Abstract
The aim of this study was to evaluate the antiprotozoal activity of essential oils from Varronia curassavica accessions against different stages of Ichthyophthirius multifiliis. Essential oils from each accession were tested in vitro at the concentrations 0, 10, 25, 50, 75, 100, and 200 mg/L. The VCUR-001, VCUR-202, VCUR-509, and VCUR-601 accessions presented the major compounds α-pinene, germacrene d-4-ol, (E)-caryophyllene and epiglobulol, and sabinene, respectively. These isolated compounds were tested in vitro at a concentration proportional to that found in the essential oil which caused 100% mortality of the parasite. The concentrations of 10 and 50 mg/L of the essential oil of accession VCUR-202 provided 100% mortality of trophonts and tomonts, respectively. For the accession VCUR-509, 100% mortality of trophonts and tomonts was observed at concentrations 75 and 200 mg/L of essential oil, respectively. The same mortality was observed at concentration 200 mg/L in both stages of the parasite for the other accessions. The major compounds α-pinene, sabinene, and the (E)-caryophyllene + epiglobulol mixture caused 100% mortality of trophonts and tomonts. The in vivo assay for white spot disease control was performed in a therapeutic bath of 1 h with the essential oil of accession VCUR-202 at concentrations of 0.5 and 2.0 mg/L. A significant reduction of about 30% of trophonts on infected fish was observed, independent of the oil concentration. The V. curassavica essential oil, especially the VCUR-202 accession, is a potential source of raw material for the formulation and commercialization of bioproducts to control freshwater white spot disease in fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The medicinal species Varronia curassavica (Cordia verbenacea DC. synonymy), native from Brazil, is commonly used to treat inflammation. In the pharmaceutical industry, the essential oil of V. curassavica is used since 2004 in the production of a commercial phytotherapeutic medicine. In addition to its proven medicinal properties, biological activities such as antibacterial (Meccia et al. 2009; Matias et al. 2010; Pinho et al. 2012; Rodrigues et al. 2012) and antifungal (Hoyos et al. 2012; Silva et al. 2012, 2014) have been reported for this species. However, there are no studies on its antiparasitic potential.
Essential oils consist of a complex mixture of several chemicals, mostly terpenes, with wide variation in composition. In general, the most abundant compound determines the properties of essential oils, and it is likely that their mode of action involves several targets in the cell (Bakkali et al. 2008). A recent study has reported the existence of high chemical diversity of compounds in the essential oils of V. curassavica, depending on the plant origin. In addition, the authors verified that depending on the chemical composition, the essential oils presented higher or lower activity against phytopathogenic fungus (Nizio et al. 2015).
For fish disease treatments, extracts of medicinal plants have been evaluated mainly to reduce or to avoid the use of chemotherapeutics which drive drug resistance in parasites and promote a negative environmental impact (Doughari et al. 2009). In this regard, several studies have been conducted, testing the use of extracts or plant compounds to control ichthyophthiriasis; these include the aqueous extract of Capsicum frutescens (Ling et al. 2012), methanol extracts of Psoralea corylifolia (Ling et al. 2013), extract and flavonoids isolated from the bark of Morus alba (Fu et al. 2014b; Liang et al. 2015); cynatratoside-C isolated from the roots of Cynanchum atratum (Fu et al. 2014a), and essential oils (Valladão et al. 2016a).
Ichthyophthiriasis is caused by the ciliate Ichthyophthirius multifiliis, popularly known as freshwater white spot disease or freshwater ich. This protozoan has caused great economic losses for the fish farming industry, in conventional and high-tech systems, or in the production of ornamental fish (Matthews 2005; Heinecke and Buchmann 2009; Martins et al. 2011). This parasite has a direct life cycle, and the water temperature dictates its life cycle which includes an infectious stage, the free-swimming theront that penetrates the skin epithelium and the gills, transforming into a trophont within a developing pustule (Shinn et al. 2012). Mature trophonts release tomonts that settle on the substrate and secrete a surrounding gelatinous cyst. The tomont multiplies by binary division producing tomites that differentiate into infectious theronts, thus repeating the cycle (Xu et al. 2012; Dickerson and Findly 2014). The objective of this study was to evaluate the antiprotozoal activity of the essential oils of different V. curassavica accessions against I. multifiliis, an important ectoparasite that causes freshwater white spot disease in fish.
Materials and methods
Fish and parasites
Tambaqui (Colossoma macropomum) fingerlings, naturally infected with I. multifiliis, were obtained from a commercial fish farm. Infected fish (4–5 g) were gently scraped to remove trophonts from their skin for in vitro and in vivo assays. The isolated trophonts were washed several times with dechlorinated tap water to remove the fish mucus. To obtain the tomonts, trophonts were incubated for 4 h at 24 °C, as described by Fu et al. (2014b).
The experimental procedures were approved by the Ethics Committee for Animals Use of Embrapa Tabuleiros Costeiros under protocol number 03.13.09.005.00.05.001.
Essential oils and major compounds
The essential oils of the following four V. curassavica accessions of the active germplasm bank of medicinal and aromatic plants of the Federal University of Sergipe were used: VCUR-001, VCUR-202, VCUR-509, and VCUR-601 (Table 1). Leaves were collected and dried in an oven with forced air circulation at 40 °C for 5 days (Ehlert et al. 2006). The extraction of the essential oil was carried out by hydrodistillation in a modified Clevenger apparatus, using 75 g of dry leaf samples.
Analyses of the essential oil compounds were carried out using a GC-MS/flame ionization detector (FID) (QP2010 Ultra, Shimadzu Corporation, Kyoto, Japan) equipped with an autosampler AOC-20i (Shimadzu). Separations were accomplished using an Rtx®-5MS Restek-fused silica capillary column (5% diphenyl–95% dimethyl polysiloxane) of 30 m × 0.25 mm I.D., 0.25-μm film thickness, at a constant helium (99.999%) flow rate of 1.2 mL/min. Injection volume of 0.5 μL (5 mg/mL) was employed, with a split ratio of 1:10. The oven temperature was programmed from 50 °C (isothermal for 1.5 min), with an increase of 4 °C/min, to 200 °C, then 10 °C/min to 250 °C, ending with a 5-min isothermal at 250 °C.
The MS and FID data were simultaneously acquired employing a detector splitting system; the split flow ratio was 4:1 (MS/FID). A 0.62 m × 0.15 mm i.d. restrictor tube (capillary column) was used to connect the splitter to the MS detector; a 0.74 m × 0.22 mm I.D. restrictor tube was used to connect the splitter to the FID detector. The MS data (total ion chromatogram, TIC) were acquired in the full-scan mode (m/z of 40–350) at a scan rate of 0.3 scan/s using the electron ionization (EI) with electron energy of 70 eV. The injector temperature was 250 °C, and the ion source temperature was 250 °C. The FID temperature was set to 250 °C, and the gas supplies for the FID were hydrogen, air, and helium at flow rates of 30, 300, and 30 mL/min, respectively. Quantification of each constituent was estimated by FID peak area normalization (%). Compound concentrations were calculated from the GC peak areas, and they were arranged in order of GC elution.
Retention indices were determined by the equation of Van den Dool and Kratz (1963) in relation to a homologous series of n-alkanes (nC9–nC18) and compared with retention indices of the literature (Adams 2007) for the identification of the compounds. Simultaneously, the three libraries WILEY8, NIST107, and NIST21 of the equipment were used, which allowed the comparison of the spectra data with the data of the libraries, using an 80% similarity index.
The major compounds of VCUR-001, VCUR-509, and VCUR-601 accessions, commercially available (Sigma-Aldrich®) and used in the tests, were (E)-caryophyllene, α-pinene, sabinene, and epiglobulol. The major compound of the essential oil of accession VCUR-202 (germacrene d-4-ol) was not commercially available, so it was not tested in the present study.
In vitro antiprotozoal activity
Two stages of the parasite were used, trophonts and tomonts. The experimental design was completely randomized with three replications. Essential oils were tested in vitro at the concentrations 10, 25, 50, 75, 100, and 200 mg/L, in addition to the control water + Tween 80. The essential oils and the major compounds were diluted in Tween 80 at the ratio: 2/3 essential oil/major compound: 1/3 Tween, to improve the dispersion in water. This mixture was vigorously stirred in vortex until complete homogenization. Solution containing the essential oil or the isolated compound mixture was added to 5-mL Petri dish with 15 parasites each. Incubation was carried out for 1 h.
The major compounds of the essential oil of three accessions were tested separately. Proportional concentrations were tested for each compound present in majority of each essential oil, according to the concentration of the essential oil that caused 100% mortality at each stage of the parasite.
The compounds (E)-caryophyllene and epiglobulol, which are the major compounds of the essential oil of the accession VCUR-509, were tested isolately and in mixture, at the concentrations 14.90 and 11.44 mg/L, respectively, for trophonts, and at the concentrations 39.74 and 30.50 mg/L for tomonts. Sabinene, the major compound of the essential oil of the accession VCUR-601, and α-pinene, the major compound of the accession VCUR-001, were tested, respectively, at the concentrations 77.34 and 84.70 mg/L for trophonts and tomonts.
Viability of parasites exposed to essential oil and to its major compounds
Viability of parasites after 1 h of incubation was measured by staining the cells with fluorescent probes SYBR-14 and propidium iodide (PI) (Molecular Probes®). Visualization was carried out in epifluorescence microscope (Nikon Eclipse 50i). This technique is an adaptation of the method described by Maria et al. (2010) for detection of sperm cell viability. Propidium iodide intercalates the DNA of the cells and can only penetrate through damaged cell membranes. SYBR-14 is a fluorescent dye which can penetrate in all cells, in intact or damaged membranes, and bind to DNA. Therefore, intact and viable parasites emit green fluorescence, while dead or damaged cells emit red fluorescence. After incubation with essential oil or isolated compounds for 1 h, the parasites were transferred with a pipette to Kline concavity slides. In each well, 2.5 μL of SYBR-14 (final concentration 250 nM) was added and incubated for 5 min. Later, 2.5 μL of iodide propidium (IP) (final concentration 30 μM) was added and incubated for an additional 5 min. The viability of the parasites was then evaluated under epifluorescence microscopy.
In vivo antiprotozoal activity
For the in vivo assay, trophonts originated from naturally infected tambaquis were collected and incubated in Petri dishes with water at 24 °C for 5 h for the formation of tomonts. In order to achieve a consistent infestation of tambaqui, tomonts were inserted in 1-L beaker at a ratio of 10 tomonts/fish. The fish (n = 20) were kept individually in beakers for 7 days in a semi-static system with constant temperature (24 ± 1 °C), pH (7.20 ± 0.30), and oxygenation (greater than 4 mg/L) and partial replacement of water. After this period, the fish showed high levels of parasitic infection, characterized by numerous white spots on the skin.
The experimental design was completely randomized with five treatments (oil concentrantion) and four replications (infected fish). The infected fish were submitted to a therapeutic bath for 1 h in solutions containing essential oil of the accession VCUR-202 in the concentrations 0 (control), 0.5, 1.0, 1.5, and 2.0 mg/L. The accession VCUR-202 was selected due the better results in in vitro assay.
Immediately after bath, the fish from all groups (including control) were transferred to other containers and maintained for 24 h until the trophonts were to be gently removed by scraping the skin and fins. To avoid interference of water quality in parasite survival, temperature, pH, and oxygen were monitored and maintained under the same conditions throughout the experiment. The mean intensity of infection was calculated as the number of trophonts per infected fish (Bush et al. 1997).
Previous tests showed that the tambaqui has low tolerance to the essential oil of V. curassavica, especially the accession VCUR-202. Therefore, only the concentrations lower than 2 mg/L were used in the therapeutic baths.
Statistical analysis
Data were analyzed with the software SPSS 17.0® and expressed as the mean ± standard deviation.
The data were assessed with regard to the assumptions of normality and homoscedasticity, using the Shapiro–Wilk and Levene’s tests, respectively. For the data with normal distribution, analysis of variance was used, followed by the Tukey test, to make comparisons between the means. Statistical significance was set at p < 0.05.
Results
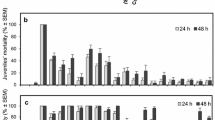
Table 2 shows the chemical composition of the essential oils of the four V. curassavica accessions. The accessions VCUR-001, VCUR-202, VCUR-509, and VCUR-601 presented the major compounds α-pinene, germacrene d-4-ol, (E)-caryophyllene and epiglobulol, and sabinene, respectively.
In addition to red and green fluorescent stages (Fig. 1a, b), there were also mixed stages in which the protozoan simultaneously emitted green and red fluorescences (Fig. 1c, d). In this case, the cell membrane is damaged; therefore green–red protozoa were considered dead.
I. multifiliis stained with fluorocromes SYBR-14 and propidium iodide (PI). Trophonts stained with SYBR-14 showing live parasite (a). Trophont stained with PI showing damage in cellular membrane and parasite death (b). Trophonts and tomonts emitting fluorescents red and green, indicating apoptosis (c). Tomites within tomonts (e, f). Live tomont without exposure to essential oil (e). Tomont exposed to 77.34 mg/L of sabinene for 1 h (f). Tomites released from tomont after 1 h of exposure to 75 mg/L of the essential oil of accession VCUR-509 (g) and extravasation of trophont cytoplasm after 1 h of exposure to 75 mg/L of the essential oil of accession VCUR-509 (h, i)
The essential oil of VCUR-202 accession exhibited more pronounced activity against the cell in the two stages, followed by VCUR-509. Concentrations of 10 and 50 mg/L of essential oil provided 100% mortality in the trophont and tomont phases, respectively. For VCUR-509 essential oil, 100% mortality was achieved for both trophonts and tomonts, respectively, at 75 and 200 mg/L of essential oil. The same mortality was observed for the other accessions at 200 mg/L for both trophonts and tomonts (Table 3). Tomites, within the tomonts, were visible and emitted green or red fluorescence (Fig. 1e, f). In all the assays, we observed cyst disruption, early release of tomites, and consequently, death (Fig. 1g).
It was also observed that in some trophonts incubated with the essential oil of V. curassavica and isolated compounds, there were disruption of the membrane and extravasation of the cellular contents (Fig. 1h, i).
The compounds α-pinene and sabinene, present in higher proportions in the VCUR-001 and VCUR-601 accessions, respectively, provided mortality similar to that shown by the essential oils at the two stages of the protozoan (Table 4). The compounds epiglobulol and (E)-caryophyllene, tested separately, provoked lower mortalities than those caused by the VCUR-509 essential oil, at both the trophont and tomont stages. However, when the protozoa were incubated in a solution containing both compounds simultaneously, mortality equal to that of the VCUR-509 essential oil was observed (Table 4).
A therapeutic bath with the essential oil of the VCUR-202 accession resulted in a significant reduction (about 30%) of the intensity of I. multifiliis on the fishes when compared to the control (Fig. 2).
Discussion
Results from in vitro and in vivo experiments show that the essential oil of V. curassavica, especially the accession VCUR-202, is effective against I. multifiliis. The in vivo test showed that therapeutic bath with essential oil reduced the I. multifiliis load of tambaqui significantly compared to the control, providing a decrease of about 30% of the number of trophonts per infected fish.
Tambaqui is the main cultivated Brazilian native species, corresponding to 28.1% of all fish production (IBGE 2015). Currently, there is a strong trend towards increased production of this species in intensive systems (Valladão et al. 2016b); however, the intensification of production has led to an increased incidence of parasites such as I. multifiliis, causing losses in fish farming. I. multifiliis is considered an obstacle to the productivity of tambaqui in intensive rearing, since it can present prevalence of 87 to 93% and abundance of 171,000 to 311,000 parasites per fish (Dias and Tavares-Dias 2015). In this context, the reduction in the number of trophonts promoted by the treatment with essential oil of V. curassavica can be considered as a relevant control option.
Although the essential oil of V. curassavica consists of many compounds in smaller amounts, it can be inferred that the monoterpenes α-pinene and sabinene are primarily responsible for the antiprotozoal activity presented by the essential oil of the VCUR-001 and VCUR-601 accessions, respectively. Mikus et al. (2000) also reported the antiparasitic activity of the monoterpenes α-pinene and sabinene against Leishmania major and Trypanosoma brucei. In general, the major compounds determine the biological properties of the particular essential oil and it is likely that their mode of action involves several targets in the cells (Bakkali et al. 2008).
The mortality observed in I. multifiliis simultaneously incubated with the compounds (E)-caryophyllene and epiglobulol evidences that there is a synergistic effect between the two compounds that confers antiprotozoal activity to the essential oil. These biological properties are the result of the synergy of all molecules or of those present in higher proportion (Bakkali et al. 2008). It is possible that the activity of a major compound is modulated by other compounds in minor proportions (Santana-Rios et al. 2001; Hoet et al. 2006). It should also be considered that the synergistic effect between the compounds may be linked to different modes of action of each compound. Due to the large number of components, essential oils appear to have no specific cellular targets (Carson et al. 2002).
The essential oils of V. curassavica and their major compounds were toxic to the two stages of I. multifillis. Most studies involving tests with substances and/or products with antiprotozoal activity against I. multifiliis use as mortality criteria the lack of motility and/or abnormal cell division (Ling et al. 2012; Fu et al. 2014a; Yao et al. 2015; Liang et al. 2015). According to De la Fuente et al. (2004), the fluorescent staining is more accurate than motility to assess protozoon viability and is recommended as a tool to quickly assess cellular damage. In the present study, we observed that damage caused to the plasma membrane of the cells of parasites has been one of the modes of action of the essential oil. At the trophont stage, disruption of the membrane and extravasation of cellular contents were visualized (Fig. 1h, i), while at the tomont stage, disruption of the cyst wall occurred, causing the release of tomites (Fig. 1g). The hydrophobicity of the essential oils allows them to pass through the cytoplasmic membrane altering its structure and permeability, leading to leakage of potassium ions and cytoplasmic content (Turina et al. 2006; Bakkali et al. 2008; Chavan and Tupe 2014).
Our study also demonstrates that the essential oils of V. curassavica accessions have different actions against I. multifiliis, all of which were directly related to the chemical composition of each essential oil. The major compounds α-pinene, sabinene, and the mixture (E)-caryophyllene + epiglobulol were toxic to I. multifiliis at the trophont and tomont stages. The major compounds tested in this study were also toxic to I. multifiliis.
Damage to membrane integrity at the trophont stage and cyst wall disruption at the tomont stage appear to be the main cause of death of I. multifiliis incubated with V. curassavica essential oils and isolated compounds. The confirmation of the antiprotozoal potential of the essential oils of V. curassavica qualifies this species as a source of promising raw material for formulation and commercialization of bioproducts to control freshwater white spot disease in fish. However, new application strategies of this essential oil should be investigated in order to improve its effectiveness.
References
Adams RP (2007) Identification of essential oil components by gas chromatograpy/mass spectroscopy, 4th edn. Carol Stream, Allured
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475
Bush AO, Lafferty KD, Lotz JM, Shostack AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83(4):575–583
Carson CF, Mee BJ, Riley TV (2002) Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 46:1914–1920
Chavan PS, Tupe SG (2014) Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeast. Food Control 46:115–120
De la Fuente G, Cebrián JA, Fondevila M (2004) A cryopreservation procedure for the rumen protozoon Entodinium caudatum: estimation of its viability by fluorescence microscopy. Lett Appl Microbiol 38:164–168
Dias MKR, Tavares-Dias M (2015) Seasonality affects the parasitism levels in two fish species in the eastern Amazon region. J Appl Ichthyol 31:1049–1055
Dickerson HW, Findly RC (2014) Immunity to Ichthyophthirius infections in fish: a synopsis. Dev Comp Immunol 43:290–299
Doughari JH, Human IS, Bennade S, Ndakidemi PA (2009) Phytochemicals as chemotherapeutic agents and antioxidants: possible solution to the control of antibiotic resistant verocytotoxin producing bacteria. J Med Plant Res 3:839–848
Ehlert PAD, Blank AF, Arrigoni-Blank MF, Paula JWA, Campos DA, Alviano CS (2006) Tempo de hidrodestilação na extração de óleo essencial de sete espécies de plantas medicinais. Rev Bras Plantas Med 8:79–80
Fu YW, Zhang QZ, Xu DX, Liang JH, Wang B (2014a) Antiparasitic effect of cynatratoside-C from Cynanchum atratum against Ichthyophthirius multifiliis on grass carp. J Agric Food Chem 62:7183–7189
Fu YW, Zhang QZ, Xu DX, Xia H, Cai XX, Wang B, Liang J (2014b) Parasiticidal effects of Morus alba root bark extracts against Ichthyophthirius multifiliis infecting grass carp. Dis Aquat Org 108:129–136
Heinecke RD, Buchmann K (2009) Control of Ichthyophthirius multifiliis using a combination of water filtration and sodium percarbonate: dose–response studies. Aquaculture 288:32–35
Hoet S, Stévigny C, Hérent MF, Quetin-Leclercq J (2006) Antitrypanosomal compounds from leaf essential oil of Strychnos spinosa. Planta Med 72:480–482
Hoyos JMA, Alves E, Rozwalka LC, Souza EA, Zeviani WM (2012) Antifungal activity and ultrastructural alterations in Pseudocercospora griseola treated with essential oils. Cienc Agrotec 36:270–284
IBGE (2015) Instituto Brasileiro de Geografia e Estatística. Produção da pecuária municipal 43:1–49
Liang JH, Fu YW, Zhang QZ, Xu DH, Wang B, Lin DJ (2015) Identification and effect of two flavonoids from root bark of Morus alba against Ichthyophthirius multifiliis in grass carp. J Agric Food Chem 63:1452–1459
Ling F, Wang JG, Lu C, Wang GX, Lui YH, Gong XN (2012) Effects of aqueous extract of Capsicum frutescens (Solanaceae) against the fish ectoparasite Ichthyophthirius multifiliis. Parasitol Res 111:841–848
Ling F, Lu C, Tu X, Yi Y, Huang A, Zhang Q, Wang G (2013) Antiprotozoal screening of traditional medicinal plants: evaluation of crude extract of Psoralea corylifolia against Ichthyophthirius multifiliis in goldfish. Parasitol Res 112:2331–2340
Maria AN, Azevedo HC, Santos JP, Silva CA, Carneiro PCF (2010) Semen characterization and sperm structure of the Amazon tambaqui Colossoma macropomum. J Appl Ichthyol 26:779–783
Martins ML, Xu DH, Shoemaker CA, Klesius PH (2011) Temperature effects on immune response and hematological parameters of channel catfish Ictalurus punctatus vaccinated with live theronts of Ichthyophthirius multifiliis. Fish Shellfish Immunol 31:774–780
Matias EF, Santos KKA, Almeida TS, Costa JGM, Coutinho HDM (2010) Atividade antibacteriana in vitro de Croton campestris A., Ocimum gratissimum L. e Cordia verbenacea DC. Rev Bras Biocienc 8:294–298
Matthews RA (2005) Ichthyophthirius multifiliis Fouquet and ichthyophthiriosis in freshwater teleosts. Adv Parasitol 59:159–241
Meccia G, Rojas LB, Velasco J, Díaz T, Usubillaga A, Arzola JC, Ramos S (2009) Chemical composition and antibacterial activity of the essential oil of Cordia verbenacea from the Venezuelan Andes. Nat Prod Commun 4:1119–1122
Mikus J, Harkenthal M, Steverding D, Reichling J (2000) In vitro effect of essential oils and isolated mono-and sesquiterpenes on Leishmania major and Trypanosoma brucei. Planta Med 66:366–368
Nizio DAC, Brito FA, Sampaio TS, Melo JO, Silva FLS, Gagliardi PL, Arrigoni-Blank MF, Anjos CS, Alves PB, Wisniewski A, Blank AF (2015) Chemical diversity of native populations of Varronia curassavica Jacq. and antifungal activity against Lasiodiplodia theobromae. Ind Crop Prod 76:437–448
Pinho L, Souza PNS, Sobrinho EM, Almeida AC, Martins ER (2012) Antimicrobial activity of hydroalcoholic extracts from rosemary, peppertree, barbatimão and erva-baleeira leaves and from pequi peel meal. Cienc Rural 42:326–331
Rodrigues FFG, Oliveira LGS, Rodrigues FFG, Saraiva ME, Almeida SCX, Cabral MES, Campos AR, Costa JGM (2012) Chemical composition, antibacterial and antifungal activities of essential oil from Cordia verbenacea DC leaves. Pharm Res 4:161–165
Santana-Rios G, Orner GA, Amantana A, Provost C, SY W, Dashwood RH (2001) Potent antimutagenic activity of white tea in comparison with green tea in the Salmonella assay. Mutat Res 495:61–74
Shinn AP, Picón-Camacho SM, Bron JE, Conway D, Yoon GH, Guo FC, Taylor NG (2012) The anti-protozoal activity of bronopol on the key life-stages of Ichthyophthirius multifiliis Fouquet, 1876 (Ciliophora). Vet Parasitol 186:229–236
Silva AC, Souza PE, Machado JC, Silva-Jr BM, Pinto JEBP (2012) Effectiveness of essential oils in the treatment of Colletotrichum truncatum-infected soybean seeds. Trop Plant Pathol 37:305–313
Silva AC, Souza PE, Resende MLV, Silva-Jr MB, Ribeiro-Jr PM, Zeviani WM (2014) Local and systemic control of powdery mildew in eucalyptus using essential oils and decoctions from traditional Brazilian medicinal plants. For Pathol 44:145–153
Turina AV, Nolan MV, Zygadlo JAP (2006) Natural terpens: self-assebly and membrane partitioning. Biophys Chem 122:101–113
Valladão GMR, Gallani SU, Ikefuti CV, Da Cruz C, Levy-Pereira N, Rodrigues MVN, Pilarski F (2016a) Essential oils to control ichthyophthiriasis in pacu, Piaractus mesopotamicus (Holmberg): special emphasis on treatment with Melaleuca alternifolia. J Fish Dis 39:1143–1152
Valladão GMR, Gallani SU, Pilarski F (2016b) South American fish for continental aquaculture. Rev Aquac 0:1–19
Van Den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr A 11:463–471
Xu DH, Shoemaker CA, Martins ML, Pridgeon JW, Klesius PH (2012) Enhanced susceptibility of channel catfish to the bacterium Edwardsiella ictaluri after parasitism by Ichthyophthirius multifiliis. Vet Microbiol 158:216–219
Yao JJ, Xu Y, Yin WL, Yuan XM, Lin LY, Xu T, Zuo ML, Pan XY, Shen JY (2015) Evaluation of nystatin isolated from Streptomyces griseus SDX-4 against the ciliate, Ichthyophthirius multifiliis. Parasitol Res 114:1425–1431
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
de Castro Nizio, D.A., Fujimoto, R.Y., Maria, A.N. et al. Essential oils of Varronia curassavica accessions have different activity against white spot disease in freshwater fish. Parasitol Res 117, 97–105 (2018). https://doi.org/10.1007/s00436-017-5673-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5673-x