to administer any type of rescue treatment or other concomitant treatment. In addition, among animals that were treated with TZR (T01 to T06), regardless of when the product was administered before experimental infections with mixed culture of Eimeria spp., there were no cases of diarrhea or other clinical signs suggestive of eimeriosis.
Mean counts of Eimeria spp. oocyst and toltrazuril efficacy
The following species of the genus Eimeria were identified both in the control and treated groups: E. bovis, E. zuernii, E. ellipsoidalis, and E. alabamensis. Regardless of treatment, the two most prevalent species were E. zuernii followed by E. bovis (Fig. 1ABCD).
Prevalence of Eimeria spp. oocysts identified in feces of bovines experimentally infected (day 0) with Eimeria spp. inoculum from groups treated (groups 1 to 6, treated on days − 42, − 35, − 28, − 21, − 14, and − 7, respectively) with 15 mg/kg of toltrazuril and control group (group 7, saline solution on day − 42). A Eimeria species identified 21 days post-inoculation; B Eimeria species identified 28 days post-inoculation; C Eimeria species identified 35 days post-inoculation; D Eimeria species identified in the entire period (21 to 35 days post-inoculation)
The long-term activity of TZR (15 mg/kg) against Eimeria ssp., obtained by the fecal samples collected on days 21, 28, and 35 post-infection, achieved efficacy greater than 95.0% up to 14 and 7 days. This formulation showed values of 78.2 to 82.3% of factor protective against Eimeria for 21 and 28 days. Between 35 and 42 days, the protective value of the TZR against Eimeria spp. was 42.5 to 53.2% (Table 2).
Analyzing the efficacy calculated by each Eimeria species, the long-term activity up to 42 days, the toltrazuril was of 56.6% for E. zuernii, 49.3% for E. bovis, 54.2% for E. alabamensis, and 0.0% for E. ellipsoidallis. Up to 35 days, the TZR showed 54.9% against E. zuernii, 10.6% against E. bovis, 28.7% against E. alabamensis, and 0.0% for E. ellipsoidallis. Up to 21 to 28 days, the long-term efficacy was respectively the following: 79.1–8.1% for E. zuernii, 74.1–81.0% for E. bovis, 70.2–87.2% for E. alabamensis, and 27.1–86.4% for E. elipsoidalis. Up to 14 days, the TRZ against E. zuernii, E. bovis, and E. alabamensis was ≥ 93%, and up to 7 days, the long-term efficacy for each Eimeria species was 100% (Table 2).
The mean OPG counts quantified in calves from T06, between days 28 and 35, were lower (P ≤ 0.05) than the other groups. The mean OPG counts of T03, T04, and T05 were lower (P ≤ 0.05) than T07. There was no difference (P > 0.05) between the mean OPG counts of T01, T02, T03, T04, and T05 (Table 3).
Discussion
This work evaluated the long-term efficacy of TZR administered prophylactically in young calves experimentally infected with Eimeria spp. The results are important from a practical point of view since it was demonstrated for how many days and how effective this formulation can prevent animals against re-infections by Eimeria species.
Regarding the therapeutic efficacy of TZR, there are studies that report a high efficacy of this compound against Eimeria spp. (Foreyt et al. 1981; Parker et al. 1986; Mckenna 1988; Hoblet et al. 1989; Mundt et al. 2003; Jonsson et al., 20,011; Philippe et al. 2014). Evaluating the excretion of Eimeria spp. oocysts for up to 78 days, from naturally infected calves, kept in a grazing system and treated with TRZ and diclazuril, Philippe et al. (2014) observed that the mean oocyst excretion decreased dramatically in the 5 days after treatments for both formulations. In addition, after 36 to 41 days post-treatment, the numerical counts of oocyst excretion by cattle in the treated and control animals was equivalent. These results are like those found in the current study, since TRZ demonstrated a long-term efficacy of 100% for 7 days. Furthermore, the mean oocyst counts among control animals compared to those treated with TRZ 35 and 42 days before challenge did not differ from each other.
In the present study, analyzing the efficacies for each Eimeria, the efficacy against E. zuernii was above 95% for 7 to 14 days, between 79 and 84% for 21 to 28 days, and between 54 and 56% for 35 to 42 days. For E. bovis, the efficacy was between 93 and 100% for 7 and 14 days, between 74 and 81% for 21 to 28 days, and between 10 and 49.3% for 35 and 42 days. As to E. alabamensis, the efficacy was 98 to 100% for 7 to 14 days, between 70 and 87% for 21 to 28 days and between 28 and 54% for 35 to 42 days. Against E. ellipsoidalis, the efficacy was 100% for 7 days, between 27 and 86% for 14 to 28 days, and no efficacy was observed for 35 to 42 days. The studies regarding TZR pharmacokinetic in cattle and pigs can help to better understand the results found in the present work: the TZR (10, 15, and 20 mg/kg), after administered orally, is absorbed and rapidly converted to the short-lived intermediary metabolite named toltrazuril sulfoxide and then metabolized to the reactive toltrazuril sulfone. These metabolites present long-terminal half-life, consequently, enable a long-term clinical efficacy in the treatment (EMEA 2004; Lim et al. 2010). In other words, maybe these TZR metabolites remained in cattle organism helping in the long-term efficacy.
The animals from the control group (without TZR treatment) showed diarrhea with streaked blood between days + 7 and + 10 after Eimeria spp. oral infection. Besides, one animal from the control group did not shed Eimeria spp. in feces after experimental inoculation on day 0. Even this period (+ 7 to + 10) does not correspond to the prepatent period of Eimeria spp., it is known that this parasite develops within the intestinal mucosa cells at certain sites during its biological cycle (Daugschies and Najdrowski 2005). It is important to highlight that the inoculum used here had almost 60% of its composition formed by E. zuernii; the schizonts of E. zuernii infect the lamina propria below the junction between crypt and villus, close to the muscular layer of the intestine, which can explain the bleeding (Maxie 2015). It is not ofen that clinical signs would develop during early merogony. The clinical signs are seen later, when the parasite cicle has been completed with gametogony. Maybe this could have happened because of the inoculum dose, or the fact that it was a mixed inoculum. Regarding the no elimination of Eimeria spp. oocysts, the dose of inoculum used, the interval between sampling time points, or perhaps even some individual factor of the animal may explain this result.
Studies have shown that primary infection in calves by Eimeria spp. occurs more frequently in animals aged ≥ 37 days (Cruvinel et al. 2019). In the age range between 35 and 55 days, Cruvinel et al. (2018b) reported, under field conditions, the presence of E. zuernii clinical outbreaks in beef calves, with animal mortality. To prevent these outbreaks occurrence, and to facilitate management, producers administer the TZR together with the management of the navel healing of these animals, which happens when the calves are with 1 to 3 days old (personal communication). This mentioned conduct, until then, achieved to suppress outbreaks in properties that routinely demonstrated consecutive outbreaks of eimeriosis in calves (Cruvinel et al. 2018b). The evidence regarding the long-term efficacy found in this work reinforces that this treatment in the newborn animals would be interesting. However, this early TRZ treatment may be specific for some operations, but the timing depends strongly on the operation’s coccidiosis history and should be adjusted to meet metaphylactic treatment for optimal efficacy.
On the other hand, it is important to highlight that the results verified in the current work occurred according to the susceptibility level of the Eimeria spp. population to TZR. Over time, more frequent the use of this coccidicide, the degree of Eimeria spp. resistance to TZR increases, and the percentage of effectiveness, consequently, decreases, and the molecule could not show the same results as those presented here. In sheep, the resistance of Eimeria spp. to TZR was reported (Odden et al. 2018).
In addition, it is important to note that in farms that have outbreaks caused by E. zuernii, these cases are recurrent and specific treatments against Eimeria spp. should be considered on calves each year (Cruvinel et al. 2018b), associated with operational changes when possible, such as changing maternity paddock each year. However, future field studies should be carried out to better understand when the treatment needs to be performed, at birth or after some animals show some clinical signs, to maintain the health and productivity of the herd and at the same time postpone the appearance of Eimeria spp. resistance to TZR.
Conclusions
In summary, the oral administration of TZR in different dates, at a dose of 15 mg/kg, demonstrated a beneficial effect against E. bovis, E. zuernii, and E. alabamensis oocyst excretion that was declining over time but significant over the study period.
References
Bangoura B, Bardsley KD (2020) Ruminant coccidiosis. Vet Clin North Am Food Anim Pract 36(1):187–203. https://doi.org/10.1016/j.cvfa.2019.12.006
Bangoura B, Daugschies A (2007) Parasitological and clinical parameters of experimental Eimeria zuernii infection in calves and influence on weight gain and haemogram. Parasitol Res 100(6):1331–1340. https://doi.org/10.1007/s00436-006-0415-5
Bruhn FRP, Lopes MA, Demeu FA, Perazza CA, Pedrosa MF, Guimarães AM (2011) Frequency of species of Eimeria in females of the Holstein-Friesian breed at the post-weaning stage during autumn and winter. Rev Bras Parasitol Vet 20(4):303–307
Cruvinel LB, Nicaretta JE, Bastos TSA, Couto LFM, Santos JB, Zapa DMB, Cavalcantes ASA, Cruz BC, Borges DGL, Borges FA, Soares VE, Lopes WDZ (2018) Eimeria species in dairy and beef cattle of different ages in Goiás state. Brazil Rev Bras Parasitol Vet 27:169–176
Cruvinel LB, Bastos TSA, Nicaretta JE, Couto LFM, Borges DGL, Borges FA, Soares VE, Lopes WDZ (2018) Surtos consecutivos ocasionado por Eimeria zuernii em bezerros de corte de uma propriedade do estado de São Paulo. Pesquisa Veterinária Brasileira 38:277–284
Cruvinel LB, Ayres H, Zapa D, Nicaretta JE, Couto L, Heller LM, Bastos T, Cruz BC, Soares VE, Teixeira WF, de Oliveira JS, Fritzen JT, Alfieri AA, Freire RL, Lopes WDZ (2019) Prevalence and risk factors for agents causing diarrhea (Coronavirus, Rotavirus, Cryptosporidium spp., Eimeria spp., and nematodes helminths) according to age in dairy calves from Brazil. Trop Anim Health Prod 52:777–791. https://doi.org/10.1007/s11250-019-02069-9
Daugschies A, Najdrowski M (2005) Eimeriosis in cattle: current understanding. J Vet Med 52:417–427
Diaferia M, Veronesi F, Morganti G, Nisoli L, Fioretti DP (2013) Efficacy of toltrazuril 5 % suspension (Baycox®, Bayer) and diclazuril (Vecoxan®, Janssen-Cilag) in the control of Eimeria spp. in lambs. Parasitol Res 112(Suppl 1):163–8. https://doi.org/10.1007/s00436-013-3440-1
EMEA (2004) Committee for medicinal products for veterinary use for cattle. Toltrazuril. Avaiable in: document EMEA/MRL/907/04-FINAL from June 2004.
Enemark HL, Dahl J, Enemark JM (2015) Significance of timing on effect of metaphylactic toltrazuril treatment against eimeriosis in calves. Parasitol Res 114(1):201–212
Epe C, von Samson-Himmelstjerna G, Wirtherle N, von der Heyden V, Welz C, Beening J, Radeloff I, Hellmann K, Schnieder T, Krieger K (2005) Efficacy of toltrazuril as a metaphylactic and therapeutic treatment of coccidiosis in first-year grazing calves. Parasitol Res 97 Suppl 1(Suppl 1):S127–S133. https://doi.org/10.1007/s00436-005-1456-x
Foreyt WJ, Gates NL, Rich JE (1981) Evaluation of lasalocid in salt against experimentally induced coccidiosis in confinement reared lambs from weaning to market weight. Am J Vet Res 42:57–60
Ghanem MM, Radwaan ME, Moustafa AM, Ebeid MH (2008) Comparative therapeutic effect of toltrazuril, sulphadimidine and amprolium on Eimeria bovis and Eimeria zuernii given at different times following infection in buffalo calves (Bubalus bubalis). Prev Vet Med 84(1–2):161–170. https://doi.org/10.1016/j.prevetmed.2007.12.013
Hoblet KL, Charles TP, Howard RR (1989) Evaluation of lasalocid and decoquinate against coccidiosis resulting from natural exposure in weaned dairy calves. Am J Vet Res 50(7):1060–1063
Joachim A, Altreuther G, Bangoura B, Charles S, Daugschies A, Hinney B, Lindsay DS, Mundt HC, Ocak M, Sotiraki S (2018) W A A V P guideline for evaluating the efficacy of anticoccidials in mammals (pigs, dogs, cattle, sheep). Vet Parasitol 15(253):102–119. https://doi.org/10.1016/j.vetpar.2018.02.029
Jonsson NN, Piper EK, Gray CP, Deniz A, Constantinoiu CC (2011) Efficacy of toltrazuril 5 % suspension against Eimeria bovis and Eimeria zuernii in calves and observations on the associated immunopathology. Parasitol Res 109(Suppl 1):S113–S128. https://doi.org/10.1007/s00436-011-2408-2
Kertz AF, Reutzel LF, Mahoney JH (1984) Ad libitum water intake by neonatal calves and its relationship to calf starter intake, weight gain, feces score, and season. J Dairy Sci 67(12):2964–2969. https://doi.org/10.3168/jds.S0022-0302(84)81660-4
Le Suer C, Mage C, Mundt HC (2009) Efficacy of toltrazuril (Baycox® 5% suspension) in natural infections with pathogenic Eimeria spp. in housed lambs. Parasitol Res 104:1157–1162
Lim JH, Kim MS, Hwang YH, Song IB, Park BK, Yun HI (2010) Pharmacokinetics of toltrazuril and its metabolites, toltrazuril sulfoxide and toltrazuril sulfone, after a single oral administration to pigs. J Vet Med Sci 72(8):1085–1087. https://doi.org/10.1292/jvms.09-0524
Lopez DF, Ayensa MC (1996) Diagnostico. Aula Veterinária Ovis, Leon 45(1):41–47
Lopes WDZ, Carvalho RF, Pereira V, Martinez AC, Cruz BC, Teixeira WF, Maciel WG, Da Costa AJ, Soares VE, Borges DGL, Rodriguez FS, Borges FA (2014) Efficacy of sulfadoxine+trimethoprim compared to management measures for the control of Eimeria parasitism in naturally infected and clinically asymptomatic sheep that were maintained in a feedlot. Small Ruminant Res 116:37–43
Maxie G (2015) Jubb, Kennedy & Palmer’s pathology of domestic animals: volume 16th edition. Saunders.
McKenna PB (1988) Eficácia del toltrazuril em cabras com infecciones por coccídios contraídas naturalmente. Noticias Médicas Veterinárias 59:157–161
Mundt HC, Daugschies A, Vebe F, Rinke M (2003) Efficacy of toltrazuril against artificial infection with Eimeria bovis in calves. Parasitol Res 9(suppl 3):s166–s167
Mundt HC, Bangoura B, Mengel H, Keidel J, Daugschies A (2005) Control of clinical coccidiosis of calves due to E. bovis and E. zuernii with Baycox® Bovis under field conditions. Parasitol Res 97:134–142
Mundt HC, Dittmar K, Daugschies A, Grzonka E, Bangoura B (2009) Study of the comparative efficacy of toltrazuril and diclazuril against ovine coccidiosis in housed lambs. Parasitol Res 105(Suppl 1):S141–S150. https://doi.org/10.1007/s00436-009-1505-y
Odden A, Denwood MJ, Stuen S, Robertson LJ, Ruiz A, Hamnes IS, Hektoen L, Enemark HL (2018) Field evaluation of anticoccidial efficacy: a novel approach demonstrates reduced efficacy of toltrazuril against ovine Eimeria spp in Norway. Int J Parasitol Drugs Drug Resist 8(2):304–311. https://doi.org/10.1016/j.ijpddr.2018.05.002
Parker RJ, Jones GW, Ellis KJ, Heater KM, Schroter KL, Tyler R, Holroyd RG (1986) Post-weaning coccidiosis in beef cattle in the dry tropics; experimental control with continous monensin supplementation via intra-ruminal devices concurrent epidemiological observations. Trop Anim Health Prod 18:198–208
Philippe P, Alzieu JP, Taylor MA, Drochies PH (2014) Comparative efficacy of diclazuril (Vecoxan®) and toltrazuril (Baycox bovis®) against natural infections of Eimeria bovis and Eimeria zuernii in French calves. Vet Parasitol 206:129–137
SAS Institute. SAS user’s guide. Version 9.4 [online]. Cary, NC: SAS Institute Inc., 2016.
Stockdale PH, Bainborough AR, Bailey CB, Niilo L (1981) Some pathophysiological changes associated with infection of Eimeria zuernii in calves. Can J Comp Med 45(1):34–7
Taubert A, Hermosilla C, Suhwold A, Zahner H (2008) Antigen-induced cytokine production in lymphocytes of Eimeria bovis primary and challenge infected calves. Vet Immunol Immunopathol 126(3–4):309–320
Ueno H, Gonçalves PC (1998) Manual para diagnóstico das helmintoses de ruminantes. Japan International Coopertion Agency
Veronesi F, Nisoli L, Diaferia M, Falcini R, Ficola E, Fioretti DP (2013) Influence of a metaphylactic treatment with Baycox® Bovis on the reproductive performances of Fresian heifers. Parasitol Res 112(6):2137–2142
Vieira LS et al (2005) Monensina sodica no controle da eimeriose em caprinos leiteiros. Ciência Animal Brasiliera, Goiania 15(1):25–31
Author information
Authors and Affiliations
Contributions
DMBZ: investigation, data curation, project administration; LFMC, LMH, HVI, RBN, AASNT, LMAG: investigation; LLF: writing — original draft, writing — review and editing; VES: formal analysis; WDZL: supervision, data curation, writing — original draft.
Corresponding author
Ethics declarations
Ethics approval
The project was approved by the Ethics Committee on the Use of Animals (CEUA) of the Federal University of Goiás (UFG) (Protocol 104/19) being in accordance with the ethical principles in animal experimentation for elaboration of the experiment and its execution.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Berit Bangoura
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zapa, D.M.B., Couto, L.F.M., Heller, L.M. et al. Long-term efficacy of toltrazuril in naïve calves prophylactically treated and experimentally infected with Eimeria spp.. Parasitol Res 121, 2571–2578 (2022). https://doi.org/10.1007/s00436-022-07601-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07601-9