Abstract
In Brazil, the Amazon region comprises 99.5% of the reported malaria cases. However, another hotspot of the disease is the Atlantic Forest regions, with the sporadic occurrence of autochthonous human cases. In such context, this study sought to investigate the role of anopheline mosquitoes (Diptera: Culicidae) in the residual malaria transmission in Atlantic Forest areas. Two rural areas in the Espírito Santo state were the surveyed sites. Mosquitoes were captured using Shannon trap and CDC light traps and identified into species based on morphological characters. Ecological indexes (Shannon–Wiener diversity, Simpson’s dominance, Pielou equability, and Sorensen similarity) were the tools used in the anopheline fauna characterization and comparison along with the two explored areas. The assessment of the sampling adequacy in the studied areas was possible through the generation of a species accumulation curve. A correlation test verified the influence of climatic variables on the anopheline species abundance. A total of 1471 female anopheline mosquitoes were collected from May 2019 to April 2020, representing 13 species. The species richness was higher in Valsugana Velha (hypo-endemic) than in Alto Caparaó (non-endemic). There was a significant variation in the species abundance between Valsugana Velha (n = 1438) and Alto Caparaó (n = 33). The most abundant species was Anopheles (Kerteszia) cruzii complex Dyar and Knab, 1908 representing 87% of the total anophelines collected. These results suggest that the Plasmodium spp. circulation in Brazilian Atlantic Forest areas occurs mainly due to the high frequency of Anopheles (K.) cruzii complex, considered the principal vector of simian and human malaria in the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mosquitoes pose a threat to public health worldwide. Vector-borne illnesses currently account for approximately 17% of all infectious diseases (WHO 2017). One of the most relevant mosquito-borne diseases is malaria, caused by the protozoan parasite Plasmodium spp. and transmitted by female Anopheles mosquitoes infected (Ashley et al. 2018). According to World Health Organization (WHO 2020) estimation, there were 229 million malaria cases in 2019 worldwide. Despite substantial progress in the fight to eliminate malaria, the disease still is a significant health problem, causing economic impacts in many countries in the region of the Americas, where the health authorities reported more than 800,000 cases in 2019, with Brazil accounting for approximately 20% of all occurrences.
Engaged in the fight against malaria since the beginning of the twentieth century, Brazil has made several attempts to control the disease over time (Deane 1988). The estimates for the epidemiologic scenario in 1940 indicated that almost all country regions were receptive and reported malaria cases (Pinotti 1951; Deane 1986). Malaria elimination from some states in 1965 was the consequence of the global eradication campaign proposed by the WHO and adopted by the Brazilian Ministry of Health, a challenging effort given the high magnitude of the disease in the previous years. At that time, dichlorodiphenyltrichloroethane (DDT) and chloroquine were the cornerstones for the control activities. Dichlorodiphenyltrichloroethane was the residual insecticide useful for vector control, and chloroquine was the drug in general use to treat the infection (Loiola et al. 2002). The actions adopted by the Brazilian authorities were enough to control and eliminate the disease from several regions of Brazil, including the Northeast, Southeast, South, and Central-West. However, due to eco-epidemiological factors, such measures failed to interrupt the transmission in some Atlantic Forest areas and mostly, in the Amazon region, still classified as an endemic area (Carlos et al. 2019; Buery et al. 2021).
Although autochthonous malaria transmission being under control in most extra-Amazonian regions, residual foci persist along with the Brazilian Atlantic Forest areas of southern and southeastern states. In such spots, howler monkeys, anophelines mosquitoes, and humans use to be infected naturally by Plasmodium vivax/Plasmodium simium, Plasmodium malariae/Plasmodium brasilianum, and, more recently, Plasmodium falciparum (Laporta et al. 2015; Buery et al. 2017; Demari-Silva et al. 2020). Some research studies have indicated that non-human primates (NHP) may participate in the human malaria transmission cycle in the Atlantic Forest (Brasil et al. 2017). The clinical aspects and spatial–temporal dynamics of the malaria incidence in these areas associated with vector behavior reinforce such a hypothesis.
The Brazilian Atlantic Forest is considered one of the most distinguished biodiversity repositories of the world. The region has geographic and climatic characteristics that benefit the development of insects, mainly mosquitoes (Ribeiro et al. 2009). Such insects belonging to the Culicidae family of the Diptera order can play a relevant epidemiological role in arboviruses and parasite transmission (WHO 2017). In this context, the Brazilian Atlantic Forest can become a hotspot for malaria, putting the disease elimination plan at risk. The present study aimed to elucidate the possible contribution of the dynamics of anopheline populations in sustaining residual malaria transmission.
Methods
Study areas
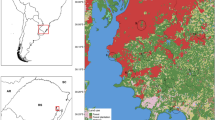
Malaria vector surveillance occurred in Valsugana Velha (19°58′05.2″S, 40°34′40.8″W) and Alto Caparaó (9°44′12.7″S, 40°58′33.1″W) (Fig. 1), two inland rural communities situated at high elevation points of Santa Teresa (hypo-endemic) and Itaguaçu (non-endemic), respectively. Both sites have proximity to Atlantic Forest fragments, potential breeding sites for Anopheles species.
Santa Teresa (altitude of 100–1050 m) and Itaguaçu (altitude of 70–1320 m) are municipalities of the central mountainous region of Espírito Santo State, southeastern Brazil (IJSN 2020). While Santa Teresa has an area of 683,032 km2 with 32.1% covered by Atlantic Forest, Itaguaçu, located 53 km away from Santa Teresa, has 535,021 km2, of which 17.7% have forest cover (SEAMA 2018; IBGE 2019).
According to the Capixaba Institute for Research, Technical Assistance and Rural Extension (Instituto Capixaba de Pesquisa, Assistência Técnica e Extensão Rural—INCAPER), the area of Valsugana Velha and Alto Caparaó experiences cold and humid weather conditions (INCAPER 2020). There is one rainy season between January and April, a dry or less rainy season between May and September, and another rainy season between October and December. The minimum and maximum average temperatures of the coldest and warmest months range from 7.3–9.4 °C and 25.3–27.8 °C, respectively. These areas receive annual precipitation of 1332 mm (Valsugana Velha) and 1066 mm (Alto Caparaó) (INCAPER 2020). Cases of residual malaria from Atlantic Forest systems occur primarily in Santa Teresa, where there are findings of infected Anopheles (K.) cruzii complex specimens (Cerutti Junior et al. 2007; Buery et al. 2018).
Adult mosquito sampling and identification of Anopheles species
During 1 year, from May 2019 to April 2020, there has been a sampling of Anopheles mosquitoes twice a month (one site per night), from 06:00 pm to 06:00 am the next morning. The setting of two types of traps was the strategy chosen for the capture of adult mosquitoes: (1) Captures performed in a Shannon light trap, with the help of an oral suction tube: the setting inside the forest allowed the capture of anophelines for 4 h (06:00–10:00 pm). (2) Captures performed by a CDC automatic (Center for Disease Control) trap with CO2 baits (200 g of dry ice): the team installed two CDC traps inside the forest, the first at 1 m above the ground, and the second at 10 m height, in the canopy. CDC traps remained turned on for 12 h, from the night (06:00 pm) until the morning (06:00 am).
Every morning after the collection, the team removed the mosquitoes from the traps. The researchers transferred the insects to polyethylene cages, transporting them to the Entomology and Malacology Centre of Espírito Santo (Núcleo de Entomologia e Malacologia do Espírito Santo—NEMES/ES), where they performed the identification of the anophelines. After the insects’ killing in a freezer, the researchers identified the adult female anophelines through the key developed by Consoli and Lourenço-de-Oliveira (1994).
Climatic variables
Santa Teresa Station of the National Meteorology Institute of Brazil provided the meteorological data. Data used included the temperature (mean of the day of the capture) and the precipitation (mean from seven days before the capture event).
Data analysis
The statistical software for data analyses was the vegan package in R Statistical Software (version 1.3.1093) and PAST software (version 4.03). The indexes used were the Shannon–Wiener diversity index (H’), Simpson’s dominance index (C), and the Pielou equability index (J) for Anopheles species diversity, dominance, and evenness, respectively. The Sorensen index (SI) allowed the assessment of the species composition similarity among the sampled areas. The Hutcheson t-test (α = 0.05) disclosed the occurrence of statistically significant differences in the Shannon–Wiener diversity index (H’) and Simpson’s dominance index (C) between the two studied areas: Valsugana Velha and Alto Caparaó.
To assess the sampling sufficiency, a species accumulation curve was generated through the EstimateS program (Colwell 2006). The species accumulation curve considered the estimator’s Sobs (Mao Tao), which represents observed species richness, and Jackknife 1, which indicates the expected species richness to be sampled. Five-hundred randomizations were used, with a confidence interval of 95%.
Data analyses included a correlation testing between climatic variables (mean temperature; rainfall) and the abundance of species in both areas. After checking the data normality through the Shapiro–Wilk test since there was no normality in the data distribution, the investigators performed a non-parametric Spearman’s correlation procedure (α = 0.05).
Results
Species composition and abundance
The sampling comprised 1471 Anopheles mosquitoes, belonging to 13 different species and three subgenera (Table 1). The three most abundant species were Anopheles (K.) cruzii complex (87%), Anopheles (Nyssorhynchus) strodei Root, 1926 (6%), and Anopheles (Nyssorhynchus) evansae Brethes, 1926 (4.21%). These three species comprised 97.2% of all collected specimens.
Valsugana Velha had the highest abundance and richness of anophelines, with 1438 (97.7%) specimens distributed in 10 species. In contrast, in Alto Caparaó, there was a collection of only 33 (2.24%) anophelines, belonging to seven different species. The sample-based species accumulation curves in both areas were close to the asymptote (Fig. 3). A total of 10 species resulted from the Valsugana Velha survey and seven species from Alto Caparaó, which gave a sampling efficiency of 78.46% (Jackknife 1 = 12.75 ± 3.86) and 71.79% (Jackknife 1 = 9.75 ± 3.86), respectively.
Anopheles (K.) cruzii complex, the primary vector of human and simian malaria in the Atlantic Forest, was the dominant and most abundant species in the two sites, constituting 87% of all captured Anopheles mosquitoes. Regarding the distribution, Valsugana Velha yielded 1259/1438 specimens (87.5%) and Alto Caparaó 21/33 (63.6%) of them.
Anopheles species diversity
Table 1 discloses the richness, abundance, and diversity of Anopheles species in each area. Four of the species (31%) were similar between the sites: Anopheles (K.) cruzii complex, Anopheles (N.) strodei, Anopheles (N.) evansae, and Anopheles (Nyssorhynchus) lutzii Cruz, 1901. The value estimated with Sorensen’s index indicated a low similarity among the areas (SI = 0.47).
Despite the trapping of the lowest numbers of species and specimens in Alto Caparaó, this area showed the highest diversity index value (H’ = 1.2912). On the other hand, Valsugana Velha, accounting for the highest richness and abundance, showed lower diversity (H’ = 0.53514). According to the Hutcheson t-test, the Shannon–Wiener diversity index was significantly different between Alto Caparaó and Valsugana Velha (p < 0.01). The Simpson dominance index was higher in Valsugana Velha than in Alto Caparaó (C = 0.77508 versus C = 0.42883, p < 0.01). The values of both the Shannon diversity index and the Simpson dominance index suffered the influence of the evident abundance and dominance of the Anopheles (K.) cruzii complex, which represented 87.5% and 63.3% of the Anopheles population of Valsugana Velha and Alto Caparaó, respectively. This observation has corroboration of the values of equability. The Pielou equability index was J = 0.6635 for Alto Caparaó and J = 0.2324 for Valsugana Velha.
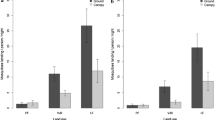
Comparing the ecological indexes of diversity and dominance by trap (Table 2) for Valsugana Velha and Alto Caparaó, we found that there was no significant difference in Simpson dominance index (C = 0.2849 versus C = 1.3863, p = 0.85) and species diversity (H’ = 1.4527 versus H’ = 1.3863, p = 0.85) for CDC trap placed on the ground. However, Simpson dominance index (C = 0.97473 versus C = 1, p < 0.05) and Shannon–Wiener diversity index (H’ = 0.074337 versus H’ = 0, p < 0.01) were significantly different for CDC traps placed in the canopy. The same observation applies to the Simpson dominance index (C = 0.37902 versus 0.18519, p < 0.05) for the Shannon light trap.
Exploration of seasonal trend in Valsugana Velha
The temporal distribution for the three most common species captured in Valsugana Velha revealed a multimodal pattern for Anopheles (K.) cruzii complex and a unimodal for Anopheles (N.) strodei and Anopheles (N.) evansae (Fig. 2). Anopheles (K.) cruzii complex was the dominant species, present throughout the year with two relevant peaks, one in May 2019 (dry season) and the other in November 2019 (rainy season). Anopheles (N.) strodei and Anopheles (N.) evansae presented only one peak in October 2019 (rainy season). There was no significant correlation between climatic variables and Anopheles abundance.
Exploration of seasonal trend in Alto Caparaó
Anopheles (K.) cruzii complex had a unimodal distribution pattern, with a higher peak in February 2020 (rainy season) (Fig. 2). There was no significant correlation between climatic variables and Anopheles (K.) cruzii complex abundance.
Discussion
This study provides updated information on the distribution and diversity of Anopheles species in the Atlantic Forest regions of Brazil. In the present study, 13 Anopheles species were found. Anopheles (K.) cruzii complex was the most common species in both areas. Although found in a distinct amount, this species represented 63.6% and 87.5% of the anopheline fauna of Alto Caparaó and Valsugana Velha, respectively. This is consistent with the existing literature. Anopheles (K.) cruzii complex has been described as the dominant species in the Brazilian Atlantic forest (Ueno et al. 2007; Reis et al. 2010; Guedes and Navarro-Silva 2014; Ceretti-Junior et al. 2020). Studies performed in Espírito Santo, Rio de Janeiro, and São Paulo have incriminated Anopheles (K.) cruzii complex as the primary malaria vector. In Espírito Santo, this vector was found naturally infected by P. vivax/P. simium on two occasions (Rezende et al. 2009; Buery et al. 2018). In 2015–2016, the state of Rio de Janeiro reported 49 autochthonous malaria cases, all from the Atlantic Forest area, a place with high Anopheles (K.) cruzii density (Brasil et al. 2017). Furthermore, specimens of Anopheles (K.) cruzii complex were found naturally infected with P. vivax/P. simium, P. malariae/P. brasilianum, and most recently P. falciparum in the Atlantic Forest areas of São Paulo (Duarte et al. 2013; Laporta et al. 2015; Demari-Silva et al. 2020).
The abundance of mosquitoes and the species richness were different between the two areas. Although Alto Caparaó and Valsugana Velha have the same biome, there was a substantial difference in the number of specimens collected in one and the other. Some hypotheses proposed to explain these differences are the forest cover and the anthropogenic environmental disturbance (e.g., deforestation, agriculture). The expressive abundance and dominance of Anopheles (K.) cruzii complex, found in Valsugana Velha, and the small number of specimens of this species in Alto Caparaó probably indicate that the first area has a lower level of environmental degradation than the second. According to Dorvillé (1996), anophelines from the subgenus Kerteszia are excellent bioindicators of anthropogenic activity. Ribeiro et al. (2012) and Chaves et al. (2016) revealed differences in the Anopheles (K.) cruzii complex abundance between preserved and degraded Atlantic Forest environment. Recently, Medeiros-Sousa et al. (2019) also observed the influence of anthropogenic landscape changes on the abundance of this vector.
Climatic factors such as temperature and precipitation can also influence the composition and abundance of anophelines added to the specific causes mentioned above. Studies conducted in areas of Atlantic Forest in São Paulo state indicated that the abundance of Anopheles (K.) cruzii complex was associated with temperature and rainfall (Guimarães et al. 2000, 2001). However, like the study of Rezende et al. (2009) developed in Atlantic Forest areas of Espírito Santo, our study also found no correlation between climate variables and the abundance of this vector. This lack of correlation might result from the low frequency of the collected specimens, especially in Alto Caparaó. Alternatively, the complex topography of the Atlantic Forest can generate environmental and microclimate conditions capable of influencing the abundance of some anophelines (Marques et al. 2012). For example, mosquitoes of the subgenus Kerteszia, mainly Anopheles (K.) cruzii complex, reproduce in waters that accumulate inside of bromeliads, a plant located under the shade of the treetops, protected from the sun’s rays, which prevents the rapid evaporation of the water contained therein (Consoli and Lourenço-de-Oliveira 1994). This specific condition of reproducing inside bromeliads can generate particular microclimate conditions (e.g., temperature and humidity) that become indistinguishable when analyzing only data from macroclimate.
The differences in Anopheles species richness and abundance observed in the present study can influence the differential epidemiological scenario of malaria observed in rural areas of Itaguaçu (non-endemic) and Santa Teresa (hypo-endemic). In general, these findings agree with those from previous entomological studies conducted in areas of malaria transmission in the Brazilian Atlantic Forest. According to Rezende et al. (2013), species richness, density of anophelines species, and dominance of the Anopheles (K.) cruzii complex are factors that can influence the dynamics of malaria transmission in the Atlantic Forest.
In the present study, the Shannon–Wiener diversity index (H’), Simpson’s dominance index (C), and the Pielou equability index (J) were used to measure the species diversity, species dominance, and species evenness. Although the Valsugana Velha area has presented higher richness, it was also where the highest species dominance was verified. In the Alto Caparaó area, the Shannon–Wiener diversity index (1.2912), Pielou equability index (0.6635), and Simpson’s dominance index (0.42883) indicated higher diversity and equitability and lower dominance compared to the Valsugana Velha area. In this sense, it is noted that the highest richness observed does not always reflect the higher existing diversity in the area since diversity is determined by both richness and equability (Melo 2008). The equitability index refers to the distribution of the number of specimens among species. It is proportional to diversity and inversely proportional to dominance (Pielou 1975). Therefore, the expressive abundance of the species Anopheles (K.) cruzii complex in Valsugana Velha gave a greater dominance and lower equitability to the area compared to Alto Caparaó, which showed greater diversity probably due to the greater equitability of the number of specimens among the species.
Considering that the species accumulation curves (Fig. 3) showed a tendency to an asymptote but did not stabilize and that the results of the Jackknife 1 showed that there may be more additional species to the ones collected, new species of genus Anopheles can be sampled in both areas with the continuation of sampling.
As expected, Simpson’s dominance index (C) for both study sites was higher in the CDC trap placed in the tree canopy (C = 1 for Alto Caparaó and C = 0.97473 for Valsugana Velha). This result reflects the high proportions of Anopheles (K.) cruzii complex collected using this method. Regarding the vertical distribution of the Anopheles (K.) cruzii complex, this species represented 60.6% and 86% of the anopheline community in the canopy strata of Alto Caparaó and Valsugana Velha, respectively. According to Forattini et al. (1968), there is evidence of the preference of this species for inhabiting the tree canopies compared with ground level, when they observed the presence of 83.3% of the specimens at 15 m in height in an area located in the state of São Paulo. This event was observed in Espírito Santo by Deane et al. (1971) and Rezende et al. (2009) and also by Guimarães et al. (1985) in Rio de Janeiro, where this species was much more numerous in the canopy than at the ground level.
Altogether, these data support the hypothesis that the specific breeding conditions of the Anopheles (K.) cruzii complex favor their predominance in the canopy, where they find, as the principal blood source, the non-human primates. On the other hand, different breeding sites occur at the ground level, favoring more species diversity. The distribution of the Anopheles (K.) cruzii complex fits the proposed malaria transmission cycle, which would take place mainly among the non-human primates, being human dwellers, accidental hosts.
In this study, other anopheline species, including Anopheles (N.) strodei, Anopheles (N.) evansae, and Anopheles (Nyssorhynchus) triannulatus Neiva and Pinto, 1922, were found in low frequency. However, it is relevant to document their occurrence, as they are also able to transmit malaria. Different authors have reported their susceptibility to infection by Plasmodium parasites in Atlantic Forest areas (Duarte et al. 2013; Laporta et al. 2015). According to Consoli and Lourenço-de-Oliveira (1994), these species possibly are secondary vectors and can participate in the transmission cycle when present at high densities.
Finally, this study reinforces that Anopheles (K.) cruzii complex is the principal vector involved in malaria transmission in the Brazilian Atlantic Forest. The high frequency of this vector found in Valsugana Velha might explain the difference observed in the epidemiological scenario of malaria between Santa Teresa (hypo-endemic) and Itaguaçu (non-endemic).
References
Ashley EA, Phyo AP, Woodrow CJ (2018) Malaria. The Lancet 391:1608–1621. https://doi.org/10.1016/S0140-6736(18)30324-6
Brasil P, Zalis MG, de Pina-Costa A et al (2017) Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: a molecular epidemiological investigation. Lancet Glob Health 5:1038–1046. https://doi.org/10.1016/S2214-109X(17)30333-9
Buery JC, Rodrigues PT, Natal L et al (2017) Mitochondrial genome of Plasmodium vivax/simium detected in an endemic region for malaria in the Atlantic Forest of Espírito Santo state, Brazil: do mosquitoes, simians and humans harbour the same parasite? Malar J 16:437. https://doi.org/10.1186/s12936-017-2080-9
Buery JC, Rezende HR, Natal L et al (2018) Ecological characterisation and infection of Anophelines (Diptera: Culicidae) of the Atlantic Forest in the southeast of Brazil over a 10 year period: has the behaviour of the autochthonous malaria vector changed? Mem Inst Oswaldo Cruz 113:111–118. https://doi.org/10.1590/0074-02760170225
Buery JC, Alencar FECd, Duarte AMRd et al (2021) Atlantic Forest Malaria: a review of more than 20 years of epidemiological investigation. Microorganisms 9:132. https://doi.org/10.3390/microorganisms9010132
Carlos BC, Rona LD, Christophides GK et al (2019) A comprehensive analysis of malaria transmission in Brazil. Pathog Glob Health 113:1–13. https://doi.org/10.1080/20477724.2019.1581463
Ceretti-Junior W, Oliveira-Christe R, Wilk-da-Silva R et al (2020) Diversity analysis and an updated list of mosquitoes (Diptera: Culicidae) found in Cantareira State Park, São Paulo. Brazil Acta Trop 212:105685. https://doi.org/10.1016/j.actatropica.2020.105669
Cerutti C, Boulos M, Coutinho AF et al (2007) Epidemiologic aspects of the malaria transmission cycle in an area of very low incidence in Brazil. Malar J 6:33. https://doi.org/10.1186/1475-2875-6-33
Chaves LSMC, Sá ILR, Bergamaschi DPL et al (2016) Kerteszia Theobald (Diptera: Culicidae) mosquitoes and bromeliads: a landscape ecology approach regarding two species in the Atlantic rainforest. Acta Trop 164:303–313. https://doi.org/10.1016/j.actatropica.2016.09.023
Colwell RK (2006). EstimateS: statistical estimation of species richness and shared species from samples. Version 8. Accessed 03 February
Consoli RAGB, Lourenço-de-Oliveira R (1994) Principais mosquitos de importância sanitária no Brasil. Fiocruz, Rio de Janeiro
Deane LM (1986) Malaria vectors in Brazil. Mem Inst Oswaldo Cruz 81:5–14. https://doi.org/10.1590/S0074-02761986000600002
Deane LM (1988) Malaria studies and control in Brazil. Am J Trop Med Hyg 38:223–230. https://doi.org/10.4269/ajtmh.1988.38.223
Deane LM, Deane MP, Neto JAF et al (1971) On the transmission of simian malaria in Brazil. Rev Inst Med Trop São Paulo 13:311–319
Demari-Silva B, Laporta GZ, Oliveira TMP et al (2020) Plasmodium infection in Kerteszia cruzii (Diptera: Culicidae) in the Atlantic tropical rain forest, southeastern Brazil. Infect Genet Evol 78:104061. https://doi.org/10.1016/j.meegid.2019.104061
Dorvillé LFM (1996) Mosquitoes as bioindicators of forest degradation in Southeastern Brazil, a statistical evaluation of published data in the literature. Stud Neotrop Fauna E 31:68–78. https://doi.org/10.1076/snfe.31.2.68.13331
Duarte AMR, Pereira DM, de Paula MB et al (2013) Natural infection in anopheline species and its implications for autochthonous malaria in the Atlantic Forest in Brazil. Parasit Vectors 6:58. https://doi.org/10.1186/1756-3305-6-58
Forattini OP, Lopes OS, Rabello EX (1968) Investigações sobre o comportamento de formas adultas de mosquitos silvestres no estado de São Paulo, Brazil. Rev Saúde Públ 2:111–173. https://doi.org/10.1590/S0034-89101968000200002
Guedes MLP, Navarro-Silva MA (2014) Mosquito community composition in dynamic landscapes from the Atlantic Forest biome (Diptera, Culicidae). Rev Bras Entomol 58:88–94. https://doi.org/10.1590/S0085-56262014000100014
Guimarães AÉ, Arlé M, Machado RNM (1985) Mosquitos no Parque Nacional da Serra dos Orgãos, Estado do Rio de Janeiro, Brasil: II. Distribuição Vertical. Mem Inst Oswaldo Cruz 80:171–185. https://doi.org/10.1590/S0074-02761985000200008
Guimarães AÉ, Mello RPD, Lopes CM et al (2000) Ecology of mosquitoes (Diptera: Culicidae) in areas of Serra do Mar State Park, State of São Paulo, Brazil. I-monthly frequency and climatic factors. Mem Inst Oswaldo Cruz 95:01–16. https://doi.org/10.1590/S0074-02762000000100001
Guimarães AÉ, Gentile C, Catarina ML et al (2001) Ecologia de mosquitos em áreas do Parque Nacional da Serra da Bocaina: II - Freqüência mensal e fatores climáticos. Rev Saúde Públ 35:392–399. https://doi.org/10.1590/S0034-89102001000400010
Instituto Brasileiro de Geografia e Estatística (2019) Área territorial brasileira. IBGE. https://www.ibge.gov.br/cidades-e-estados. Accessed 25 Nov 2020
Instituto Capixaba de Pesquisa, Assistência Técnica e Extensão Rural (2020) Programa de Assistência Técnica e Extensão Rural – PROATER 2020 – 2023. INCAPER. https://incaper.es.gov.br/proater. Accessed 23 Nov 2020
Instituto Jones dos Santos Neves (2020). Limites e Regionalizações – Divisão Regional do Espírito Santo. IJSN. http://www.ijsn.es.gov.br/mapas/. Accessed 25 Nov 2020
Laporta GZ, Burattini MN, Levy D et al (2015) Plasmodium falciparum in the southeastern Atlantic Forest: a challenge to the bromeliad-malaria paradigm? Malar J 14:181. https://doi.org/10.1186/s12936-015-0680-9
Loiola CCP, Silva CJ, Tauil PL (2002) Controle da malária no Brasil: 1965 a 2001. Rev Panam Salud Publica 11:235–244. https://doi.org/10.1590/S1020-49892002000400005
Marques TC, Bourke BP, Laporta GZ et al (2012) Mosquito (Diptera: Culicidae) assemblages associated with Nidularium and Vriesea bromeliads in Serra do Mar, Atlantic Forest, Brazil. Parasit Vectors 5:41. https://doi.org/10.1186/1756-3305-5-41
Medeiros-Sousa AR, de Oliveira CR, de Castro Duarte AMR et al (2019) Effects of anthropogenic landscape changes on the abundance and acrodendrophily of Anopheles (Kerteszia) cruzii, the main vector of malaria parasites in the Atlantic Forest in Brazil. Malar J 18:110. https://doi.org/10.1186/s12936-019-2744-8
Melo AS (2008) What do we win ‘confounding’ species richness and evenness in a diversity index? Biota Neotrop 8:21–27. https://doi.org/10.1590/S1676-06032008000300001
Pielou EC (1975) Ecological diversity. Wiley, New York
Pinotti M (1951) The biological basis for the campaign against the malaria vectors of Brazil. Trans R Soc Trop Med Hyg 44:663–682. https://doi.org/10.1016/0035-9203(51)90003-X
Reis M, Müller GA, Marcondes CB (2010) Inventário de mosquitos (Diptera: Culicidae) da Unidade de Conservação Ambiental Desterro, Ilha de Santa Catarina, Sul do Brasil. Biota Neotrop 10:333–337. https://doi.org/10.1590/S1676-06032010000300031
Rezende HR, Soares RM, Cerutti Junior C et al (2009) Entomological characterization and natural infection of anophelines in an area of the Atlantic Forest with autochthonous malaria cases in mountainous region of Espírito Santo State. Brazil Neotrop Entomol 38:272–280. https://doi.org/10.1590/S1519-566X2009000200017
Rezende HR, Falqueto A, Urbinatti PR et al (2013) Comparative study of distribution of anopheline vectors (Diptera: Culicidae) in areas with and without malaria transmission in the highlands of an extra-Amazonian region in Brazil. J Med Entomol 50:598–602. https://doi.org/10.1603/ME12085
Ribeiro MC, Metzger JP, Martensen AC et al (2009) The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol Cons 142:1141–1153. https://doi.org/10.1016/j.biocon.2009.02.021
Ribeiro AF, Urbinatti PR, de Castro Duarte AMR et al (2012) Mosquitoes in degraded and preserved areas of the Atlantic Forest and potential for vector-borne disease risk in the municipality of São Paulo, Brazil. J Vector Ecol 37:316–324. https://doi.org/10.1111/j.1948-7134.2012.00233.x
Secretaria do Meio Ambiente e Recursos Hídricos (2018) Atlas da Mata Atlântica do estado do Espírito Santo. SEAMA. https://seama.es.gov.br/atlas-da-mata-atlantica-es. Accessed 25 Nov 2020
Ueno HM, Forattini OP, Kakitani I (2007) Distribuição vertical e sazonal de Anopheles (Kerteszia) em Ilha Comprida, SP. Rev Saúde Públ 41:269–275. https://doi.org/10.1590/S0034-89102007000200014
World Health Organization (2017) Global vector control response 2017–2030. Geneva: World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/259205/9789241512978eng.pdf;jsessionid=D635DEF4975FDB33F49EA2F6951AE74B?sequence=1. Accessed 03 Jan 2021
World Health Organization (2020) World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization. https://www.who.int/docs/default-source/malaria/world-malaria-reports/9789240015791-double-page-view.pdf?sfvrsn=2c24349d_5. Accessed 03 Jan 2021
Funding
This research was supported by the Research and Innovation Support Foundation of Espírito Santo (FAPES-Brazil; grant number 344/2018). L.M.F. has a Coordination for the Improvement of Higher Education Personnel master’s scholarship (CAPES-Brazil; grant number 88882.385044/2019-01). J.C.B. has a Research and Innovation Support Foundation of Espírito Santo post-doctoral scholarship (FAPES-Brazil; grant number PROFIX 10/2018).
Author information
Authors and Affiliations
Contributions
L.M.F. and C.C.J. conceptualized the article; L.M.F., H.R.R., and L.S.d.S. performed the research; L.M.F. performed the data analysis; L.M.F. and C.C.J. drafted the article; A.M.R.d.C.D., B.F., C.C.J., H.R.R., J.C.B., and T.C.C.F. critically revised the article; all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Section Editor: Helge Kampen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferreira, L.M., Rezende, H.R., Buery, J.C. et al. Residual malaria of Atlantic Forest systems and the influence of anopheline fauna. Parasitol Res 120, 2759–2767 (2021). https://doi.org/10.1007/s00436-021-07238-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07238-0