Abstract
This study evaluated the technology of detection of Giardia spp. cysts and Cryptosporidium spp. oocysts in environmental matrices obtained after water treatment on a bench scale. Calcium carbonate flocculation with immunomagnetic separation was the selected method to quantify the protozoa, and the importance of the number of acid dissociations in the immunomagnetic separation was assessed. When adding the third acid dissociation, an increase of 71% ± 6 in floated residue and 31.9% ± 28.7 in filter backwash water in cyst recovery was observed, while in oocyst recovery, a non-significant increase was detected. In the filtered water, this increased dissociation was important in the protozoa recovery with increases greater than 33%. The results showed that there is a strong interaction of these target organisms with the magnetic microspheres, since protozoa were still recovered in the third acid dissociation and some of them were still adhered to the magnetic microspheres.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water quality degradation in supply sources worldwide has increased the presence of pathogenic protozoa, such as Giardia spp. and Cryptosporidium spp., causing serious public health implications. Outbreaks of cryptosporidiosis and giardiasis have been reported in various countries (Karanis et al. 2007; Baldursson and Karanis 2011; Rosado-García et al. 2017; Efstratiou et al. 2017a), and events of this type may occur due to faults in water supply and treatment systems (Daly et al. 2010; Cho et al. 2013; Efstratiou et al. 2017a). Such problems with piped water supply can lead governments to rely on bottled and packaged water as a solution for human consumption; nonetheless, challenges in management and social inequalities are generated (Kooy and Walter 2019).
Giardia and Cryptosporidium appear in the water ecosystems in their resistance forms in the cyst and oocyst stages, respectively, and can cause enteric infections. Although these parasites require a host to maintain their life cycles, Koh et al. (2013) demonstrated that Cryptosporidium can reproduce in aquatic biofilms, causing new and serious implications in the sanitation field worldwide, since there is a risk of increased water-borne outbreaks. Furthermore, these protozoa can survive for months in soil and water (Walker et al. 1998; Olson et al. 1999); they are resistant to the action of disinfectants commonly used in water treatment plants (WTP) (Korich et al. 1990) and can pass through WTP filters due to the small size (Andreoli and Sabogal-Paz 2019).
The challenge in setting up surveillance systems for these protozoa starts with the available analytical methods, which are expensive and also have a high variability and low reproductivity, as well as a delayed response and require specialized technical personnel. For example, the reagents used in the tests, such as the Dynabeads® kit (Dynabeads® Life Technologies AS, Oslo) sufficient for only 50 samples, can cost, on average, U$5000 in countries such as Brazil (August, 2019 price). These and other products used in detection methods are considered foreign in Latin American countries. In accredited laboratories, these materials can take up to 3 months to be purchased. Furthermore, some of them, such as EasySeed™ (BTF) or ColorSeed™ (BTF), can arrive at the laboratories a few days before the expiry date, which is a difficult aspect for the analysis organization.
The US Environmental Protection Agency Method 1623.1 (USEPA 2012) is usually used for protozoa detection in water. However, applications are infeasible in some countries due to the high cost and technical and analytical complexity. Efstratiou et al. (2017b) observed that since 2001, Method 1623 has been adopted in almost one third of the publications on Cryptosporidium and Giardia monitoring in water worldwide. However, only 5% of these publications come from Latin American and African countries. Therefore, the study of simplified protocols adapted to the local conditions of these countries and that meet the criteria of Method 1623.1 can provide subsidies to health surveillance agencies concerning the estimation and prevention of waterborne diseases.
Protozoa detection methods in environmental samples involve concentration (e.g. filtration or flocculation), purification such as immunomagnetic separation (IMS), and enumeration such as fluorescein isothiocyanate (FITC) with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) staining and differential interference contrast (DIC). In the IMS, magnetic microspheres containing antibodies link to target organisms and separate them from the debris (e.g. Dynabeads kit), and thermal or acid dissociation may be used to separate the microsphere-protozoa aggregate. Two acid dissociations in the IMS are recommended by USEPA (2012); nevertheless, parasite recovery increase with more acid dissociations has not been widely explored for both protozoa in the scientific literature. Regarding concentration methods, calcium carbonate flocculation (CCF), developed by Vesey et al. (1993), has been used in environmental samples with protozoa in conjunction with IMS (Feng et al. 2011; Andreoli and Sabogal-Paz 2019). CCF has been efficient in turbid matrices (Feng et al. 2011).
Drinking water treatment through flotation is usually used to purify water from rivers with high values of colour and protozoa (Schofield 2001). Such treatment can be reproduced in laboratory scale by dissolved air flotation (DAF) jar test, and the treated water and sludge samples may contain protozoa (Andreoli and Sabogal-Paz 2019).
In this context, the authors in this manuscript evaluated CCF with IMS performance with two and three acid dissociations in environmental samples (filtered water, floated residue and filter backwash water) obtained after water treatment on a bench scale to confirm if increased dissociations might improve the protozoa recovery.
Materials and methods
Water preparation
The research is divided into three steps (1 to 3), according to Fig. 1. Step 1 consisted of preparing the studied water adding kaolinite and humic acid to the water of a well (without the presence of protozoa) in the proportion of 0.09 g L−1 and 0.01 g L−1, respectively. This water was prepared with the aim of eliminating possible interferences inherent to the natural samples, as the main objective was to evaluate the performance of the protocol to detect protozoa.
DAF jar test and quality assays
In Step 2, assays were performed in a DAF jar test to optimize the parameters associated with the treatment (coagulation and flocculation conditions, flotation time and recirculation rate), and analytical quality assays were carried out in the studied water to evaluate the recoveries of the CCF with the IMS method.
Polyaluminium chloride content Al2O3 (17.66%) was used as coagulant in the assays of the DAF jar test. In the filtration, laboratory filters filled with sand (grains between 0.30 and 0.59 mm and effective size of 0.42 mm) were used, which were operated with a rate flow of 100 m3 m−2d−1. Several assays were carried out testing different parameters of treatability (coagulant dosage, velocity gradient in rapid mixing, rapid mixing time, velocity gradient for flocculation, flocculation time, flotation time and recirculation rate) until obtaining the lowest values of colour and turbidity of the treated water. Samples of filtered water, floated residue and filter backwash water were characterized with physical-chemical and microbiological analyses following the procedures described in the Standard Methods (APHA 2012).
The quality assessment of the protozoan detection method (CCF with IMS) was carried out using the USEPA Method 1623.1 criteria (USEPA 2012). Protozoa from the EasySeed® were inoculated into 1 L samples of the studied water following the manufacturer’s instructions. The samples were concentrated by CCF, the floc formation occurred by adding sodium bicarbonate (1 M, 10 mL L−1) and calcium chloride (1 M, 10 mL L−1), increasing the pH to 10 with sodium hydroxide. After standing at room temperature and overnight, the supernatant was discarded, and sulfamic acid (10%, 20 mL.L−1) was added. The sample was centrifuged at 1500×g for 20 min, and phosphate-buffered saline (PBS) was added for pH neutralization. Then, a further centrifugation was performed, and the supernatant was removed to 5 mL. A total of 0.5 mL pellet was obtained, and the concentrated sample was sent for purification phase. IMS was performed using a Dynabead® kit (life Technologies™), as stated in the manufacturer’s instructions, considering two and three acid dissociations with strong shaking. At the end of the IMS, the microscope slide was prepared. In this case, the sample was labelled with fluorescent monoclonal antibody for Giardia and Cryptosporidium fluorescein isothiocyanate (FITC), according to the manufacturer’s protocol (Merifluor® kit, Meridian Bioscience, Inc.), also used by Giglio and Sabogal-Paz (2018). The internal structures of cysts and oocysts were confirmed as follows. A volume of 50 μL of Fluoroshield™ with DAPI (Sigma Aldrich, St. Louis, MO) was placed in each well and allowed to stand at room temperature for 15 min. Slides were placed in the dark, mounted with mounting medium and examined × 400 magnification using epifluorescence microscopy (Olympus® BX51 microscope, Tokyo, Japan). A FITC filter (excitation, 480 nm; emission, 520 nm) was used to detect FITC-conjugated MAb-labelled cysts and oocysts, and a UV-filter block was used for DAPI (excitation, 350 nm; emission, 420 nm). DIC microscopy was also used to confirm the presence of cysts and oocysts.
Recovery evaluation
Step 3 consisted of the protozoa inoculation in the DAF assays, evaluating these microorganisms in treated water and waste. Initially, a known number of target organisms were inoculated into the jars of the DAF jar test (102 to 103 cysts L−1 and oocysts L−1). The inoculum volume, to obtain the desired concentration in the jars, was determined by the arithmetic mean of the protozoa counted in three identical aliquots taken after homogenization of the suspensions. The purified suspension of Giardia spp. cysts was obtained from the Laboratory of Protozoology at the University of Campinas (Brazil), and the suspension of Cryptosporidium spp. oocysts was purchased from Waterborne™ Inc. (USA). The counting was performed by FITC, DAPI and DIC. At the end of the above procedure, the inoculum was added to the studied water in each vessel of the DAF jar test, and the treatability assays were carried out using the parameters optimized in Step 2 (the assays were performed in triplicate). At the end of the aforementioned tests, the environmental matrices were obtained (filtered water, floated residue and filter backwash water).
CCF with IMS protocol was applied to each of the studied matrices (filtered water, floated residue and filter backwash water), and at the end of the IMS, the microscope slide was prepared. Parasites were enumerated under a microscope, and the visualization was performed in FITC, DAPI and DIC as described above.
Results
Studied water shows turbidity around 50 NTU and true colour nearby 80 HU (Table 1). The treatability parameters obtained in phase 2 were 25 mg L−1 of coagulant dosage; velocity gradient in rapid mixing of 700 s−1; rapid mixing time of 10 s; velocity gradient for flocculation of 60 s−1; flocculation time of 4 min; flotation time of 10 min; and recirculation rate of 5%.
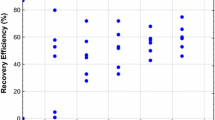
Quality assays for two acid dissociation resulted in recoveries of 16.7 ± 2.9% for cysts and 32.3 ± 5% for oocysts. With the increase of the third acid dissociation, such recoveries improved to 24.7 ± 4.1% and 46.5 ± 6.1%, respectively. These values meet the criteria recommended by the USEPA Method 1623.1 (USEPA 2012).
The influence of acid dissociations on the IMS is shown in Table 2 for Giardia cysts and Table 3 for Cryptosporidium oocysts.
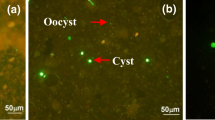
Microscopic images obtained throughout the dissociations are shown in Fig. 2.
Discussion
Data in Table 2 suggest a strong interaction of cysts and oocysts with the Dynabeads® microspheres, as recoveries were still possible after the second acid dissociation. In this context, Chang et al. (2007) evaluated the influence of the number of acid dissociation on the IMS in relation to the C. parvum recovery using a deionized water matrix. The authors observed that from the third dissociation onwards, the cumulative number of oocysts remained stable.
The third dissociation increased, on average, the recovery of Giardia spp. cysts by more than 31% in the matrices tested, and it was observed that the floated residue had the highest recovery percentage recorded for the protozoan (71.4% ± 6.1). This fact shows the influence of this dissociation on the final recovery.
The increase in Cryptosporidium spp. oocyst recovery was lower for the floated residue and the filter backwash water (5.6% ± 3.5 and 11.9% ± 8.7, respectively). However, this procedure was important in filtered water, with an increase in recovery of 52.8% ± 45.9, higher than that recorded for the cysts (33.3% ± 57.7).
In the filtered water view (Fig. 2), the presence of anti-Cryptosporidium and anti-Giardia microspheres is only observed in the DIC visualizations in all dissociations, highlighting the third dissociation, where the microsphere still appears to be attached to the cyst. Maciel and Sabogal-Paz (2016) and Rochelle et al. (1999) also observed microspheres after dissociations with cysts and oocysts, respectively.
According to the results, the Dynabeads® kit performance should be evaluated worldwide, since Anti-Giardia microspheres remain attached to the cysts after the third acid dissociation. The presence of microspheres throughout the dissociations, although following the kit manufacturer’s recommendations, is also an aspect that must be analysed in relation to the true efficiency of the kit.
The best result of the CCF with the IMS quality test (24.7% for cysts and 46.5 for oocysts) met the recovery criteria recommended by the USEPA Method 1623.1. However, the Dynabeads® kit (GC-Combo, Life Technologies™) comes with a quality control that designates, as acceptable, a requirement of oocyst and cyst recovery in water greater than or equal to 70% (certificate of analysis #73012, 98-14289-00). Therefore, more research is needed to optimize the detection methods aiming to have reliable results at affordable costs. It is emphasized that the use of purification is of great importance for the analyses in this type of samples.
The average cost of a CCF with IMS and two dissociation analysis (considering only an application of Dynabeads® kit and two of Merifluor® kit) is U$170 in Brazil (September 2020 price). When used with a third dissociation, the cost increases to U$210. Despite increasing the percentage of recovery from the analysis, the third acidic dissociation increases costs and operational complexity.
According to Rochelle et al. (1999), it is essential that the methods used to recover protozoa do not have harmful effects on the viability or infectivity of protozoa, as these assays are important for public water services to assess the efficiency of disinfection and the risks to public health. According to the authors, IMS processed with two acid dissociations did not alter the viability of the C. parvum oocysts. Despite this, it is not known about these effects when the third dissociation is added, as protozoa are exposed for a longer time to acid. Although the images showed no differences in the structure of the microorganisms (Fig. 2), specific viability and animal infectivity testing should be carried out to assess whether there is an influence of the third acid dissociation on these parameters.
The presence of cysts and oocysts in the treatment residues was expected and was higher in the float sludge when compared to the filter backwash water. This result reinforces the need for proper treatment and disposal and besides improves the protozoa detection methods aiming to reduce of microbiological risks. According to Keegan et al. (2008), viable and potentially infectious cysts and oocysts may be present in WTP residues.
Conclusions
CCF method quality tests for protozoan recovery met USEPA recommendations, and the three acid dissociation obtained greater recovery than two dissociations.
The increase of the number of acid dissociations in IMS improved the protozoa recovery; nevertheless, more research is needed to confirm if this increase influences the viability and infectivity assays. Likewise, additional studies should be carried out to optimize the protozoa detection methods to obtain reliable results at affordable costs, especially in less-developed countries.
References
Andreoli FC, Sabogal-Paz LP (2019) Coagulation, flocculation, dissolved air flotation and filtration in the removal of Giardia spp. and Cryptosporidium spp. from water supply. Environ Technol 40(5):654–663
APHA - American Public Health Association/American Water Works Association/Water Environment Federation. Standard methods for the examination of water and wastewater; Washington DC, USA, 2012
Baldursson S, Karanis P (2011) Waterborne transmission of protozoan parasites: review of worldwide outbreaks – an update 2004–2010. Water Res 45:6603–6614
Chang CY, Huang C, Pan JR, Wu BJ (2007) Modification of immunomagnetic separation procedures for analysis of Cryptosporidium at spiked oocysts and turbid sample conditions. J Environ Eng Manag 17:333
Cho EJ, Yang JY, Lee ES, Kim SC, Cha SY, Kim ST, Park YS (2013) A waterborne outbreak and detection of Cryptosporidium oocysts in drinking water of an older high-rise apartment complex in Seoul. Korean J Parasitol 51:461–466
Daly ER, Roy SJ, Blaney DD, Manning JS, Hill VR, Xiao L, Stull JW (2010) Outbreak of giardiasis associated with a community drinking-water source. Epidemiol Infect 138:491–500
Efstratiou A, Ongerth JE, Karanis P (2017a) Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2011–2016. Water Res 114:14–22
Efstratiou A, Ongerth J, Karanis P (2017b) Evolution of monitoring for Giardia and Cryptosporidium in water. Water Res 123:96–112
Feng Y, Zhao X, Chen J, Jin W, Zhou X, Li N, Xiao L (2011) Occurrence, source, and human infection potential of Cryptosporidium and Giardia spp. in source and tap water in Shanghai, China. Appl Environ Microbiol 77:3609–3616
Giglio GL, Sabogal-Paz LP (2018) Performance comparison of three methods for detection of Giardia spp. cysts and Cryptosporidium spp. oocysts in drinking-water treatment sludge. Environ Monit Assess 190:686
Karanis P, Kourenti C, Smith H (2007) Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health 5:1–38
Keegan A, Daminato D, Saint CP, Monis PT (2008) Effect of water treatment processes on Cryptosporidium infectivity. Water Res:42, 1805–1811
Koh W, Clode PL, Monis P, Thompson RA (2013) Multiplication of the waterborne pathogen Cryptosporidium parvum in an aquatic biofilm system. Parasit Vectors 6:270
Kooy M, Walter CT (2019) Towards a situated urban political ecology analysis of packaged drinking water supply. Water 11:225
Korich DG, Mead JR, Madore MS, Sinclair NA, Sterling CR (1990) Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl Environ Microbiol 56:1423–1428
Maciel PMF, Sabogal-Paz LP (2016) Removal of Giardia spp. and Cryptosporidium spp. from water supply with high turbidity: analytical challenges and perspectives. J Water Health 14:369–378
Olson ME, Goh J, Phillips M, Guselle N, McAllister TA (1999) Giardia cyst and Cryptosporidium oocyst survival in water, soil, and cattle feces. J Environ Quality 28:1991–1996
Rochelle PA, De Leon R, Johnson A, Stewart MH, Wolfe RL (1999) Evaluation of immunomagnetic separation for recovery of infectious Cryptosporidium parvum oocysts from environmental samples. Appl Environ Microbiol 65:841–845
Rosado-García FM, Guerrero-Flórez M, Karanis G, Hinojosa MDC, Karanis P (2017) Water-borne protozoa parasites: the Latin American perspective. Int J Hyg Environ Health 220:783–798
Schofield T (2001) Dissolved air flotation in drinking water production. Water Sci Technol 43:9–18
USEPA – United Stated Environmental Protection Agency. Method 1623.1: Cryptosporidium and Giardia in water by filtration/IMS/FA; Office of Water, EPA 816-R-12-001, 2012
Vesey G, Slade JS, Byrne M, Shepherd K, Fricker CR (1993) A new method for the concentration of Cryptosporidium oocysts from water. J Appl Bacteriol 75:82–86
Walker MJ, Montemagno CD, Jenkins MB (1998) Source water assessment and nonpoint sources of acutely toxic contaminants: a review of research related to survival and transport of Cryptosporidium parvum. Water Resour Res 34:3383–3392
Funding
The authors are grateful to the São Paulo Research Foundation (FAPESP) (Process 12/50522-0), the Global Challenges Research Fund (GCRF) UK Research and Innovation (SAFEWATER; EPSRC Grant Reference EP/P032427/1) for the research support and the National Council for Scientific and Technological Development (CNPq-Brazil) for the Master’s scholarship awarded to Fernando César Andreoli.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andreoli, F.C., Sabogal-Paz, L.P. Detection of Giardia and Cryptosporidium in environmental matrices with immunomagnetic separation: two or three acid dissociations. Parasitol Res 120, 629–635 (2021). https://doi.org/10.1007/s00436-020-06999-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06999-4