Abstract
Schistosomiasis still affects a lot of people in many developing countries. Reducing the disease dissemination has been the target of various studies. As methyl gallate has antioxidant properties, it is assumed that it can be a good candidate for stimulating the immune response of snails. So, the aim of this work is to investigate the potential of using methyl gallate as an immunostimulant to Biomphalaria alexandrina snails in order to prevent the development of invading miracidia into infective cercariae. The infected snails were exposed to three concentrations of methyl gallate for two periods: 24 and 72 h. The results indicated that the most effective concentration was the lowest one: 125 mg/L of methyl gallate for 72 h, as it reduced both infection rate and mean number of shed cercariae. Also, it increased the total number of snails’ hemocytes in hemolymph, which were observed in head-foot region and digestive gland of treated snails surrounding degenerated sporocysts and cercariae. In addition, hydrogen peroxide showed its highest content in tissues of snails exposed to 125 mg/L of methyl gallate for 72 h. In conclusion, methyl gallate can be considered as one of the most promising immunostimulants of B. alexandrina snails against infection with Schistosoma mansoni.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis is a chronic parasitic disease caused by trematodes of the genus Schistosoma. It is the second most devastating disease in terms of morbidity and mortality in the world. It is affecting approximately 260 million people worldwide, and more than 90% of cases occur in the African region (WHO 2016), especially in poor communities lacking adequate sanitation (WHO 2013; Obare et al. 2016) and suffering low social and economic status (dos Santos et al. 2014). The parasite life cycle starts when Schistosoma eggs are deposited in water by an infected vertebrate host. The miracidium hatches from the egg and actively searches for its intermediate host (snails of the genus Biomphalaria) where it penetrates the tegument of the snail, preferentially targeting head-foot region; then, morphological and physiological changes occur in the miracidium, and it develops into a mother sporocyst. Two weeks after penetration, the mother sporocysts give birth to daughter sporocysts that migrate to the hepatopancreas (digestive gland) and reproductive organs. Approximately 30 days after this migration, the snails start to shed cercariae into the water, and these cercariae infect the vertebrate host (Nelwan 2019).
In Egypt, Biomphalaria alexandrina (Ehrenberg, 1831) is the main intermediate host of Schistosoma mansoni (Sambon, 1907) (Lotfy et al. 2005; Mohamed et al. 2012; Abou-El-Naga 2013). Recently, Haggag et al. (2017) reported that the average prevalence of S. mansoni infection in five Egyptian governorates of the Nile Delta region was relatively high as it equaled 10.7%. The conventional control programs of schistosomiasis include repeated mass chemotherapy using praziquantel, public health education focusing on behavior changes toward risk factors, improving sanitation, provision of clean water supply, and snail control (Kiros et al. 2014). Despite the availability of praziquantel as a treatment for schistosomiasis infection, there is no vaccine, and reinfection is common in areas where the parasite occurs (Wu and Halim 2000). Regarding snail control, chemical molluscicides are commonly applied, but they have many drawbacks such as the high cost, their negative impact on the environment, their toxicity to non-target organisms, and the possibility of emergence of snail resistance to these compounds (Molla et al. 2013). Therefore, researchers have tried another approach which focuses on reducing the numbers of shed cercariae by controlling transmission, and tackling the parasite development at the snail stage of its life cycle (King et al. 2006; Kjetland et al. 2006; Rollinson et al. 2009). Hence, the spread of infection can be stopped.
Despite the lack of an adaptive immune system, invertebrates respond to infection by activation of various defense mechanisms (Little et al. 2005). The Biomphalaria internal defense system is composed of soluble components of hemolymph and circulating cells, termed hemocytes, which work in association during the snail responses against infectious agents (Van der Knaap and Loker 1990). In snails, circulating hemocytes, especially the phagocytic cell population, are the principal line of cellular defense involved in destruction of S. mansoni larvae inside the intermediate host (Bayne et al. 1980; Negrão-Corrêa et al. 2007). Hemocyte activation also triggers the production of cytotoxic molecules, the best known being the reactive oxygen species (ROS) (Adema et al. 1994). It is known that hydrogen peroxide and nitric oxide are the species that mediate the killing of S. mansoni sporocysts (Hahn et al. 2001a, b). In this context, it is important to find substances which induce snails’ immunity to sequester and destroy as many invading miracidia as possible, besides hindering the development of those that escaped the action of hemocytes.
Methyl gallate is a phenolic compound which is a derivative of gallic acid. It was previously isolated from plants showing medicinal properties, e.g., Toona sureni (Ekaprasada et al. 2015) and Spondias pinnata (Chaudhuri et al. 2015). Also, it was identified and isolated from the filtrates of the fungi Penicillium janthinellum (Saad et al. 2016), Penicillium implicatum, Aspergillus niveus, and Aspergillus petrakii (Abdel-Wareth and Ghareeb 2018). Many studies showed its multiple biological activities as antioxidant, antiproliferative, and anticancer agent (Chaudhuri et al. 2015; Ekaprasada et al. 2010; Kamatham et al. 2015). Having antioxidant properties, we hypothesized that methyl gallate could stimulate the immune response of snails based on previous studies carried out on Punica granatum peels and Eucalyptus camaldulensis plant extract, where the ability of these plants to stimulate the immune response of snails was attributed to their antioxidant effects (Mossalem et al. 2017, 2018). So, the objective of the current study is to investigate the effectiveness of methyl gallate as an immunostimulant against S. mansoni infection. This will be achieved through studying the histopathological, hematological, and physiological responses of B. alexandrina snails treated with different concentrations of methyl gallate after their exposure to S. mansoni miracidia.
Materials and methods
Experimental materials
Methyl gallate which is also known as methyl 3,4,5-trihydroxybenzoate (CAS Number: 99-24-1) was purchased from Sigma-Aldrich CoTM. Its linear formula is (HO)3 C6H2 CO2 CH3, and its molecular weight is 184.15.
Snails
Laboratory-bred Biomphalaria alexandrina (Ehrenberg, 1831) snails (4–6 mm in shell diameter) were obtained from colonies maintained in the Medical Malacology Laboratory, Theodor Bilharz Research Institute (TBRI). They were kept in plastic aquaria (16 × 23 × 9 cm), provided with dechlorinated aerated tap water (as 10 snails/L), and covered with glass plates. Oven-dried lettuce leaves were used for feeding. Water in the aquaria was weekly changed, and photoperiodicity of 12 h light/12 h dark, besides water temperature of 25 ± 1 °C was adjusted. Dead snails were removed daily.
Schistosoma mansoni ova
The Egyptian strain of Schistosoma mansoni (Sambon, 1907) was obtained from Schistosome Biological Supply Centre (SBSC) at TBRI. Eggs and freshly hatched miracidia were collected according to the method of El-Sheikha et al. (2008), from the liver of ten CD-1TM mice livers infected 6–8 weeks earlier with 300 S. mansoni cercariae. About 200 mL of 0.85% saline solution was added to the minced tissue, and the suspension was homogenized for 5–10 s at very low speed using a warring blender. The homogenate was sieved using a tiered column of sieves arranged in a descending order of mesh opening: 420, 177, 105, and 45 μm. The eggs were washed through bottom sieve with 100 mL of 0.85% saline solution and rinsed with 100 mL of aerated tap water. The eggs were pipetted into a small 1.5 × 6 cm Petri dish and kept for hatching under ceiling illumination for about 5 min.
Exposure of snails to miracidia and methyl gallate
Three hundred fifty of Biomphalaria alexandrina snails (4–6 mm in shell diameter) were exposed individually to 8–10 miracidia of S. mansoni in multi-dish plates, filled with 2 mL dechlorinated tap water for 24 h (Anderson et al. 1982). After 24 h of the miracidial exposure, snails were divided into seven experimental groups; for each group, five replicates, each of 10 snails/L (three replicates for cercarial shedding and two replicates for hematological investigation), were prepared. The seven groups are as follows: group 1 represented infected snails without treatment (control), and groups 2, 3, and 4 were infected snails exposed to 125, 250, and 500 mg/L of methyl gallate, respectively for 24 h, while groups 5, 6, and 7 were infected snails exposed to 125, 250, and 500 mg/L of methyl gallate, respectively for 72 h. Figure 1 shows a flow chart of the experimental design.
Examination of snails for cercarial shedding
After 21 days post-infection, survived snails were individually examined for cercarial shedding in multi-dish plates. After 3 h of exposure to light (desk lamp) using 2 mL dechlorinated water for each snail/well, positive snails were removed, marked and transferred to clean aquaria with dechlorinated water, and maintained in the dark under laboratory conditions. Few drops of iodine solution were added to each well containing cercariae to count them under a stereomicroscope and record their number for each snail. This examination was carried out once weekly to avoid exhausting snails. The survival rate was calculated by dividing the number of snails at the first shedding on the total number of exposed snails at the beginning of the experiment. The snail’s infection rate was calculated at the end of the experiment by dividing the number of shedding snails on the number of survived exposed snails at the first shedding (Yousif et al. 1998).
Hemolymph collection
The infected snails’ shells from each experimental group were cleaned with 70% alcohol and dried, and the hemolymph was collected from at least 7–10 snails/group by a cardiac puncture using a 21-gauge needle (Martins-Souza et al. 2006). About 700 μL of hemolymph was collected in a 1.5-mL Eppendorf tube for the hematological examination.
Count and differentiation of hemocytes
For total hemocyte count, 20 μL of hemolymph was used, and the number of cells in each tested group was counted by diluting freshly collected hemolymph in leucocyte count solution of 1:20 ratio. Using a Bürker-Turk hemocytometer, the total hemocytes were counted in three replicates and the mean number of circulating hemocytes was calculated. For differential hemocyte investigation, hemocyte monolayers were prepared by placing 10 μL of hemolymph on a glass slide, and hemocytes were allowed to adhere to the slide in a moist chamber for 15 min at room temperature, then rinsed with Snail Wringer buffer/10 mMCa+2 (SR), pH 7.3, and incubated in the same buffer for 10 min (Zelck and Becker 1992). Hemocytes were dehydrated with methanol for 5 min at room temperature, rinsed several times with Snail Wringer buffer (SR), and stained with 10% Giemsa stain (Sigma-Aldrich CoTM) in buffered distilled water (0.021 M Na2HPO4/0.015 M KH2PO4) with pH 7.2 for 30 min (Barracco et al. 1993). Differential hemocyte counts were recorded in each treated and control group.
Histopathological examination
Infected B. alexandrina snails that were exposed to different concentrations of methyl gallate for 24 and 72 h were used for histopathological studies. After 4 weeks of recovery from methyl gallate exposure, randomly selected three to five snails from each experimental and control group were dissected. Each snail was carefully crushed between two microscopic slides, and the broken shell was pulled away from the body. The soft bodies of snails were fixed in Bouin’s solution for at least 24 h, placed in gradually increasing concentrations of ethanol, cleared with xylol, then embedded in paraffin, and finally sectioned at 6 μm. Sections were stained with hematoxylin and eosin stain and dried, and then, the slides were examined microscopically for the histopathological effects (Borges et al. 1998).
Hydrogen peroxide (H2O2) assay
After exposure of infected snails to methyl gallate for 24 and 72 h, the shells of treated and control snails were gently crushed between two glass slides, and the soft bodies of 3–5 snails from each experimental group were pooled in a 1-mL Eppendorf tube. Then, they are weighted and homogenized in 10% ice-cold (50 mM potassium phosphate buffer pH 7.5, 1 mM EDTA) using a glass homogenizer for 30 s. The homogenates were centrifuged at 4000 rpm for 15 min at 4 °C. The resultant supernatant was used for hydrogen peroxide (H2O2) assay according to Fossati et al. (1983). The reagents of H2O2 test were obtained from Biodiagnostic CompanyTM for Diagnostic and Research Reagents, Egypt.
Statistical analysis
Data of survival and infection rates were presented as percentages, and analyzed using chi-square values of contingency tables to determine the significant differences between the control and the experimental groups and between each two similar treatments after two periods. Data of cercarial shedding, total hemocyte count, and hydrogen peroxide content were analyzed using one-way ANOVA to determine the significant differences in means between the control and the experimental groups, and among the experimental groups at P < 0.05. All statistical analyses were performed by the SPSS computer program (version 20 for Windows).
Results
Survival rate, infection rate, and cercarial shedding
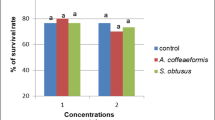
Generally, the survival rate showed no significant differences neither between control and treated groups (P > 0.05) in the same period, nor between each two similar concentrations in the two periods. Only the survival rate of snails exposed to 500 mg/L of methyl gallate for 72 h showed significant difference from control, and also from that of snails exposed to the same concentration after 24 h at P < 0.05 (Fig. 2).
Survival rate of infected Biomphalaria alexandrina snails treated with methyl gallate for 24 and 72 h post-infection (data are presented as percentages); different letters in each period indicate significance between the control and treatment groups at P < 0.05. The asterisk symbol (*) refers to significant difference at P < 0.05 between snails exposed to 500 mg/L of methyl gallate after 24 and 72 h, while no significant differences were observed between other treatments after 24 and 72 h at P > 0.05
Exposure of infected snails to different concentrations of methyl gallate for 24 and 72 h led to a significant reduction in their infection rate as compared to the control (P < 0.001). Moreover, it was observed that the effect of methyl gallate was more pronounced when snails were exposed to its concentrations for 72 h, where the lowest infection rate was observed in snails exposed to 250 mg/L of methyl gallate for 72 h (Fig. 3). Statistical analysis of the infection rate showed no significant differences in each group neither after 24 h nor after 72 h.
Infection rate of Biomphalaria alexandrina snails treated with methyl gallate for 24 and 72 h post-infection (data are presented as percentages); different letters in each period indicate significance between the control and treatment groups at P < 0.05. No significant differences were observed between each two similar treatments after 24 and 72 h at P > 0.05
On the other hand, along 5 weeks, the mean number of cercariae for all treated snails was significantly lower than that of the control (P < 0.05) (Fig. 4). It was observed that the least mean cercarial number was recorded in snails treated with 125 mg/L of methyl gallate for 72 h. Moreover, after 72 h, the mean number of cercariae in each group was significantly lower than that observed after 24 h of exposure at P < 0.05 (Fig. 4).
Mean number of cercariae/B. alexandrina snail exposed to methyl gallate for 24 and 72 h (data are presented as mean ± SE); different letters in each period indicate significance between the control and treatment groups at P <0.05, while similar letters indicate insignificant differences. The asterisk symbol (*) refers to significant difference at P < .05 between each two similar treatments after 24 and 72 h
Hematological investigation
Examination of the hemolymph of healthy B. alexandrina snails by light microscopy revealed the presence of three types of hemocytes. They are classified according to their shape and granular contents into granulocytes, hyalinocytes, and amoebocytes. Granulocytes are either with few granules or densely granulated. Hyalinocytes are characterized by their transparent cytoplasm, while amoebocytes appear with clear pseudopodia (Fig. 5a–d). When B. alexandrina snails were exposed to S. mansoni infection, some hemocytes were activated, as their cytoplasm became vacuolated, and they showed few pseudopodia and dense granules (Fig. 6).
Figure 7 shows high activation of hemocytes in infected B. alexandrina snails exposed to different concentrations of methyl gallate for 72 h, many vacuoles appeared in the cytoplasm of hemocytes, and many pseudopodia were observed, besides the formation of dense granules.
The results of differential hemocyte count indicated that the highest percentage among the three types of hemocytes was that of granulocytes in the control group, while in all treatment groups the percentages of granulocytes and amoebocytes were higher than that of hyalinocytes. On the other hand, hyalinocyte percentages were higher in snails exposed to low and medium concentrations of methyl gallate for 24 h (Table 1).
The total hemocyte count of the snail groups exposed to methyl gallate for 24 and 72 h significantly increased when compared to the control group at P < 0.05. The highest total hemocyte counts were in snails exposed to 125 mg/L of methyl gallate, as their values were 4933.3 and 7266.6/mm3 after 24 and 72 h, respectively (Fig. 8).
Total hemocyte count in infected Biomphalaria alexandrina snails exposed to methyl gallate for 24 and 72 h (data are presented as mean ± SE). The differences among groups were dependent on one-way ANOVA test. For each period, the same letters refer to insignificant results and the different letters refer to significant results at P < 0.05
Histopathological examination
The histological examination of normal infected snails shedding cercariae at the 4th week post-exposure to miracidia indicated the presence of large numbers of sporocysts with increasing size and number of dividing germ cells, in addition to the appearance of cercariae at different developmental stages. Each of sporocysts and developed cercariae was observed without evident cellular responses surrounding them. The highest number of sporocysts appeared in the digestive gland tissues (Fig. 9a–c).
Photomicrographs of transverse sections in the digestive gland of control infected Biomphalaria alexandrina snails showing a numerous multiplying sporocysts distributed between tubules (thin arrows) and differentiation of cercariae (thick arrow), b absence of tissue reaction around both the intact sporocysts rich in dividing germ cells (s) and immature cercariae (c), and c development of cercariae inside sporocysts into head (H) and tail (T)
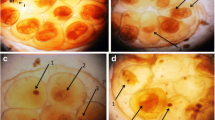
In contrast, in the infected snails treated with methyl gallate, most of sporocysts showed morphological damage. The degenerated sporocysts which were still settled in the head-foot region and tentacles due to retarded infection dynamics appeared as round eosinophilic masses; their teguments were destroyed and numerous hemocytes surrounded them (Fig. 10a). Formation of a granuloma around remnants of sporocysts was observed. This granulomatous tissue consists of fibrous cells and hemocytes (Fig. 10b). Also, a small number of host fibrous cells surrounded destroyed sporocysts as a thin layer (Fig. 10c). Moreover, a tissue reaction in the form of loose hemocyte-rich nodules in the cephalopodal tissues was detected (Fig. 10d).
Photomicrographs of transverse sections in the head-foot region of infected B. alexandrina snails exposed to 125 mg/L of methyl gallate for 72 h showing both parasites and tissue reaction. a Only one degenerated sporocyst (s) surrounded by numerous hemocytes (arrow). b A granuloma consists of fibrous cells (thick arrow) and hemocytes (thin arrows) around remnants of sporocysts. c Aggregation of layers of host fibrous cells (arrow) around degenerated sporocysts (s). d Loose hemocyte-rich nodules (arrows)
In addition, few numbers of sporocysts migrated to digestive gland tissues, as they appeared with few number of dividing germ cells. Also, few developmental stages of cercariae were detected. Moreover, strong tissue reactions were observed where hemocyte proliferation with focal thickening of the stroma was observed in the digestive gland tissues. Many dense granular amoebocytes aggregated around both disintegrated sporocysts and immature cercariae (Fig. 11a, b); also, phagocytic cells (macrophage-like amoebocytes) were observed (Fig. 11c). They varied in shape from round to fusiform, with little variation in size, and found to engulf small parts of dead sporocysts. Moreover, several dead sporocysts and highly affected cercariae were observed with different numbers of flattened hemocytes attached over their surfaces (Fig. 11d–f).
Photomicrographs of transverse sections in the digestive gland of infected B. alexandrina snails exposed to 125 mg/L of methyl gallate for 72 h showing a degenerated immature cercariae (c) surrounded by dense granular amoebocytes (arrow), b aggregation of host granulocytes (arrows) around both degenerated sporocysts (s) and immature cercariae (c), c phagocytic cell engulfed small part of dead sporocyst (arrow), d completely destroyed immature cercariae surrounded by hemocytes (arrows), e completely disintegrated sporocysts with large number of flattened hemocytes attached over their surfaces (arrows), and f highly affected cercariae (c) due to tissue reaction mediated by the hemocytes scattered upon their surfaces (arrow)
Hydrogen peroxide production
All treated snail groups showed significant increase in H2O2 content when compared to control group after 24 h of exposure to methyl gallate. Moreover, after 72 h of exposure to methyl gallate, a pronounced significant increase in H2O2 content was observed in the snail group exposed to 125 mg/L of methyl gallate, as it was 1.682 Mm/g (Table 2).
Discussion
As an alternative approach to control schistosomiasis, this study focuses on investigating the ability of a phenolic compound, methyl gallate, in raising the immunity of infected Biomphalaria alexandrina (Ehrenberg 1831) snails to reduce the numbers of shed cercariae. Three concentrations were tested for two periods of exposure: 24 and 72 h post-infection. The results showed that generally survival rate of treated snails was not significantly different neither from control in the same period, nor between the same treatment in different periods. Only the survival rate of snails exposed to 500 mg/L of methyl gallate for 72 h showed significant difference from control, and also from the same concentration after 24 h. These results match the findings of Saad et al. (2016), as they reported that LC50 of methyl gallate against B. alexandrina snails was 10,300 mg/L, and its sublethal concentrations which affect snails on prolonged exposure were 1000, 3000, and 5000 mg/L. This indicated that methyl gallate affects snails at concentrations higher than the concentrations investigated in the current work.
The present study demonstrated that the most effective concentrations in reducing infection rates of snails and mean number of cercariae were the lowest and the median concentrations: 125 and 250 mg/L of methyl gallate for 72 h, respectively. El Sayed et al. (2017) found a similar effect of sodium alginate, as exposure of B. alexandrina snails to its concentrations for different periods resulted in reduction of infection rate, where the most effective concentrations were the lowest (0.1 mg/mL) and the median (0.5 mg/mL) concentrations after 7 days of exposure. By the same token, Soliman et al. (2017) recorded a reduction of infection rate in B. alexandrina snails exposed to β-glucan, where the lowest concentration, 0.1 mg/mL, was the most effective after 3 days of exposure. In pharmacology, there is a term known as the effective dose (in vivo) or the effective concentration (in vitro); it is defined as the concentration of a drug that produces a biological response. It was also stated that any substance could be toxic at a high enough concentration, but efficacy is specific to a certain concentration (Rang et al. 2015). So, in the present work, the low and medium concentrations of methyl gallate can be considered the effective ones rather than the higher concentration. The observed activity of methyl gallate in the current study coincides with the results of Mossalem and Mossa (2014), as they recorded a decrease in infection rate of B. alexandrina when treated with rice bran methanol extract, and demonstrated that it had high total phenolic content. Similarly, Mossalem et al. (2018) reported a pronounced reduction in infection rate of B. alexandrina snails treated with ethyl acetate extract of Eucalyptus camaldulensis plant, and they attributed that to the high antioxidant activity of the plant which is rich in phenolic compounds.
The immune system of Biomphalaria is composed of cellular and humoral components acting independently or together to fight invading microbes or parasites (Coustau et al. 2015). The first line of defense is mediated by circulating hemocytes found in the hemolymph of the snail. These cells have an important role in phagocytosis and encapsulation reactions. Many hematological studies have been carried out to evaluate the effectiveness of potential immunostimulants (Sritunyalucksana et al. 1999; El Sayed et al. 2011; Mossalem et al. 2017). In the present work, hematological examination of snails’ hemolymph showed the formation of vacuoles in hemocytes of infected B. alexandrina snails exposed to the tested concentrations of methyl gallate, in addition to the appearance of many pseudopodia, and the formation of dense granules. Generally, it was claimed that formation of vacuoles and pseudopodia in hemocytes of infected B. alexandrina snails might be a pathological response (Bakry 2009). The observed effect of methyl gallate on hemocytes matches the findings of Ibrahim and Abdel-Tawab (2020), as they demonstrated that exposure of B. alexandrina snails to LC50 of Cystosiera barbata algal extract resulted in irregular cell membrane and formation of pseudopodia in the hemocytes, and they also mentioned that the tested algal extract had high phenolic content. Similarly, Helal et al. (2003) observed many cytoplasmic inclusions and long spike-like pseudopodia in hemocytes of B. alexandrina snails treated with 20 ppm of Euphorbia peplus water extract. Moreover, Mossalem and Mossa (2014) postulated that exposure of B. alexandrina snails to rice bran extract before, during, and after infection activated their hemocytes and resulted in altering their shape, and formation of vacuoles and pseudopodia.
The present study showed that the hemolymph of B. alexandrina snails contains three types of hemocytes: granulocytes, hyalinocytes, and amoebocytes. This classification is in accordance with that reported by El Sayed et al. (2011), Mossalem et al. (2017), and Bahgat et al. (2018).
The current results indicated that the highest percentage among the three types of hemocytes in the control snails was that of granulocytes. It was declared that granulocytes are the primary hemocyte type in contact with the sporocyst, as they are involved in parasite encapsulation (Bayne et al. 1980; Loker et al. 1982). Regarding the snails treated with methyl gallate, it was found that the percentages of granulocytes and amoebocytes were higher than that of hyalinocytes. The current result agrees with Sparks (1972) who found that the successful elimination of potential infective agents requires granulocytes and amoebocytes to engulf particles and further eliminate living pathogens through enzymatic or oxidative degradation. Also, Barçante et al. (2012) mentioned that granulocytes are the most abundant cell type and perform the most phagocytosis. Moreover, Feng et al. (1971) reported that the dense granules found in granulocytes could be considered as lysosomes containing hydrolase enzymes that are responsible for intracellular digestion of extracellular substances. In the same vein, El Sayed et al. (2017) and Saad et al. (2017) reported an increase of granulocytes as compared to other hemocyte types in infected snails. In addition, Loker et al. (2004) and Yoshino and Coustau (2011) found that the first line of defense in Biomphalaria snails was mediated by circulating phagocytic cells known as amoebocytes, and these cells had an important role in phagocytosis and encapsulation reactions.
The current results indicated that hyalinocyte percentages were higher in snails exposed to low and medium concentrations of methyl gallate for 24 h. Sparks (1972) claimed that hyalinocytes were responsible primarily for wound repair, as they aggregate at the injury site. Also, hyalinocytes were reported to be unable to adhere to substrates or emit pseudopods on the contrary to granulocytes (Barracco et al. 1993; Bezerra et al. 2003; Helal et al. 2014).
The total hemocyte count in the current work was significantly higher in infected snails exposed to methyl gallate than control. Also, it showed its highest values in snails exposed to 125 mg/L of methyl gallate. This may be due to the flow of the originated hemocytes with the hemolymph to concentrate themselves at locations of infection (Souza and Andrade 2012). It was also mentioned that maintaining sufficient hemocyte numbers is an important element of the snail immune response, as it represents the driving force behind successful immune reaction. In this way, a higher concentration of hemocytes was observed in the hemolymph of treated snails. This means that methyl gallate stimulated amoebocytes producing organs to synthesize more hemocytes. El Sayed et al. (2017) reported a similar increase in total hemocyte count for B. alexandrina snails treated with 0.5 mg/mL of sodium alginate. In the same vein, Mossalem et al. (2017) noticed a significant increase in the hemocyte numbers following exposure of infected B. alexandrina snails to Punica granatum peels extract, and that was attributed to enhancing defense mechanisms such as encapsulation and phagocytosis of the parasite carried out by hemocytes. Furthermore, it was noticed that hemocyte count in uninfected B. alexandrina snails treated with sodium alginate was higher than that of untreated snails (El Sayed et al. 2017). It was also demonstrated that increased number of hemocytes indicated low susceptibility (Saad et al. 2017). These findings are in line with the present results, as exposure of snails to methyl gallate stimulated their immune response, the effect which was mirrored in the higher number of hemocytes.
The histological examination of infected snails treated with methyl gallate showed that the tissue reaction which indicated the immune response of snails might be happened by two different mechanisms, an early and a delayed one. In the present work, an early defense mechanism was observed, the one that initiated direct miracidial destruction soon after their penetration. In this case, the intense hemocyte aggregation and the hemocyte-rich nodules were found at the site of the penetration of the miracidia. This was observed as degenerated sporocysts in the head-foot region with adjacent numerous hemocytes. Similarly, in Biomphalaria tenagophila, a diffuse and focal hemocytic infiltration was observed in the cephalopodal tissue of the infected highly resistant snails, and was found to be associated with rapid parasite destruction after penetration (Negrão-Corrêaet al. 2007). In the same vein, Abou-El-Naga and Radwan (2012) found that the histopathological response of infected B. alexandrina snails which were not able to shed cercariae was characterized by intense diffuse hemocytic reaction at the penetrating sites of miracidia, and formation of hemocyte-rich nodules in the cephalopodal tissues, in addition to appearance of dead parasites surrounded by several layers of flattened hemocytes. These effects coincide with those reported by Hussein et al. (2016), as they found that exposure of infected B. alexandrina snails to 1/4 LC50 of inorganic fertilizers enhanced the tissue response represented by some hemocytes, which disseminated around many mother sporocysts trying to destroy them, besides damaging the teguments of sporocysts. Similarly, Osman et al. (2014) demonstrated that the treatment with Mirazid combined with S. mansoni infection delayed sporocyst maturation in B. alexandrina snails, and resulted in formation of vacuoles. The second type of immune defense mechanisms was observed in the digestive gland tissues. It was demonstrated as hemocyte proliferation with focal thickening of the stroma, besides aggregation of many dense granular amoebocytes around both disintegrated sporocysts and immature cercariae. Borges et al. (1998) considered these reactions as a delayed development of resistance that happened after spread of sporocysts in the snail tissues. They considered this delayed resistance an alternative sort of host internal defense mechanism against S. mansoni miracidia. Moreover, Abdel-Wareth and Ghareeb (2018) found that methyl gallate was effective on miracidia and cercariae of Schistosoma mansoni (in vitro) at relatively high concentrations.
A comparison between the histopathology of susceptible and refractory Biomphalaria spp. showed massive proliferation of amoebocytes, with encapsulation and destruction of sporocysts, besides encircling differentiated cercariae by strong stromal reactions in refractory species (Souza et al. 1997). These findings are in line with the current effects of methyl gallate which confirms the potential of using it as an immunostimulant.
Regarding physiological response, it was observed that all infected snail groups treated with methyl gallate showed significant increase in H2O2 content when compared to the control group after 24 h of exposure to methyl gallate. Moreover, after 72 h of exposure to methyl gallate, an increase in H2O2 content was observed in all snails including control ones, and this can be explained in the light of the role of H2O2 in killing S. mansoni sporocysts (Humphries and Yoshino 2008), as its content increases in infected tissues of snails as the time extends to counteract the effect caused by the invading miracidia. Moreover, the maximum increase in H2O2 content was recorded in snails exposed to 125 mg/L of methyl gallate after 72 h, and this reflects the stimulation of snails’ immunity by exposure to methyl gallate. In the same vein, it was found that H2O2 production by B. glabrata hemocytes has been associated with resistance to S. mansoni (Adema et al. 1994). Moreover, it was shown that inhibition of H2O2 production favors sporocyst survival, indicating that this reactive species would be toxic to trematode larvae (Hahn et al. 2001a, b). Many researchers have postulated that hydrogen peroxide is the main reactive oxygen species involved in killing S. mansoni sporocysts (Hahn et al. 2000; Humphries and Yoshino 2008). Additionally, Bender et al. (2005) investigated the difference between hemocytes from resistant and susceptible strains in their capacity to produce extracellular H2O2, and found that the rate of H2O2 production by resistant hemocytes was significantly higher than that of susceptible ones. The present results match these findings as the highest total hemocyte count and the highest level of H2O2 production were observed in the infected snails exposed to 125 mg/L of methyl gallate. This indicated that this treatment was effective in increasing the ability of snails to overcome the invading parasite, and ensuring its success in killing sporocysts. All in all, the low infection rate and the less cercarial output observed in the current work were a reflection of what has occurred inside the tissues of snails treated with methyl gallate, specially the lowest concentration. Aggregation of hemocytes around sporocysts and destroying them by both encapsulation and production of hydrogen peroxide were recorded in the sections of head-foot region and digestive gland, and also, it was declared by the high H2O2 content in tissues of treated snails.
It is worth mentioning that methyl gallate was previously tested against Daphnia pulex to determine its toxicity to aquatic fauna, and the results indicated that it is environmentally safe, as its high concentrations, 1000 and 3000 mg/L, did not kill any of exposed Daphnia (Saad et al. 2016).
Conclusion
Immunostimulants are critical in eliciting immune responses of aquatic organisms and enhancing their resistance to diseases. Methyl gallate which is a phenolic compound naturally produced by many plants and fungal species is capable of increasing immunity of B. alexandrina to S. mansoni infection at 125 mg/L and 250 mg/L for 72 h. This was reflected in hematological, histopathological, and physiological responses of treated snails. Methyl gallate represents a good candidate as an immunostimulant, with the advantages of being a natural compound, environmentally friendly, and effective in a small concentration. Further studies on immunostimulants in the aquatic system should be considered, as they can be applied in a small scale such as the controlled semi-field conditions.
References
Abdel-Wareth MTA, Ghareeb MA (2018) Bioprospecting certain freshwater-derived fungi for phenolic compounds with special emphasis on antimicrobial and larvicidal activity of methyl gallate and p-coumaric acid. Egypt J Chem 61(5):773–784
Abou-El-Naga IF (2013) Biomphalaria alexandrina in Egypt: past, present and future. J Biosci 38:665–672. https://doi.org/10.1007/s12038-013-9329-4
Abou-El-Naga IF, Radwan EH (2012) Defense response of susceptible and resistant Biomphalaria alexandrina snails against Schistosoma mansoni infection. Revista de biología tropical 60(3):1195–1204
Adema CM, van Deutekom-Mulder EC, Van Der Knaap WPW, Sminia T (1994) Schistosomicidal activities of Lymnaea stagnalis haemocytes: the role of oxygen radicals. Parasitol 109(4):479–485
Anderson RM, Mercer JG, Wilson RA, Carter NP (1982) Transmission of Schistosoma mansoni from man to snail: experimental studies of miracidial survival and infectivity in relation to larval age, water temperature, host size and host age. Parasitol 85:339–360
Bahgat DM, Mossalem HS, Al-Sayed E, Eldahshan OA, Singab AN, Abu El Einin H (2018) Influence of saponin fraction from Albizia anthelmintica on Biomphalaria alexandrina snail; the intermediate host of Schistosoma mansoni in Egypt. Egypt J Aqua Biol Fish 22(5):231–240
Bakry FA (2009) Effect of infection with Schistosoma mansoni on some biological parameters in Biomphalaria alexandrina snails. Amer Eur J Sci Res 4(3):180–190
Barçante TA, Barçante JMP, Fujiwara RT, Lima WS (2012) Analysis ofcirculating haemocytes from Biomphalaria glabrata following Angiostrongylus vasorum infection using flow cytometry. J Parasitol Res https://doi.org/10.1155/2012/314723
Barracco MA, Steil AA, Gargioni R (1993) Morphological characterization of the hemocytes of the pulmonate snail Biomphalaria tenagophila. Memórias do Instituto Oswaldo Cruz 88(1):73–83
Bayne CJ, Buckley PM, DeWan PC (1980) Macrophagelike hemocytes of resistant Biomphalaria glabrataare cytotoxic for sporocysts of Schistosoma mansoni in vitro. J Parasitol 66:413–419
Bender RC, Broderick EJ, Goodall CP, Bayne CJ (2005) Respiratory burst of Biomphalaria glabrata hemocytes: Schistosoma mansoni–resistant snails produce more extracellular H2O2 than susceptible snails. J Parasitol 91(2):275–279
Bezerra FSM, Nogueira-Machado JA, Chaves MM, Correa RF, Coelho PMZ (2003) Effect of gamma radiation on the activity of hemocytes and the course of Schistosoma mansoni in resistant Biomphalaria glabrata. Mem. Inst Oswaldo Cruz 98:73–75
Borges CM, Souza CP, Andrade ZA (1998) Histopathological features associated with susceptibility and resistance of Biomphalaria snails to infection with Schistosoma mansoni. Mem Inst Oswaldo Cruz 93:117–121
Chaudhuri D, Ghate NB, Singh SS, Mandal N (2015) Methyl gallate isolated from Spondias pinnata exhibits anticancer activity against human glioblastoma by induction of apoptosis and sustained extracellular signal-regulated kinase 1/2 activation. Pharmaco Magaz 11:269–277
Coustau C, Gourbal B, Duval D, Yoshino TP, Adema CM, Mitta G (2015) Advances in gastropod immunity from the study of the interaction between the snail Biomphalaria glabrata and its parasites: a review of research progress over the last decade. Fish Shellfish Immunol 46(1):5–16
dos Santos AF, Fonseca SA, César FA, Albuquerque MC, Santana JV, Santana AE (2014) A penta-substituted pyridine alkaloid from the rhizome of Jatropha elliptica (Pohl) Muell. Arg. is active against Schistosoma mansoni and Biomphalaria glabrata. Parasitol Res 013:37–43
Ekaprasada MT, Nurdin H, Ibrahim S, Dachriyanus D (2010) Antioxidant activity of methyl gallate isolated from the leaves of Toona sureni. Indonesian J Chem 9:457–460
Ekaprasada MT, Nurdin H, Ibrahim S, Dachriyanus D (2015) Antibacterial activity of methyl gallate isolated from the leaves of Toona sureni. Int J Adv Sci Engineer Info Technol 5:280–282
El Sayed KA, Mahmoud MB, Mossalem HS (2011) Cryptostegia grandiflora affecting compatibility of Biomphalaria alexandrina and Biomphalaria glabrata to infection with Schistosoma mansoni with emphasis on some hematological effects. Aust J Basic Appl Sci 5(12):3357–3365
El Sayed K, Soliman MG, Elfekky FA, Ouf NAA, Gad RM (2017) Susceptibility of Biomphalaria alexandrina snails to infection with Schistosoma mansoni miracidia under the effect of sodium alginates as immunostimulant. Europ J Biom 4(05):115–123
El-Sheikha HM, Hussein HS, Rahbar MH (2008) Clinico-pathological effects of Schistosoma mansoni infection associated with simultaneous exposure to malathion in Swiss outbred albino mice. Acta Trop 108:11–19
Feng SY, Feng JS, Burke CN, Khairallah LH (1971) Light and electron microscopy of the leucocytes of Crassostrea virginica (Mollusca: Pelecypoda). Z Zellforsch 120:222–245
Fossati P, Prencipe L, Berti G (1983) Enzymic creatinine assay: a new colorimetric method based on hydrogen peroxide measurement. Clin Chem 29(8):1494–1496
Haggag AA, Rabiee A, Elaziz KMA, Gabrielli AF, Hay RA, Ramzy RM (2017) Mapping of Schistosoma mansoni in the Nile Delta, Egypt: assessment of the prevalence by the circulating cathodic antigen urine assay. Acta Trop 167: 9–17. 10.1016/j. actatropica.2016.11.038
Hahn UK, Bender RC, Bayne CJ (2000) Production of reactive oxygen species by hemocytes of Biomphalaria glabrata: carbohydrate-specific stimulation. Developm Comp Immunol 24(6-7):531–541
Hahn UK, Bender RC, Bayne CJ (2001a) Killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata: role of reactive oxygen species. J Parasitol 87(2):292–299
Hahn UK, Bender RC, Bayne CJ (2001b) Involvement of nitric oxide in killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata. J Parasitol 87(4):778–785
Helal I, EL Mehlawy M, Rizk E, El-Khodary G (2003) Effect of Euphorbia peplus plant extract and the antihelmenthic prazequantel on the defence system of Biomphalaria alexandria snail. Egypt J Aqua Biol Fish 7(4):501–527
Helal EG, El-Dafrawy SM, Mohamed AH, Abou-El-Nour BM, Ibrahim S (2014) Ultrastructural study on Biomphalaria alexandrina hemocytes infected with Schistosoma mansoni in Egypt and its correlation with nitric oxide level. J Egypt Soc Parasitol 44:113–124
Humphries JE, Yoshino TP (2008) Regulation of hydrogen peroxide release in circulatinghemocytes of the planorbid snail Biomphalaria glabrata. Develop Comp Immunol 32:554–562. https://doi.org/10.1016/j.dci.2007.09.001
Hussein RM, Marie MAS, El-Deeb FAA, Hasheesh W, Sayed SSM (2016) Effects of three inorganic fertilizers on the biology and histopathology of infected Biomphalaria alexandrina snails. Res J Pharm Biol Chem Sci 7:2564–2574
Ibrahim AM, Abdel-Tawab H (2020) Cystoseira barbata marine algae have a molluscicidal activity against Biomphalaria alexandrina snails supported by scanning electron microscopy, hematological and histopathological alterations, and larvicidal activity against the infective stages of Schistosoma mansoni. Biologia 1-10.
Kamatham S, Kumar N, Gudipalli P (2015) Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol Rep 2:520–529
King CH, Sturrock RF, Kariuki HC, Hamburger J (2006) Transmission control for schistosomiasis-why it matters now. Trends Parasitol 22:575–582
Kiros G, Erko B, Giday M, Mekonnen Y (2014) Laboratory assessment of molluscicidal and cercariacidal effects of Glinus lotoides fruits. BMC Res Notes 7:220–227
Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N (2006) Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 20:593–600
Little J, Hultmark D, Read AF (2005) Invertebrate immunity and the limits of mechanistic immunology. Nat. Immunol. 6:651–654
Loker ES, Bayne CJ, Buckley PM, Kruse KT (1982) Ultrastructure of encapsulation of Schistosoma mansoni mother sporocysts by hemocytes of juveniles of the 10-R2 strain of Biomphalaria glabrata. J parasitol 68:84–94
Loker ES, Adema CM, Zhang SM, Kepler TB (2004) Invertebrate immune systems – not homogeneous, not simple, not well understood. Immunol Rev 198:10–24
Lotfy WM, DeJong RJ, Abdel Kader AA, Loker ES (2005) A molecular survey of Biomphalaria in Egypt: is B. glabrata present? Amer. J. Trop. Med. Hyg. 73:131–139
Martins-Souza RL, Pereira CA, Martins Filho OA, Coelho PMZ, Correa A (2006) Differential lectin labeling of circulating hemocyte from Biomphalaria glabrata and Biomphalaria tenagophila resistant or susceptible to Schistosoma mansoni infection. Mem Inst Oswaldo Cruz 101:185–192
Mohamed AH, Sharaf El-Din AT, Mohamed AM, Habib MR (2012) The relationship between genetic variability and the susceptibility of Biomphalaria alexandrina snails to Schistosoma mansoni infection. Mem. Inst. Oswaldo Cruz 107: 326–337. 10.1590/S0074-02762012000300006
Molla E, Giday M, Erko B (2013) Laboratory assessment of the molluscicidal and cercariacidal activities of Balanites aegyptiaca. Asian Pac J Trop Biomed 3:657–662
Mossalem HS, Mossa ATH (2014) Effect of rice bran extract on immunological and physiological parameters of Biomphalaria alexandrina snails infected with Schistosoma mansoni. Afr J Pharm Pharmacol 8(22):621–628
Mossalem HS, Ghareeb MA, Refahy LA, Mohamed AS, Habib MR (2017) Gas chromatography-mass spectrometry analysis and antioxidant activity of Punica granatum L. peels and its role as immunostimulant against Schistosoma mansoni infection in Biomphalaria alexandrina. GAS, 10 (1)
Mossalem HS, Habib MR, Ghareeb MA (2018) Control of infection of Biomphalaria alexandrina (Ehrenberg, 1831) with Schistosoma mansoni Sambon, 1907 using Eucalyptus camaldulensis. Folia Malacologica 26(3):155–165
Negrão-Corrêa D, Pereira CAJ, Rosa FM, Martins-Souza RL, Andrade ZA, Coelho PMZ (2007) Molluscan response to parasite, Biomphalaria and Schistosoma mansoni interaction. Inverteb Surv J 4:101–111
Nelwan M. L. (2019) Schistosomiasis: life cycle, diagnosis, and control. Curr Ther Res Clin Exp 91: 5–9. 10.1016/j.curtheres.2019.06.001
Obare B, Yole D, Nonoh J, Lwande W (2016) Evaluation of cercaricidal and miracicidal activity of selected plant extracts against larval stages of Schistosoma mansoni. JNSR 6(22):24–31
Osman GY, Mohamed AZ, Sheir KS, Hassab EL, Nabi SE, Allam AS (2014) Molluscidal activity of Mirazid on Biomphalaria alexandrina snails: biological and molecular studies. Inter J Adv Res 2(2):977–989
Rang HP, Dale MM, Flower RJ, Henderson G (2015) Rang and Dale's pharmacology (Eighth ed.). United Kingdom: Elsevier Churchill Livingstone.
Rollinson D, Webster JP, Webster B, Nyakaana S, Jorgensen A (2009) Genetic diversity of schistosomes and snails: implications for control. Parasitol:1–11
Saad AA, Khalil MT, Ragab FM, Mekawey AA, Abdel-Wareth MTA (2016) Separation of a compound effective against Biomphalaria alexandrina snails from the filtrate of Penicillium janthinellum. Inter J Environ Stud 73:1–17
Saad AH, El Einin HA, Abdel-Gaber R, Ayoub M, Mansour S (2017) Assessment of Bulinus truncatus immune response against Schistosoma haematobium infection by tissue reaction and hemocytes count. Res J Pharm Biol Chem Sci 8(3):1186–1196
Soliman MG, El Sayed K, Abou Ouf NA, El Fekky FAEH, Gad RM (2017) Influence of immunostimulatory β-glucan on Biomphalaria alexandrina snails under laboratory and simulated field conditions. Europ J Biom 4(12):41–50
Souza SD, Andrade ZA (2012) The significance of the amoebocyte-producing organ in Biomphalaria glabrata. Mem Inst Oswaldo Cruz 107(5):598–603
Souza CPD, Borges CC, Santana AG, Andrade ZA (1997) Comparative histopathology of Biomphalaria glabrata, B. tenagophila and B. straminea with variable degrees of resistance to Schistosoma mansoni miracidia. Memórias do Instituto Oswaldo Cruz 92: 517-522.
Sparks AK (1972) Invertebrate pathology: noncommunicable diseases. (1st eds), Academic Press, New York, USA
Sritunyalucksana K, Sithisarn P, Withayachumnarnkul B, Flegel TW (1999) Activation of prophenoloxidase, agglutinin and antibacterial activity in haemolymph of the black tiger prawn, Penaeus monodon, by immunostimulants. Fish Shellfish Immunol 9(1):21–30
Van der Knaap WPW, Loker ES (1990) Immune mechanisms in trematode-snail interactions. Parasitol Today 6(6):175–182
WHO (2013) World Health Organization. Schistosomiasis: progress report 2001–2011 and strategic plan 2012–2020. Geneva, Switzerland
WHO (2016) World Health Organization. Schistosomiasis: number of people treated worldwide in 2014. Weekly Epidemiological Record 91: 53–60. http://www.who.int/wer/2016/wer9105.
Wu GY, Halim MH (2000) Schistosomiasis: progress and problems. World J Gastroenterol 6(1):12–19. https://doi.org/10.3748/wjg.v6.i1.12
Yoshino TP, Coustau C (2011) Immunobiology of Biomphalaria-trematode interactions. In: Biomphalaria snails and larval trematodes. New York: Springer 159-89.
Yousif F, Ibrahim A, El-Bardicy SN (1998) Compatibility of Biomphalaria alexandrina, Biomphalaria glabrata and a hybrid of both to seven strains of Schistosoma mansoni from Egypt. J Egypt Soc Parasitol 28:863–881
Zelck U, Becker W (1992) Biomphalaria glabrata: influence of calcium, lectins, and plasma factors on in vitro phagocytic behavior of hemocytes of noninfected or Schistosoma mansoni-infected snails. Exper parasitol 75(1):126–136
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national, and international guidelines for the care and use of animals were followed.
Additional information
Section Editor: Christoph G. Grevelding
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mansour, S.M., Sayed, S.S.M. & Abdel-Wareth, M.T.A. Effect of methyl gallate on immune response of Biomphalaria alexandrina (Ehrenberg, 1831) snails to infection with Schistosoma mansoni (Sambon, 1907). Parasitol Res 120, 1011–1023 (2021). https://doi.org/10.1007/s00436-020-07037-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-07037-z