Abstract
Paratanaisia are eucotylidae digeneans that affect the upper urinary tract of birds. This genus contains three species (Paratanaisia bragai, P. robusta, and P. confusa) with similar morphological features. Macroscopic and microscopic damage caused by these parasites ranges from the irrelevant to significant lesions. This study aimed to describe the histological, morphological, and molecular features of the renal tissues and parasite specimens obtained from naturally infected free-ranging and captive wild birds in Brazil. Histopathological evaluations were performed on 103 slides containing kidney tissue sections from parasitized birds. Parasites were observed inside the collecting ducts, causing the dilation and destruction of the lining epithelial cells and alterations in other structures of the renal parenchyma. Such findings indicate that Paratanaisia have pathogenic potential in a wide range of hosts, suggesting low host specificity. The parasites recovered from the kidneys of 10 birds, including Columbiformes, Galliformes, Strigiformes, and Cuculiformes, were morphologically evaluated and identified as Paratanaisia sp. Formalin-fixed paraffin-embedded kidney fragments were subjected to conventional PCR assays targeting the 18S and 28S rDNA genes. A Bayesian inference analysis based on an 800-bp 18S rDNA gene fragment separated the trematode genus accurately, clustering all of the parasites tested with a previously described P. bragai specimen. Analyses on a small fragment of the 28S rDNA gene did not allow for accurately differentiating the Paratanaisia species. Therefore, further morphological studies with additional molecular markers are necessary to improve our understanding of the alpha-taxonomy of this group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites are important pathogens in wildlife and significantly impact the health of their hosts (Hudson et al. 1998). If an affected system such as the urinary tract shows little regeneration capability, then the health impact may be more evident and result in the mortality of some individuals (Unwin et al. 2013).
Paratanaisia are digenetic eucotylid parasites that affect the upper urinary tract of several bird species (Menezes et al. 2001; Luppi et al. 2007; Unwin et al. 2013; Prastowo et al. 2014). This genus contains three flattened and elongated species with similar morphological features: Paratanaisia bragai (Santos 1934) Freitas 1959, Paratanaisia robusta (Freitas 1959), and Paratanaisia confusa. The teguments of P. bragai (Brandolini and Amato 2007) and P. robusta are covered with flattened scales, while spines are found on that of P. confusa (Travassos et al. 1969).
Since their original description, these digeneans have been recorded parasitizing several bird species, including those belonging to the orders Galliformes (Menezes et al. 2001; Gomes et al. 2005), Psittaciformes (Luppi et al. 2007), Tinamiformes (Mapeli et al. 2003; Momo and Werther 2013; Momo et al. 2016), Ciconiiformes (Abdo and Sultan 2013), Passeriformes (Unwin et al. 2013; Tavela et al. 2014), Columbiformes (Taroda et al. 2013; Unwin et al. 2013; Xavier et al. 2015), and Cuculiformes (Santi et al. 2017), suggesting low host specificity.
Like other digeneans, Paratanaisia have a heteroxenous life cycle, with gastropod mollusks acting as the intermediate hosts and birds as the definitive hosts. The infection occurs when an avian definitive host ingests metacercariae-infected mollusks. In Brazil, the land snails Leptinaria unilamellata (Keller and Araújo 1992) and Subulina octona (Brandolini and Amato 2007) play the role of intermediate host for the Paratanaisia species.
The macroscopic effects of Paratanaisia infections are variable and range from non-specific alterations (Pinto et al. 2004; Gomes et al. 2005) to relevant changes such as alterations in kidney size and shape and brownish discolorations (Abdo and Sultan 2013). Microscopically, the dilation of renal collecting ducts with morphological alterations and abundant inflammatory reactions has been described (Menezes et al. 2001; Mapeli et al. 2003; Gomes et al. 2005).
This study aimed to identify the renal trematode Paratanaisia spp. that parasitize wild birds in Brazil using morphological and molecular methods. Additionally, this work aimed to describe the histopathology of the urinary tracts of infected birds.
Materials and methods
Histopathological analysis
To perform the histopathological evaluation, slides containing kidney tissue sections from parasitized wild birds were evaluated by light microscopy. The slides were obtained from archival material (1994 to 2014) and freshly necropsied birds (2015 and 2016) in the Veterinary Pathology Department of São Paulo State University (Unesp), School of Agricultural and Veterinarian Sciences, Jaboticabal, São Paulo, Brazil. Slides were prepared by fixing the urinary tissue fragments in 10% buffered formalin. After 24–48 h, this material was dehydrated, embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin (HE).
Morphological identification of trematodes
The remaining tissue from the freshly necropsied birds was sliced and evaluated for the presence of parasites under a stereoscopic microscope. The parasites were collected in microtubes with Railliet & Henry solution (92% deionized distilled water, 5% commercial formaldehyde, and 3% glacial acetic acid) and stored for later staining with chloridric carmine (Travassos 1950) and morphological identification according to Travassos et al. (1969). Images were obtained with an Olympus BX-51 light microscope and an Olympus SZX7 stereoscopic microscope and processed with Image Pro Plus v. 4.0. Some of the collected parasites were preserved in 70% ethanol at − 20 °C for further molecular analysis.
DNA extraction
DNA was extracted from the formalin-fixed paraffin-embedded (FFPE) kidney tissue samples from the parasitized wild birds using the QIAamp DNA FFPE™ Tissue Kit (Qiagen™, Valencia, California, USA), while the DNeasy™ Blood & Tissue Kit (Qiagen™, Valencia, California, USA) was used to extract DNA from the pooled trematode samples (1–30 parasites obtained from the kidney) obtained from each freshly necropsied bird. Both protocols were performed according to the manufacturer instructions.
Conventional PCR (cPCR) assay for an endogenous gene
The presence of amplifiable DNA in the FFPE kidney tissue samples was verified by a conventional PCR assay targeting the endogenous glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (Birkenheuer et al. 2003). The PCR assays were conducted in a 25-μL total reaction volume containing 5 μL of target DNA, 0.2 mM mixed deoxynucleotide triphosphates (dNTPs; Life Technologies®, Carlsbad, CA, USA), 3.0 mM MgCl2 (Life Technologies®, Carlsbad, CA, USA), 1.25 U Taq Platinum DNA Polymerase (Life Technologies®, Carlsbad, CA, USA), 0.4 μM of each primer [GAPDH-F (5′-CCTTCATTGACCTCAACTACAT-3′) and GAPDH-R (5′-CCAAAGTTGTCATGGATGACC-3′)] (Síntese Biotecnologia, Belo Horizonte, MG, Brazil), 2.5 μL of 10X reaction buffer, and sterile ultra-pure water (Promega) qsp 25 μL. The cycling conditions comprised an initial denaturation of 5 min at 95 °C; 37 cycles of 30 s at 95 °C, 30 s at 50 °C, and 1 min at 72 °C; and a final extension for 5 min at 72 °C. The PCR reactions were performed in a conventional thermocycling device (T100™Thermal Cycler, BioRad™, Hercules, CA, USA).
Trematode cPCR assays
The GAPDH-positive FFPE samples and parasite specimens were subjected to conventional PCR assays targeting two gene regions, 18S ribosomal DNA (rDNA; Routtu et al. 2014) and 28S rDNA (Unwin et al. 2013). The first PCR protocol was based on a 310-base pair (bp) fragment of the 28S rDNA gene (Unwin et al. 2013). These PCR reactions contained the same concentration of reagents as described for the endogenous GAPDH gene, except that 0.25 μL of the 0.3-μM forward [Para28S_F (5′-AAGCCTGTGTCCACTTGGTC-3′)] and reverse [Para28S_R (5′-CGTGCTGTTTACCCTCTCTTC-3′)] primers (Síntese Biotecnologia, Belo Horizonte, MG, Brazil) were used. The cycling conditions comprised an initial denaturation of 5 min at 95 °C; 34 cycles of 15 s at 95 °C, 30 s at 50 °C, 30 s at 72 °C; and a 5-min extension at 72 °C (Unwin et al. 2013).
The second PCR protocol was based on an 800-bp fragment of the 18S rDNA gene (Routtu et al. 2014). This PCR protocol also used 0.25 μL of 0.3-μM forward [C_for (5′-ATGGCTCATTAAATCAGCTAT-3′)] and reverse [A_rev (5′-TGCTTTGAGCACTCAAATTTG-3′)] primers (Síntese Biotecnologia, Belo Horizonte, MG, Brazil) in a reaction mixture otherwise identical to that used for the GAPDH gene. The cycling conditions comprised an initial denaturation of 2 min at 94 °C; 30 cycles of 15 s at 94 °C, 15 s at 60 °C, and 15 s at 72 °C; and a final extension of 1 min at 72 °C (Routtu et al. 2014).
PCR products were separated by electrophoresis on 1% agarose gels stained with ethidium bromide (Life Technologies®, Carlsbad, CA, USA). To prevent PCR contamination, the DNA extraction, PCR-reaction setup, PCR amplification, and electrophoresis steps were performed in separate rooms.
Purification, sequencing, and phylogenetic analyses
The amplified DNA fragments were purified with the Silica Bead DNA Gel Extraction Kit” (Fermentas®, São Paulo) according to the manufacturer’s instructions and prepared with the BigDye® Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific®, Waltham, MA, USA) for Sanger sequencing (Sanger et al. 1977) in an ABI PRISM 310 DNA Analyzer (Applied Biosystems®, Foster City, CA, USA).
Consensus sequences were obtained using PHRED software (Ewing et al. 1998), and homologous sequences were searched for using the NCBI BLAST service (Altschul et al. 1990). The MAFFT (multiple alignment program for amino acid or nucleotide sequences) program (Kazutaka et al. 2002) was used to align the sequences retrieved from the GenBank database.
Phylogenetic analyses based on Bayesian inference were conducted using both the 18S and 28S rDNA genes fragments. The Bayesian inference analysis was performed with the program MRBAYES 3.1.2 (Huelsenbeck and Ronquist 2001) on the CIPRES Science Gateway (Miller et al. 2010) using the best models selected by the IQ-TREE web server under the corrected Akaike information criterion. Markov chain Monte Carlo simulations were run for 108 generations with a sampling frequency of every 100 generations and a burn-in of 25%. The trees were examined in Treegraph 2.0.56-381 beta (Stöver and Müller 2010).
Results
A total of 103 kidney tissue sections from parasitized wild birds were evaluated by light microscopy. The slides were obtained from the Veterinary Pathology Department archives (n = 88) and from freshly necropsied birds (n = 15). In total, 73 birds (71%) were free-living and 30 (29%) lived in captivity. The birds belonged to 10 different orders and 24 avian species (Table 1). The cause of death of the birds was not taken into consideration, as the emphasis of this study was on the presence of parasites and alterations in the kidney tissue.
All of the evaluated kidneys showed histopathological alterations (Table 2), the most prominent being located at the parasitized collecting ducts (Fig.1). In some birds, the remaining renal parenchyma and interstitial tissue were also affected, with findings of prominent inflammatory reactions, hemorrhage, necrosis, and fibrosis in addition to glomerular alterations. The most significant alterations were to the collecting duct diameters and the epithelial structure.
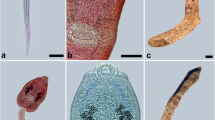
Photomicrographs of kidney renal tissue of Paratanaisia-infected birds. a Columba livia renal tissue showing a parasite inside the collecting duct (arrow) causing dilatation. Hematoxylin-eosin (HE) stain. Scale bar = 50 μm. b Amazona amazonica renal tissue showing epithelial hyperplasia of the collecting ducts (arrow) and a papilliform formation projecting into the lumen of the duct (arrow head). Hematoxylin-eosin (HE) stain. Scale bar = 50 μm. c Columba livia renal tissue showing a parasite inside the collecting duct (arrow); observe the spines covering the tegument (arrow heads). HE stain. Scale bar = 20 μm. d Guira cuckoo renal tissue showing the destruction of the collecting duct lining the epithelial cells (arrows). HE stain. Scale bar = 50 μm
The highest parasitic burden was found in the Strigiformes specimens, with an average of 18.2 parasitic structures per slide, which was followed by the Columbiformes specimens, with 17.9 parasitic structures per slide. The small number of Galliformes, Cariamiformes, Musophagiformes, Passeriformes, and Cuculiformes samples precluded an accurate evaluation.
A morphological evaluation was performed with carmine-stained parasites obtained from 10 of the 15 freshly necropsied birds. Six parasite specimens recovered from each bird were morphologically identified. A well-developed, sub-terminal, oral sucker with a muscular and wider than long pharynx was observed on the initial portion of the parasite body, while sinuous ceca were fused at the caudal end. Testes were lobed, pre-equatorial, and post-ovarian, most of them being intracecal. There was a pre-testicular, irregularly shaped, and laterally displaced ovary. Vitelline extended from the bifurcal zone to the end of the parasite body. A well-developed uterus filled with ellipsoid brownish eggs covered most of the parasite body (Fig. 2).
Photomicrographs of Paratanaisia spp. a Numida meleagridis kidney fragment showing parasites (P. confusa) inside collecting ducts (arrow). Fresh tissue. Scale bar = 500 μm. b Paratanaisia sp. showing oral sucker (1), vitelline (2), uterus filled with eggs (3), ovary (4), and testes (5). Chloridric carmine staining. Scale bar = 200 μm
In four birds (Guira guira, Numida meleagris, Columba livia, and Zenaida auriculata), all of the parasites (#1, #2, #3, and #4) had spines covering the tegument and were therefore morphologically identified as P. confusa. In contrast, the parasites (#5, #6, and #7) in three birds (all C. livia) had scale-covered tegument and were morphologically identified only at the genus level as Paratanaisia sp. (Travassos et al. 1969). In three further cases (#8, #9, and #10) recovered from two C. livia and one Athene cunicularia, two distinct morphological types were observed parasitizing the same bird, denoting the occurrence of co-infection. Due to the small number of samples, no morphological identification was possible for samples #11, #12, #13, and #14.
The cPCR amplifications targeting the endogenous GAPDH gene were negative in six FFPE samples, which were therefore excluded from the molecular analyses. The cPCR amplification targeting the 310-bp 28S rDNA gene fragment yielded positive results in 14 of 15 parasite samples (93.3%) and 52 of 73 FFPE samples (71.2%). In addition, 14 of the 15 parasite samples (93.3%) assayed for the 800-bp 18S rDNA gene fragment yielded positive results.
A BLAST search was performed for each sequence using the default parameters. All sequences retrieved from the BLAST results showed 100% coverage of the fragments. Analyzing the sequenced products for the 18S-rDNA region showed 13 of the fragments (#1 to #13) had 99% sequence identity with P. bragai (GenBank accession: JX231100) and one (#14) had 98% identity. All of the amplified sequences in this study were positioned in the same P. bragai clade detected in Zenaida graysoni (GenBank accession: JX231100). The positioning of the sequences was supported by a posterior probability greater than 62 in the Bayesian analysis (Fig. 3). Eurytrema coelmaticum was used as the outgroup.
Phylogenetic analysis of 18S-rDNA sequences based on Bayesian inference. The supporting values for a posterior probability greater than 50% are shown on each branch. The sequences from this work are highlighted in bold and form a single cluster grouped with one sequence obtained from Zenaida graysoni (67% probability). Eurytrema coelomaticum was used as the outgroup
The BLAST analysis of the sequenced products from the 28S-rDNA region had sequence identities of 98 to 100% with the P. bragai sequence reported in GenBank accession JX231098 and of 97 to 99% with the P. bragai sequence reported in GenBank accession JX231099. However, the phylogenetic inferences using this small fragment were not robust.
Discussion
Since the first description by Santos in 1934 (Giovannoni and Malheiro 1952), Paratanaisia spp. have been described in several avian species (Gomes et al. 2005; Tavela et al. 2014; Xavier et al. 2015; Momo et al. 2016, Santi et al. 2017). However, most of the reports referred to P. bragai or to undetermined species (Paratanaisia spp.). Additionally, studies on taxonomic classification and host-parasite relationships are scarce. In this study, Paratanaisia parasites were observed in 24 avian species, including some that have not previously been described as hosts for these trematodes. Thus, this work provides a new record of Paratanaisia hosts in several orders: Ramphastos toco and Colaptes campestris (Piciformes); Athene cunicularia and Megascops choliba (Strigiformes); Cariama cristata (Cariamiformes); Thraupis sayaca (Passeriformes); Amazona amazonica, Amazona aestiva, Psittacara leucophtalmus, Eupsittula aurea, Brotogeris chiriri, and Nymphicus hollandicus (Psittaciformes); Pavo cristatus (Galliformes); Patagioenas picazuro and Columbina squammata (Columbiformes); and one species in the order Musophagiformes.
Columbiformes represented the most frequently infected birds, perhaps due to their feeding habits. The diets of these species, along with Galliformes and Tinamiformes, vary widely in nature and are composed of grains, feeds, insects, and small invertebrates (Sick 1997), increasing the probability of infection by the digeneans whose intermediate hosts are gastropod mollusks (Keller and Araújo 1992). However, because the snails are small, their accidental ingestion by birds not usually expected to feed on them such as Psittaciformes and Piciformes should be considered (Gomes et al. 2005).
Wild free-living birds such as Columbiformes, which were widely infected in this study, are widespread and frequently in close contact with domestic and wild captive birds. Free-living birds could be found living around zoos and poultry and other breeding facilities, sometimes sharing the food and environment, and may act as reservoirs and carriers of trematodes to these hosts. The maintenance of different species together with different known susceptibilities could promote helminth cross-infection and the spread of the parasites, especially if suitable snail hosts are also present.
This study demonstrated the wide incidence of infection in both captive (30/103) and free-living (73/103) avian species, suggesting the parasitosis may have implications for veterinary care and aviary management, as well as for free-living endangered birds. Due to their low host specificity, these trematodes could significantly affect conservation programs if infected captive birds are released into the wild, making the accidental introduction of these trematodes into naïve avian populations possible.
It is also important to consider the role of bird migration in the spread of these parasites. Although there were no reports of migratory birds infected in this study, they have been reported as Paratanaisia hosts (Routtu et al. 2014) and could become important carriers, spreading the parasites to locations where bird migration is common.
Microscopically, the observed histological alterations at the collecting ducts were probably caused by the direct actions of the parasites. Their relatively large body size may result in physical compression, which could lead to alterations such as a decrease in lumen diameter or its replacement by connective tissue, even in the non-parasitized collecting ducts (Unwin et al. 2013; Tavela et al. 2014) as was observed in some of the sampled birds in this study. Those cases where ductal dilatation was not observed were likely related to a low parasite burden or the small size of the immature parasites in the ducts.
While most previous studies indicate a relatively benign disease focused only on structures directly affected by the parasite (Pinto et al. 2004; Brener et al. 2006; Tavela et al. 2014), we observed prominent inflammatory reactions in the interstitial renal tissue along with hemorrhage, necrosis, fibrosis, and glomerular alterations. However, because all the birds were naturally infected and most were free-living, it was not possible to confirm if these alterations were directly caused by the parasite interference or if they were related to causes other than parasitism such as the conditions of the host organism, its nutritional status, or concomitant infections. Moreover, because the sampled birds were naturally infected by trematodes, it was not possible to determine the course of infection or make inferences regarding how long the birds had been infected or how much damage the parasites had caused.
It was possible to differentiate two morphological types based on the tegument covering during the microscopic evaluation of the carmine-stained parasites; one type was covered with flattened scales, while the other was covered with spines. The identification of Paratanaisia species can be difficult when based only on morphological characters. Paratanaisia confusa can be accurately differentiated from the other two species of the genus because its tegument is covered with spines, while the teguments of the other species are covered with scales (Travassos et al. 1969; Brandolini and Amato 2007). Otherwise, the three species present many similar features regarding their digestive and reproductive tract morphology. The overlap in the morphometric measures of the three species precludes an accurate morphological identification. However, the use of PCR to amplify ribosomal gene fragments and sequencing the products should allow for a proper taxonomic assessment (Routtu et al. 2014).
Although amplifying the 310-bp fragment of the 28S-rDNA gene is considered suitable for identifying Paratanaisia spp. in kidney tissues, the short amplicons proved to be unreliable for species identification and phylogenetic inferences. In contrast, the primers targeting an 800-bp fragment of the 18S-rDNA gene were specifically designed for trematodes, and the sequence of the region covered is highly polymorphic among trematode species (Routtu et al. 2014).
The phylogenetic analysis based on Bayesian inference separated the trematode genus accurately. However, some discrepancies were observed between the morphological and molecular evaluations at the species level. For instance, specimens #2, #3, and #4 were morphologically classified as P. confusa and clustered together with P. bragai, but were separated from specimen #1, also morphologically classified as P. confusa. The discrepancies in parasite identity could be due to the presence of more than one species cohabiting the same kidney, as observed in Specimens #8, #9, and #10. Furthermore, the lack of P. confusa and P. robusta 28S and 18S rDNA sequences in the GenBank database precluded any robust phylogenetic inferences. Moreover, these two trematodes have rarely been described in the literature. It is possible the targeted genetic regions used in our study were conserved, precluding accurately differentiating between Paratanaisia species. The Paratanaisia genus would benefit from additional molecular data from each of its constituent species. Further molecular studies may elucidate the specific genetic differences, thereby improving the alpha-taxonomy and indicating whether three species or a single polymorphic species is valid.
A histopathological evaluation was used in this study as a screening tool for further molecular analysis; therefore, all the samples were known to contain trematode parasites. However, not all the samples yielded positive results from the DNA amplification. The failure to successfully amplify some of the FFPE samples may be attributed to the absence of parasites in the small fragment of kidney tissue removed from the histology block. In addition, processing the material for histopathology using chemical reagents such as formalin may disrupt long DNA chains, thereby reducing sensitivity and preventing the amplification of long fragments. This might explain the negative results for the 18S rDNA fragment amplifications from the FFPE samples. Nevertheless, the results suggest there is value in screening FFPE tissues.
Conclusion
The histopathological findings in the urinary tissue of infected birds suggested that Paratanaisia species have pathogenic potential in a wide range of hosts. Although species determination was not successful, the phylogenetic inferences showed that all parasites examined in this study belonged to the same genus. More studies aiming to amplify larger ribosomal DNA fragments, as well as other genes, will add to the ability to make phylogenetic inferences and help to clarify the taxonomy.
References
Abdo W, Sultan K (2013) Histopathological findings of the kidney Trematoda Paratanaisia spp. (Digenea: Eucotylidae) in cattle egret (Bubulcus ibis). Bras J Vet Parasitol 22(2):312–313. https://doi.org/10.1590/S1984-29612013000200050
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1006/jmbi.1990.9999
Birkenheuer AJ, Levy MG, Breitschwerdt EB (2003) Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian genotype) and B. canis DNA in canine blood samples. J Clin Microbiol 41(9):4172–4177. https://doi.org/10.1128/JCM.41.9.4172-4177.2003
Brandolini SVPB, Amato SB (2007) Morfologia externa de espécimes adultos de Paratanaisia bragai (Santos, 1934) (Digenea: Eucotylidae). Bras J Vet Parasitol 16(4):129–132. https://doi.org/10.1590/S0101-81752006000400017
Brener B, Tortelly R, Menezes RC, Muniz-Pereira LC, Pinto RM (2006) Prevalence and pathology of the nematode Heterakis gallinarum, the trematode Paratanaisia bragai, and the protozoan Histomonas meleagridis in the turkey, Meleagris gallopavo. Mem Inst Oswaldo Cruz 101(6):677–681. https://doi.org/10.1590/S0074-02762006000600017
Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185. https://doi.org/10.1101/gr.8.3.175
Giovannoni M, Malheiro DM (1952) Incidência de parasitas em Columba livia domestica. Rev Fac Med Vet 4(4):595–598. https://doi.org/10.11606/issn.2318-5066.v4i4p595-598
Gomes DC, Menezes RC, Tortelly R, Pinto RM (2005) Pathology and first occurrence of the kidney trematode Paratanaisia bragai (Santos, 1934) Freitas, 1959 (Digenea: Eucotylidae) in Phasianus colchicus L., 1758, from Brazil. Mem Inst Oswaldo Cruz 100(3):285–288. https://doi.org/10.1590/S0074-02762005000300013
Hudson PJ, Dobson AP, Newborn D (1998) Prevention of population cycles by parasite removal. Science 282(5397):2256–2258. https://doi.org/10.1126/science.282.5397.2256
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17(8):754–755. https://doi.org/10.1093/bioinformatics/17.8.754
Kazutaka K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30(14):3059–3066. https://doi.org/10.1093/nar/gkf436
Keller DG, Araújo JLB (1992) Ciclo evolutivo de Paratanaisia bragai (Santos, 1934) (Trematoda, Eucotylidae) com novo hospedeiro intermediário no Brasil: Leptinaria inilamellata (D’Orbigny, 1835) (Gastropoda, Pulmonata, Sebulinidae) em condições de laboratório. Bras J Vet Parasitol 1:89–92
Luppi MM, Melo AL, Motta ROC, Malta MCC, Gardiner CH, Santos LR (2007) Granulomatous nephritis in psittacines associated with parasitism by the trematode Paratanaisia spp. Vet Parasitol 146(3-4):363–366. https://doi.org/10.1016/j.vetpar.2007.03.011
Mapeli EB, Nascimento AA, Szabó MPJ, Tebaldi JH (2003) Infecções naturais por helmintos em perdizes (Rhynchotus rufescens Temminck, 1815) de cativeiro, no município de Jaboticabal, estado de São Paulo. Arq Inst Biol 70:415–418
Menezes RC, Mattos DG Jr, Gomes DC, Tortelly R, Muniz-Pereira LC, Pinto RM (2001) Trematodes of free range reared guinea fowls (Numida meleagris Linnaeus, 1758) in the state of Rio de Janeiro, Brazil: morphology and pathology. Avian Pathol 30(3):209–214. https://doi.org/10.1080/03079450124448
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), IEEE, pp 1–8
Momo C, Werther K (2013) Parasitismo renal em perdizes (Rhynchotus rufescens) criadas em cativeiro. PUBVET 7:1571–1652
Momo C, Garrido E, Werther K (2016) Anatomopathological findings in captive-raised winged tinamou (Rhynchotus rufescens). Braz J Vet Res Anim Sci 53(3):227–234. https://doi.org/10.11606/issn.1678-4456.v53i3p227-234
Pinto RM, Menezes RC, Tortelly R (2004) Systematic and pathologic study of Paratanaisia bragai (Santos, 1934) Freitas, 1959 (Digenea, Eucotylidae) infestation in ruddy ground dove Columbina talpacoti (Temminck, 1811). Arq Bras Med Vet Zootec 56(4):472–479. https://doi.org/10.1590/S0102-09352004000400008
Prastowo J, Sahara A, Marganingsih C, Ariyadi B (2014) Identification of renal parasite and its blood urea-creatinine profile on the Indonesian indigenous pigeons. Int J Poult Sci 13:385–389
Routtu J, Grunberg D, Izhar R, Dagan Y, Guttel Y, Ucko M, Ben-ami F (2014) Selective and universal primers for trematode barcoding in freshwater snails. Parasitol Res 113(7):2535–2540. https://doi.org/10.1007/s00436-014-3903-z
Sanger F, Nicklen S, Coulson AR (1977) Dna sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A 74(12):5463–5467. https://doi.org/10.1073/pnas.74.12.5463
Santi M, André MR, Hoppe EGL, Werther K (2017) Occurrence of Paratanaisia confusa Freitas, 1951 in free-living guira cuckoo (Guira guira, Cuculiformes: Crotophagidae). Bras J Vet Parasitol 26(2):248–251. https://doi.org/10.1590/S1984-29612017014
Sick H (1997) Ornitologia brasileira. Nova Fronteira, Rio de Janeiro
Stöver BC, Müller KF (2010) TreeGraph 2: combining and visualizing evidence from different phylogenetic analyses. BMC Bioinf 11(1):1–9. https://doi.org/10.1186/1471-2105-11-7
Taroda A, Barros LD, Zulpo DL, Cunha IAL, Paiva MCDC, Sammi AS, Santos JR, Yamamura MH, Vidoto O, Garcia JL (2013) Occurrence of gastrointestinal and renal helminths in Zenaida auriculata (Des Murs, 1847) trap-captured from Brazil. Bras J Vet Parasitol 22(3):415–419. https://doi.org/10.1590/S1984-29612013000300016
Tavela AO, Carretta M Jr, Oliveira AR, Carneiro FT, Silva VHD, Braga FR, Peixoto JV, Carvalho GD, Araújo JV, Paula TAR (2014) Parasitism by Paratanaisia bragai (Digenea, Eucotylidae) in common waxbill (Estrilda astrild). Arq Bras Med Vet Zootec 66(4):1276–1280. https://doi.org/10.1590/1678-7136
Travassos L (1950) Introdução ao estudo da helmintologia. Rev Bras Biol 173 p
Travassos L, Freitas JFT, Kohn A (1969) Gênero Paratanaisia Freitas, 1969. Mem Inst Oswaldo Cruz 67:340–343
Unwin S, Chantrey J, Chatterton J, Aldhoun JA, Littlewood DTJ (2013) Renal trematode infection due to Paratanaisia bragai in zoo housed Columbiformes and a red bird-of-paradise (Paradisaea rubra). Int J Parasitol Parasites Wildl 2:32–41. https://doi.org/10.1016/j.ijppaw.2012.11.001
Xavier VB, Oliveira-Menezes A, Santos MAJ, Amato SB, Torres EJL, Pinheiro J, Brandolini SVPB (2015) Histopathological changes in the kidneys of vertebrate hosts infected naturally and experimentally with Paratanaisia bragai (Trematoda, Digenea). Bras J Vet Parasitol 24(2):241–246. https://doi.org/10.1590/S1984-29612015017
Funding
This work was supported by the National Counsel of Technological and Scientific Development–CNPq and São Paulo Research Foundation–FAPESP (Process No. 2015/22851-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal procedures and management protocols were approved by the Ethics Committee on Animal Use (CEUA) of the School of Agricultural and Veterinarian Sciences (FCAV/Unesp) (protocol number 009414/15) and by Institute Chico Mendes for Conservation of Biodiversity (ICMBio no. 49343-1
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Section Editor: Christoph G. Grevelding
Rights and permissions
About this article
Cite this article
De Santi, M., André, M.R., Lux Hoppe, E.G. et al. Renal trematode infection in wild birds: histopathological, morphological, and molecular aspects. Parasitol Res 117, 883–891 (2018). https://doi.org/10.1007/s00436-018-5767-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5767-0