Abstract
Cephenemyia stimulator larvae cause a specific myiasis in roe deer, which is widely distributed in Europe. In Spain, this parasite was detected by the first time in 2005, coinciding with a high mortality of this ruminant especially in northwest of the country. The aim of this study was to analyse the results obtained by necropsy and ELISA to elucidate when the first infestation by C. stimulator in roe deer from northwestern Spain occurred, as well as to determine the influence of some intrinsic factors on the prevalence and intensity of infestation. During 1994–2000, none seropositive roe deer was observed by ELISA. However, from 2007 to 2014, 38 % of animals were seropositive. The results of the necropsy pointed that prevalence and intensity of infestation had increased over the years. There was a positive and significant correlation between the number of animals harbouring C. stimulator larvae and seroprevalence values. This significant correlation was also observed between the seroprevalence and mean intensity of infestation. Adult roe deer showed higher prevalence and intensity of infestation than younger reaching statistical significance. It is also detected that the prevalence of infestation was significantly higher in males than in females although the mean number of larvae found in females were higher than in males. The combined use of direct and indirect techniques demonstrated a high prevalence of C. stimulator infestation in roe deer in the northwest of Spain, which certainly highlights the importance of this myiasis during the last years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The progressive abandonment of agricultural activity, especially in mountain areas, has provided the promotion of complementary activities to traditional agricultural and livestock system, such as hunting of wild ruminants. Therefore, it is important to improve knowledge about the infections and parasites affecting these animals (Panadero et al. 2007, 2010; Alasaad et al. 2009; Pérez-Creo et al. 2013; Arias et al. 2014a; Cassini et al. 2015; Díaz et al. 2015). Flies of the genus Cephenemyia (Diptera: Oestridae) are obligate parasite of several species belonged to the Cervinae subfamily. Females fly close to the host head while depositing larvae into nostrils. After that, larvae start their development to second and third instars in upper respiratory tracts (Papp and Szappanos 1992). They then pupate in the soil, and after 2 to 3 weeks, the new imagines emerge. Four species of Cephenemyia have been described in Palearctic region including Cephenemyia stimulator described as specific for roe deer (Dudziňski 1970; Barth et al. 1976; Szappanos and Papp 1991; Papp and Szappanos 1992; Lamka et al. 1997; Minář 2000; Maes and Boulard 2001; Vaca 2000; Király and Egri 2003, 2004, 2007). According to Arias et al. (2014b), the mean intensity of infestation was 24.27 (ranging from 1 to 75) larvae/infested host in northwest Spain. Fully developed larvae are about 30 mm long.
Cephenemyiosis is widely distributed throughout Europe (Király and Egri 2007); nevertheless, in Spain, no case was detected until the mid-2000s (Pajares 2009). This myiasis was observed by Notario and Castresana (2001) in the Iberian Peninsula in one roe deer imported from France. Roe deer (Capreolus capreolus, Linnaeus, 1758) is the main wild ungulate in northwest (NW) forests of Spain (Vázquez et al. 2011; Pato et al. 2013), despite these ruminant populations had suffered an important change in last years. The traditional grazing system of transhumance caused a severe depletion of roe deer stocks but subsequently, the recovery of woodland areas and regulation of hunting (Fandos and Burón 2013) allowed a great expansion of this small wild ruminant. However, since 2005 when the first record of C. stimulator was noted in NW Spain (Pajares 2009), the number of roe deer has been reduced again. This accords with other European authors who tested that 30 to 80 C. stimulator larvae may cause a high morbidity and mortality, since roe deer have poor body condition, weakness, lethargy and lack of vitality (Vaca 2000; Sol et al. 2001; Nilssen et al. 2008; Calero-Bernal and Habela 2013; Farina and Giovannini 2013; Hoekman 2013; Ahaduzzaman et al. 2015). Parasitization by C. stimulator in roe deer is of great clinical importance because the presence of larvae in the upper airways causes sinusitis, sneezing, nasal discharge, coughing, dyspnoea and swallowing problems (Calero-Bernal and Habela 2013). The massive presence of the larvae in roe deer could cause harmful consequences on their health and general condition and state of immunosuppression similar to that observed in other hosts due to parasitism by Oestridae (Alcaide et al. 2003). Furthermore, Cephenemyia causes high levels of stress and unrest which affect to food intake when females deposited first instars (L1) (Hughes et al. 2009). In Europe, by necropsy, some authors have reported C. stimulator in roe deer from Poland (Dudziňski 1970; Cepelák and Macicka 1979), Slovakia (Čurlík et al. 2004), Hungary (Sugár 1975, 1978; Király and Egri 2007), and the Czech Republic (Lamka et al. 1997; Vaca 2000; Salaba et al. 2013). Nevertheless, seroprevalence studies are scarce, only carried out in France by Maes (2000) and Maes and Boulard (2001) and in Spain by our research group (Arias et al. 2014b). A positive correlation between immunoglobulin G (IgG) values and total number of larvae was found by enzyme-linked immunosorbent assay (ELISA) using second instars larvae (L2) of C. stimulator excretory/secretory antigens (Arias et al. 2014b). As it usually happens with other parasites, the infestation prevalence of cephenemyiosis have been influenced by intrinsic factors like age, gender, body condition and immunological status (Jahn et al. 2002; Király and Egri 2007; Vázquez et al. 2011; Pato et al. 2013).
The main aim of this study was to elucidate when C. stimulator infestation appeared in roe deer in the northwest of Spain. For that purpose, we analyse the results obtained by necropsy and ELISA along the years. In addition, we determine the influence of some intrinsic factors on the prevalence and intensity of infection.
Materials and methods
Animals and study area
Northwest Spain is an important livestock-rearing area, where domestic animals share pastures with other wild ruminants, being roe deer the most abundant (Vázquez et al. 2011; Pato et al. 2013). This area has an oceanic climate characterised by mild temperatures and high precipitation. Summers are generally warm and humid, with considerable sunshine and some rain. Winters are cold, especially in the mountains, in which snow may be present from October until May. The study area is basically comprised of scrubland, fields and woodland. The characteristics of the forest are changing because of human activity. Many Eucalyptus globules and Pinus pinaster plantations have been established (Arias et al. 2014b).
Serological procedures
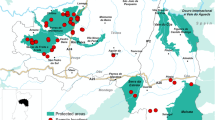
Blood samples of 1094 roe deer from northwestern Spain (Galicia, Asturias and León) were collected between 1994 and 2014. Samples were taken directly from the heart of each animal with a syringe during evisceration. After clotting, they were centrifuged and sera were collected and stored at –20 °C. The number of sera analysed each year, as well as the age and gender of the animals are shown in Table 1. Thus, Fig. 1 shows the location of hunting reserves of the animals analysed in the present study.
Antibodies [immunoglobulin G (IgG)] were detected by ELISA following the protocol described by Arias et al. (2014b). This probe has high sensitivity; negative predictive value and negative likelihood ratio were obtained using C. stimulator excretory/secretory antigens (CsES) by incubating second instars larvae (L2) of this parasite (Arias et al. 2014b). L2 were previously selected according to size (Zumpt 1965; Colwell and Scholl 2006a, b) and washed in phosphate-buffered saline (PBS, pH 7.4).
Larvae collection and identification
Ninety-eight roe deer from northwestern Spain (León, Asturias and Galicia) were examined for nasopharyngeal myiasis between 2012 and 2014. The number of animals analysed each year, as well as their age and gender are shown in Table 2. Necropsies were performed just after death, the skin of the ventral side of the necks and heads of ruminants was removed and the oesophagi and trachea were opened. The soft palate was opened to allow the observation of the oral cavity, pharynx and larynx in order to find larvae of C. stimulator (Arias et al. 2014b). The collected bot fly larvae were identified to species level following the keys reported by Zumpt (1965) and Colwell and Scholl (2006a, b). The three different larvae instars, first (L1), second (L2) and third instars (L3), were identified according to morphological and morphometric characteristics (Calero-Bernal and Habela 2013). L2 exhibit an unarmed tenth segment, and between four and six rows of spines on the remaining segments. L3 present a characteristic rear reniform peritreme. Regarding the size of the larvae, L1 (1–3 mm in length), L2 (3–13 mm long) and L3 (13–30 mm). In addition, the results at necropsy were compared to the seroprevalence reported in 240 sera obtained between 2012 and 2014 (Table 1).
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows Version 19.0 (IBM Corp., Armonk, NY, U.S.A.). The differences in prevalence values between hunting season, age and gender, were analysed using the chi-squared test and expressed as percentages with 95 % confidence intervals (CIs) (Thursfield 2005). Differences were considered as significant if they achieved a P value of <0.05. The ANOVA (F) test was used to study the mean intensity and differences of infestation by L1, L2 and L3 instars of C. stimulator larvae.
The possible relationship between the numbers of larvae found in different studies was established by the Spearman correlation test (ρ). The same test was performed to determine the relationship between the percentage of infested roe deer by necropsy and seroprevalence, as well as mean intensity and seroprevalence.
Results
Detection of the first positive cases by ELISA
Four hundred and fourteen roe deer from León province correspond to 1994 and 2000 were studied and none animal was seropositive to C. stimulator. However, 38 % (CI 35–42) of ruminants hunted in León, Asturias and Galicia between 2007 and 2014 had C. stimulator antibodies. Seroprevalence varied significantly from year to year and oscillated between 36 % (28–43) in 2007 and 60 % (51–69) in 2014. A progressive increase in seroprevalence was observed since the average percentage of positive sera raised from 33 % in the three first years (2007–2009) to 50 % in the last three years of our study (2012–2014). These differences were significant (X 2 = 46.482, P = 0.001).
Regarding age and gender, seroprevalence was slightly higher in adults (52 %, 47–57) and males (40 %, 36–44) than in young (45 %, 34–55) and females (33 %, 23–43); however, no significant differences were observed.
Necropsy
A total of 610 larvae were collected in 98 heads. C. stimulator was the only bot fly found in the sampled roe deer. The number of larvae ranged between 2 and 75 and the mean intensity of infestation was 19.67 ± 21.0. Regarding different larvae instars, the percentages of L2 and L3 were higher than the percentage of L1, probably because of their small size. A positive and significant correlation was observed between the number of L1 and L2 (ρ = 0.618, P = 0.001), L1 and L3 (ρ = 0.210, P = 0.038) and L2 and L3 (ρ = 0.567, P = 0.001). Moreover, a positive and significant correlation between the total number of larvae (TL) and L1 (ρ = 0.558, P = 0.001), TL and L2 (ρ = 0.916, P = 0.001) and TL and L3 (ρ = 0.823, P = 0.001) was found.
Considering the prevalence of C. stimulator according to hunting year, the prevalence was significantly higher (χ2 = 13.016, P = 0.005) in 2012 and 2013 (57 %, 36–78 and 41 %, 22–59, respectively) than in 2014 (16 %, 6–26). Similarly, the mean intensity of infestation in 2012 \( \left(\overline{x} = 26^{\prime }75 \pm 27^{\prime }93\right) \) was higher than in 2013 \( \left(\overline{x} = 13 \pm 10.68\right) \) and in 2014 \( \left(\overline{x} = 18.75 \pm 18.15\right) \). Statistical analysis showed significant differences (F = 3.763, P = 0.013). The prevalence and intensity of infestation were higher in adults \( \left(62\ \%,\ 41\hbox{--} 83;\overline{x} = 23.3 \pm 23.7\right) \) than in young roe deer \( \left(23\ \%,\ 14\hbox{--} 33;\overline{x} = 14.6 \pm 15.9\right) \). However, only prevalence rates were significant (X 2 = 11.325, P = 0.001). Concerning the gender of ruminants, significant differences were observed in prevalence values (χ2 = 18.181, P = 0.001) between males (55 %, 40–70) and females (14 %, 5–23). On the contrary, females presented higher larvae counts \( \left(\overline{x} = 36.1 \pm 21.1\right) \) than males \( \left(\overline{x} = 14.1 \pm 18.0\right) \), but no statistical significance was found.
The current study detected a positive and significant correlation (ρ = 0.683, P = 0.029) between percentage of infested roe deer by C. stimulator larvae at necropsy and seroprevalence. In addition, correlation between mean intensity of infestation and seroprevalence by ELISA was found (ρ = 0.742, P = 0.014).
Discussion
According to our results, no roe deer was seropositive to C. stimulator between 1994 and 2000 in different national hunting preserves located in León province. This seems to indicate the absence of this myiasis before the decade of 2000s, confirmed by the negative results obtained by ELISA and the absence of direct descriptions of Cephenemyia infestation in animals examined by hunters or researchers. The origin of this infestation (between years 2000 and 2007) is unknown, but in 2007, this myiasis arised in roe deer (Pajares 2009). These results pointed that something unexpected happened in the early 2000 causing a radical change and the emergence of more C. stimulator infestation cases every year (Arias et al. 2012, 2014b). Calero-Bernal and Habela (2013) suggest that its appearance is due to the reintroduction of roe deer from areas where the parasite is endemic. An irregular importation of carrier roe deer (Notario and Castresana 2001; Pajares 2012) results in the infestation by C. stimulator in native roe deer. Although, other possible explanations related with climate change provoking modifications in the phenology of the fly could be involved, but there are no studies regarding this hypothesis. In this sense, we need more entomological backup information. Immune problems of native roe deer or competition with other taxa should be considered.
Seroprevalence values obtained in our research were similar to those pointed by Maes and Boulard (2001) in hunted roe deer in France in years 1998 and 1999 (3243 %). C. stimulator was the only oestrid harboured in the 98 roe deer examined in accordance with authors who pointed that C. stimulator is specific for roe deer (Dudziňski 1970; Barth et al. 1976; Szappanos and Papp 1991; Papp and Szappanos 1992; Lamka et al. 1997; Minář 2000; Maes and Boulard 2001; Vaca 2000; Király and Egri 2003, 2004, 2007; Arias et al. 2014b). The percentage of infected roe deer in our study was similar to those observed in Czech Republic by Vaca (2000) and Salaba et al. (2013), but it was noticeably lower to those pointed by Lamka et al. (1997) and Sugár (1975, 1978) in Czech Republic and Hungary. Besides, the mean intensity of C. stimulator infestation was similar to other researches in roe deer from diverse European countries (Dudziňski 1970; Barth et al. 1976; Szappanos and Papp 1991; Papp and Szappanos 1992; Minář 2000; Maes and Boulard 2001; Király and Egri 2003, 2004, 2007). Furthermore, significant differences were observed in prevalence values and intensity of infestation with respect to hunting years. On the contrary, no significant differences were pointed by Király and Egri (2003) in Hungarian roe deer hunted in two successive years. The highest prevalence of infestation was found in adult roe deer, according to the results from Panadero et al. (2010) in small ruminants infested by Hypoderma spp. Thus, these authors observed that seroprevalence increased with the age of the animals. However, in our study, mean intensity of C. stimulator larvae was higher in young animals, in accordance with Vaca (2000) and Király and Egri (2007), who suggested that young roe deer revealed a less efficient immune system and worse defence against the deposit of larvae by C. stimulator females into the nostrils (Király and Egri 2004, 2007; Sugár et al. 2004). Males showed the highest prevalence and differences were considered significant. It could be related to the fact that males are more active than females, and bucks exhibit a pronounced territorial behaviour, aggressively expelling other males (Melis et al. 2005). The expelled males are then forced to disperse, increasing the chance of infection. Moreover, it has been also suggested that certain sexual hormones, such as sex steroids, modulate several aspects of host immunity, so males could become more susceptible than females to many infectious pathogens, including this myiasis (Király and Egri 2007) and another infestations by ectoparasites as ticks (Vázquez et al. 2011). In a previous work concerning gastrointestinal parasites in roe deer, Pato et al. (2013) observed that the highest prevalence corresponded to males, although the intensity of infestation was higher in females than in males. Thus, it would be interesting to investigate further about the differences in the immune system by gender. A positive correlation between total number of larvae and the results achieved using ELISA and excretion-secretion antigens obtained from L2 of C. stimulator agree with results from a previous study (Arias et al. 2014b), consequently the usefulness of this technique to carry out epidemiological surveys concerning cephenemyiosis and other myiasis in wild ruminants is confirmed (Panadero et al. 2010; Arias et al. 2014b).
This research revealed that there is a high prevalence of C. stimulator infestation, borne out by both direct and indirect techniques. This fact ensures the increasing importance of this infestation in recent years. Moreover, age and gender determine appreciable differences in prevalence and intensity of infestation. Further studies are required to get more knowledge about possible factors that influence on nasopharyngeal bot infestation, such as sexual hormones and immune response of wild ruminant.
References
Ahaduzzaman M, Islam MS, Akter S, Uddin MJ, Sharif SMO, Mannan A (2015) Asphyxial death by Oestrus ovis in a pneumonic goat. J Adv Parasitol 2:48–51
Alasaad S, Morrondo P, Dacal-Rivas V, Soriguer RC, Granados JE, Serrano E, Zhu XQ, Rossi L, Pérez JM (2009) Bronchopulmonary nematode infection of Capra pyrenaica in the Sierra Nevada massif, Spain. Vet Parasitol 164:340–343
Alcaide M, Reina D, Sánchez J, Frontera E, Navarrete I (2003) Seasonal variations in the larval burden distribution of Oestrus ovis in sheep in the southwest of Spain. Vet Parasitol 118:235–241
Arias MS, Sánchez-Andrade R, Paz-Silva A, Suárez JL, Cazapal-Monteiro C, Prieto JM, Casais R, Díez-Baños P, Morrondo P (2012) Assessment of Cephenemyia stimulator infection in roe deer (Capreolus capreolus) from Asturias (North Spain) by ELISA. Mapp Parassitol 18:129
Arias MS, Moreno V, Sarasa M, Paz-Silva A, Sánchez-Andrade R, Morrondo P, Díez-Baños P, Granados JE, Sánchez A, Pérez JM (2014a) Reliability of an ELISA test for diagnosing oestrosis in Iberian ibex. J Parasitol 100:235–238
Arias MS, Pajares G, Paz-Silva A, Díez-Baños N, Suárez JL, Díez-Baños P, Sánchez-Andrade R, Morrondo P (2014b) Antigen characterization from second instar larvae of Oestridae flies for the detection of anti-Cephenemyia stimulator antibodies by ELISA in roe deer (Capreolus capreolus). Med Vet Entomol 28:83–89
Barth D, Kudlich H, Schaich K (1976) Occurrence and significance of nasal bot infestation in roe bucks (Capreolus capreolus). In: Page LA (ed). Wildlife Diseases. Plenum Press, New York, London, pp 609–613
Calero-Bernal R, Habela MA (2013) First report of Cephenemyia stimulator (Diptera, Oestridae) parasitizing roe deer (Capreolus capreolus) in Extremadura (Spain). Galemys 25:29–34
Cassini R, Párraga MA, Signorini M, Frangipane di Regalbono A, Sturaro E, Rossi L, Ramanzin M (2015) Lungworms in Alpine ibex (Capra ibex) in the eastern Alps, Italy: an ecological approach. Vet Parasitol 214:132–138
Cepelák J, Macicka O (1979) Ksezönnemu vyskytu a ekológii streckov raticovej zveri na lesnej správe v Kamenici nad Hronom. Folia Venatoria 9:293–299
Colwell DD, Hall MJR, Scholl PJ (2006a) A synopsis of the biology, hosts, distribution, disease significance and management of the genera. In: Colwell DD, Hall MJR, Scholl PJ (eds) The oestrid flies: biology, host-parasite relationships, impact and management. CAB International, Wallingford, pp 220–305
Colwell DD, Scholl PJ (2006b) Introduction. In: Colwell DD, Hall MJR, Scholl PJ (eds) The oestrid flies: biology, host-parasite relationships, impact and management. CAB International, Wallingford, pp 1–7
Čurlík J, Letková V, Ciberej J, Lazar P, Goldová M, Kočišová A, Košuthová L, Trávniček M, Bhide M, Lazar G, Pošivak J, Konjevič D (2004) The occurrence of the genera Hypoderma, Cephenemyia and Pharyngomyia in deer in the Slovak Republic. Folia Vet 48:92–94
Díaz JM, Prieto A, López C, Díaz P, Pérez A, Panadero R, Pajares G, Díez-Baños P, Morrondo P, Fernández G (2015) High spread of Schmallenberg virus among roe deer (Capreolus capreolus) in Spain. Res Vet Sci 102:231–233
Dudziňski W (1970) Studies on Cephenemyia stimulator (Clark, 1815) (Diptera, Oestridae), the parasite of European roe deer, Capreolus capreolus (L.). I Biology Acta Parasitol Polon 18:555–572
Fandos P, Burón D (2013) Corzos. Edición propia, Sevilla
Farina G, Giovannini R (2013) Principale patologie evidenziate nella fauna selvática dal 2001 al 2011 in Provincia di Trento. Servizio Foreste e Fauna della Provincia Autonoma di Trento, Italy
Hoekman ED (2013) Dutch roe deer (Capreolus capreolus), review of cases presented at the Dutch Wildlife Health Centre. Dissertation, Veterinary Faculty, University of Utrecht, The Netherlands
Hughes J, Albon SD, Irvine RJ, Woodin S (2009) The cost of parasites to caribou. Parasitology 136:253–265
Jahn P, Minář J, Gelbič I (2002) Napadení koní larvami střečka srnčího (Hypoderma diana). Veterinarstvi 52:476–477
Király I, Egri B (2003) Data on the nasopharyngeal bot infestation of roe deer in Tolna county in 2003. Vadbiológia 10:55–60
Király I, Egri B (2004) A Tolna megyei őzállomány orrgaratbagócs fertőzöttségéről (Nasopharyngeal bot infestation of the roe deer population of Tolna county). Magy Allatorvosok Lapja 126:433–438
Király I, Egri B (2007) Epidemiological characteristics of Cephenemyia stimulator (Clark, 1815) larvae infestation in European deer (Capreolus capreolus) in Hungary. Acta Zool Acad Sci Hung 53:271–279
Lamka J, Suchý J, Štaud F (1997) Efficacy of orally administered Ivermectin against larval stages of bot fly (Cephenemyia stimulator C.) in roe deer. Acta Vet Brno 66:51–55
Maes S (2000) Etude sero-epidemiologique de l’Hypodermose et des myiases naso-pharyngees (cephenemyiose et pharyngomyiose) des cervides en France. DissertationUniversity of Paris-Est, Creteil
Maes S, Boulard C (2001) Deer myiasis in France. In: Good M, Hall MJ, Losson B, O’Brien D, Pithan K, Sol J (eds) Proceedings of COST Action 83, Mange and myiasis of livestock. Commission of the European Communities, Brussels, pp 181–186
Melis C, Cagnacci F, Lovari S (2005) Do male roe deer clump together during the rut? Acta Theriol 50:263–262
Minář J (2000) Family oestridae. In: Papp L, Darvas B (eds) Contributions to a manual palaearctic Diptera. Science Herald, Budapest, pp 467–478
Nilssen AC, Isomuru M, Oksanen A (2008) The moose throat bot fly Cephenemyia ulrichii larvae (Diptera: Oestridae) found developing in roe deer (Capreolus capreolus) for the first time. Acta Vet Scand 50:14
Notario A, Castresana L (2001) Contribution to the knowledge of Cephenemyia stimulator Clark, 1815 (Diptera, Oestridae) in Spain. Folia Ven 30–31:325–326
Pajares G (2009) Apuntes de Biología. Primera cita en España de Cephenemyia stimulator en corzos. Bol ACE 11:36
Pajares G (2012) Las larvas de la catástrofe: así empezó, qué signos tiene y cómo debemos actuar ante esta enfermedad. Caza Mayor 160:26–32
Panadero R, Vázquez L, Colwell DD, López C, Dacal V, Morrondo P, Díez-Baños P (2007) Evaluation of an antigen capture ELISA for the early diagnosis of Hypoderma lineatum in cattle under field conditions. Vet Parasitol 147:297–302
Panadero R, Martínez C, León-Vizcaíno L, López C, Díez Baños P, Morrondo P, Alonso F (2010) Use of a crude extract or purified antigen from first-instars cattle grubs, Hypoderma lineatum, for the detection of anti-Hypoderma antibodies in free-ranging cervids from southern Spain. Med Vet Entomol 24:418–424
Papp L, Szappanos A (1992) Bagócslegyek: Gasterophilidae, Oestridae, Hypodermatidae. Magyar Természettudományi Múzeum, Budapest
Pato FJ, Panadero R, Vázquez L, López CM, Díaz P, Vázquez E, Díez-Baños P, Morrondo P, Fernández G (2013) Seroprevalence of Borrelia burgdorferi sensu lato in roe deer (Capreolus capreolus) from northwestern Spain. J Zoo Wildl Med 44:660–665
Pérez-Creo A, Panadero R, López C, Díaz P, Vázquez L, Díez-Baños P, Morrondo P (2013) Prevalence and identity of Sarcocystis spp. in roe deer (Capreolus capreolus) in Spain: a morphological study. Res Vet Sci 95:1036–1040
Salaba O, Vadlejch J, Petrtyl M, Valek P, Kudrnacova M, Jankovska I, Bartak M, Sulakova H, Langrova I (2013) Cephenemyia stimulator and Hypoderma diana infection of roe deer in the Czech Republic over an 8-year period. Parasitol Res 112:1661–1666
Sol J, Sampimon OC, Martínez-Moreno J (2001) Hypoderma diana in roe deer in the Netherlands. Tijdschr Diergeneeskd 126:500–501
Sugár L (1975) Adatok a magyarországi szarvasfélék (Cervidae) parazitás fertózöttségéhez (Data on the parasitic infection of Cervidae in Hungary). In: Izrael G (ed) Nagyvadgazdálkodás (Big game management). MÉM Vadászanti és Vadgazdálkodási Fóosztály, Budapest, pp 85–102
Sugár L (1978) A vadon élő kérődzők orr-garat (torok) bagócs-fertőzöttsége (oestridosis) (Nasopharyngeal bot infestation of wild ruminants (oestridosis). In: Hőnich M, Sugár L, Kemenes F (eds) A vadon élő állatok betegségei (Diseases of Wildlife). Mezőgazdasági Kiadó, Budapest, pp 156–158
Sugár L, Kovács SZ, Kovács A, Kőrös A, Varga GY (2004) Distribution of the occurrence of nasopharyngeal bots by age and season in a red deer population in the Bakony mountains, Hungary. Vadbiológia 11:24–29
Szappanos A, Papp L (1991) Bot flies and warble flies (Diptera: Gasterophilidae, Oestridae, Hypodermatidae) in the collection of the Hungarian Natural History Museum. II. Larvae. Parasitol Hung 24:89–98
Thursfield M (2005) Veterinary epidemiology. Blackwell, Oxford
Vaca D (2000) Biology of nasopharyngeal bot fly Cephenemyia stimulator Cl. (Diptera, Oestridae) and its distribution in the Czech Republic. In: Good M, Hall MJ, Losson B, O’Brien D, Pithan K, Sol J (eds) Proceedings of COST Action 83. Mange and myiasis of livestock. Commission of the European Communities, Brussels, pp 189–194
Vázquez L, Panadero R, Dacal V, Pato FJ, López C, Díaz P, Arias MS, Fernández G, Díez-Baños P, Morrondo P (2011) Tick infestation (Acari: Ixodidae) in roe deer (Capreolus capreolus) from northwestern Spain: population dynamics and risk stratification. Exp Appl Acarol 53:399–409
Zumpt F (1965) Myiasis in man and animals in the old world. Butterworths, London
Acknowledgments
This work was supported by the Programme for Consolidating and Structuring Competitive Research Groups: Competitive Group (GRC 2015/003, Xunta de Galicia) and a ‘Parga Pondal’ post-doctoral research grant (XUGA) to Dr. M.S. Arias. The authors express their gratitude to the Asociación del Corzo Español (ACE) and the Consellería de Medio Ambiente, which facilitated sample collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Sol, A.M., Gerardo, P., Natividad, DB. et al. Cephenemyiosis, an emergent myiasis in roe deer (Capreolus capreolus) from northwestern Spain. Parasitol Res 115, 4605–4610 (2016). https://doi.org/10.1007/s00436-016-5251-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5251-7