Abstract
A new species of myxozoan, Henneguya melini sp. n. (Myxosporea: Myxobolidae), was described based on morphologic and ultrastructural features. This is a parasite of the ornamental freshwater fish C. melini from the Rio Negro, and it was found in five of 30 (16.7 %) C. melini examined. The parasite was found in the gill filaments, and the plasmodia had form of round to ellipsoid, with mature and immature spores inside them. The average spore body was 15.5 ± 0.2 μm in length, 4.7 ± 0.1 μm in width, and the tail measured 25.3 ± 0.1 μm in length. The spores showed typical features of the genus Henneguya, with two valves of equal size and two symmetrical polar capsules of 4.8 ± 0.7 μm in length and 1.7 ± 0.3 μm in width. Each polar capsule had a polar filament with five to six turns. Based on morphology (morphologic and ultrastructural data) of the plasmodia and spores and the fact that this is the first report of a Henneguya species in a fish species of the genus Corydoras, it was considered a new myxozoan species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in the Myxozoa as significant fish parasites is still increasing with the growing importance of fish farm and wild fisheries around the world. Henneguya Thélodan, 1982 (Myxozoa: Myxosporea) is one of the genera from family Myxobolidae with the highest diversity (Eiras 2002). Currently, more than 200 species are known (Eiras 2002; Eiras and Adriano 2012), and in recent years, South America has been the continent where more than 50 % of the new species reported (Eiras and Adriano 2012). Species of the genus Henneguya are predominantly histozoic, invading different organs and causing considerable pathological changes (Dyková and Lom 1978; Molnár 1998; Pote et al. 2000; Adriano et al. 2005; Naldoni et al. 2009; Barassa et al. 2012; Morsy et al. 2012).
Corydoras melini Lönnberg and Rendahl, 1930 (English name = bandit corydoras), is a Siluriformes freshwater fish belonging to the Callichthyidae family that harbor freshwater fishes with occurrence restricted to the Neotropical Region (Panama and South America) (Reis 1998). C. melini is endemic to the upper Rio Negro and Rio Meta Basins in Brazil and Colombia (Reis 1998; Froese and Pauly 2011), and it is popular in the aquarium trade industry (Froese and Pauly 2011). Despite the commercial importance of species of the genus Corydoras, only two myxozoans have been reported infecting them (Mathews et al. 2015, 2016).
Herein, as part of a continuous study of freshwater myxozoans biodiversity, was described a new species of Myxozoa, Henneguya melini n. sp., which was found in the gill filaments of C. melini from the Rio Negro, Amazonas State, Brazil. The parasite was characterized based on critical morphological features using light and transmission electron microscopy.
Materials and methods
Thirty wild C. melini specimens (ranging from 4.5 to 5 cm in length) caught in the Rio Negro, municipality of Santa Isabel do Rio Negro (0° 24′ 50″ S, 65° 01′ 08″ O), Amazonas state, Brazil, were examined in June 2014. Fish were transported live to the field laboratory, where they were euthanized in a benzocaine solution overdose (400 mg L−1) (methodology approved by the ethics research committee of the Federal University of São Paulo – UNIFESP, CEUA N 9209080214), in accordance with Brazilian law (Federal Law No. 11.794, dated 8 October 2008 and Federal Decree No. 6899, dated 15 July 2009).
All organs and body fluids were examined for myxosporeans infection. Mature myxospores were examined on fresh wet mounts by light microscopy. Morphological and morphometric analyses were performed on mature myxospores obtained from two different plasmodia based on the criteria outlined by Lom and Arthur (1989). Measurements were taken of 30 myxospores using a computer equipped with Axivision 4.1 image capture software coupled to an Axioplan 2 Zeiss microscope. Smears containing free spores were air-dried and stained with Giensa solution and mounted in a low-viscosity mounting medium (Cytoseal™) on permanent slides.
For ultrastructural examination, plasmodia were fixed for at least 12 h in 2.5 % glutaraldehyde with 0.1-M buffered cacodylate (pH 7.4). After this, the plasmodia were washed in the same buffer and post-fixed with osmium tetroxide (OsO4). All procedures were performed at 4 °C. After dehydration in an ascending ethanol series, the samples were embedded in EMbed 812 resin (Electron Microscopy Sciences, Hatfield, PA, USA). Ultrathin sections, double stained with uranyl acetate and lead citrate, were examined in an LEO 906 electron microscope operating at 60 kV.
Results
Five out of 30 (16.7 %) specimens of C. melini caught from the Rio Negro had plasmodia of an unknown Henneguya species in the gill filaments. The intensity ranged of one to three cyst per fish and they were not found in any other organs.
Henneguya melini n. sp. (Figs. 1, 2, 3, and 4).
Electron micrography of plasmodia (P) of Henneguya melini n. sp. parasite of the gill filaments of Corydoras melini showing the host–parasite interface. Externally can be seen connective tissue layer (ct) with collagenic fibers (f) and fibroblasts cells (asterisk), surrounding the plasmodial wall (large black arrows). Internally are seen a thin ectoplasm (ec), fragments of mature spores (ms), and disporic sporoblasts (sb) with immature spores (is). In a, note young sporoblast developmental stage with polar filaments (large white arrows) still out of the polar capsules (pc). In b, the sporoblast is in more advanced developmental stage, with the polar filament inside of the polar capsule, abundant valve-forming material (vm), and conspicuous sutural line (white thin arrows). The binucleate sporoplasms (sp) have numerous sporoplasmosomes (thin black arrows). cp capsulogenic cells, nc nucleus of capsulogenic cell, n nucleus Scale bars = 2 μm
Electron micrography of plasmodia (P) of Henneguya melini n. sp. parasite of the gill filaments of Corydoras melini. a Almost mature spores (sp). Note the sutural lines (large arrows), polar capsules (pc), and sporoplasm (spl) with its nucleus (n) and sporoplasmosomes (thin arrow). Scale bar = 2 μm. b Amplified area of a showing longitudinal and transversal distributions of the collagenic fibers (f) in the connective tissue layer (ct), single membrane of the plasmodial wall (large arrow), and pinocytotic channels (thin arrows) in the ectoplasm (ec). Scale bar = 1 μm. c Spores (sp) with details of the polar filaments (arrows) in the polar capsules (pc). Scale bar = 2 μm
Description
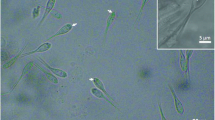
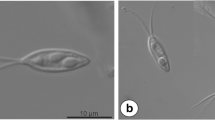
Round to ellipsoidal cysts measured up to 260 μm in diameter and with numerous myxospores were found in the gill filaments of C. melini (Fig. 1a). Mature myxospores were ellipsoidal in shape from the frontal view and had a total length of 40.8 ± 0.3 μm (40.3–41.1), a body length of 15.5 ± 0.2 μm (15.3–15.7), width of 4.7 ± 0.1 μm (4.6–4.8), and the tail measured 25.3 ± 0.1 μm (25.2–25.4) in length. The two polar capsules were elongated and equal in size, and have 4.8 ± 0.5 μm (4.3–5.3) in length and 1.7 ± 0.3 μm (1.4–2.0) in width. They occupied only the anterior third of the myxospore body, and helical filament with five to six coils was arranged obliquely to the longitudinal axis (Figs. 1b, c and 4).
Ultrastructural analysis showed a connective capsule surrounding the plasmodia, which was composed by collagen fibrils and some fibroblasts (Figs. 2 and 3a, b). The wall of the plasmodia consisted of a single membrane and the periphery of the ectoplasm had several pinocytic channels (Figs. 2 and 3b). The development of the plasmodia was asynchronous, with numerous mature spores in the central area and young developmental in the periphery (Figs. 1a, 2, and 3). Sections of immature myxospores indicated a polar capsule with five to six coils positioned obliquely to the longitudinal axis of the capsule and binucleated sporoplasms that contained several sporoplasmosomes (Figs. 2 and 3a). Capsulogenic and valvogenic cells were readily recognized in the developing sporoblast by the presence of bulbous capsular primordia and associated external tube in the first and by valve-forming materials in the second (Fig. 2).
Type host: Corydoras melini Lönnberg and Rendahl, 1930 (Callichthyidae: Corydoradinae).
Site of infection: Gill filaments
Type locality: Rio Negro, Municipality of Santa Isabel do Rio Negro, state of Amazonas, Brazil.
Prevalence: Five out of 30 (16.7 %).
Type material: glass slides with stained spores (syntype) deposited in the collection of the Museum of Zoology of State University of Campinas, “Adão José Cardoso,” State University of Campinas (UNICAMP), state of Sao Paulo, Brazil (accession number Zuec Myx 51).
Etymology: The specific name is derived from the name of the host species (“melini”).
Discussion
Several studies have reported infections of species of the genus Henneguya Thélodan, 1982, in Amazonian fishes (Rocha et al. 1992; Azevedo and Matos 1995; Casal et al. 1997; Vita et al. 2003; Feijó et al. 2008; Videira et al. 2015). However, to our knowledge, this is the first report of a Henneguya species parasitizing fish of the family Callichthyidae in the Amazon basin and the first report of this parasite infection in Corydoras fish in South America. In the present study, based on morphological features obtained by light and electron microscopy, Henneguya melini n. sp. is described parasitizing gill filaments of C. melini, a callichthyid fish endemic to the upper Rio Negro and Rio Meta basins in Brazil and Colombia (Reis 1998; Froese and Pauly 2011).
The morphological and morphometric data of H. melini n. sp. were compared firstly with all Henneguya spp. described previously infecting South America Siluriformes freshwater fish (Matos et al. 2005; Abdallah et al. 2007; Eiras et al. 2009; Naldoni et al. 2009; Naldoni et al. 2011; Adriano et al. 2012; Carriero et al. 2013; Naldoni et al. 2014). The most similar species to the new species were Henneguya maculosus Carriero et al., 2013, which infects Pseudoplatystoma corruscans and Henneguya eirasi Naldoni et al., 2011, which infect Pseudoplatystoma fasciatum and Pseudoplatystoma corrucans. However, these differ from H. melini n. sp. in the size of the spore (13.3 ± 0.7 μm in length to H. maculosus, 37.1 ± 1.8 μm to H. eirasi and 40.8 ± 0.3 μm to the new species), number of coils in the polar filament (six to seven to H. maculosus, 12 to 13 H. eirasi and five to six to the new species), and because the tail is substantially greater in length (25.3 ± 0.1 μm in the new species and 17.5 ± 1.0 μm in H. maculosus and 23.6 ± 2.2 μm in H. eirasi). Considering Henneguya guanduensis Abdallah et al., 2007, which was described from Hoplosternum littorale, the closest host species to C. melini, the comparison showed shorter and wider spores for H. guanduensis, with 33.6 × 6.5 μm, while 40.8 × 4.7 μm for H. melini n. sp. The tail was smaller for H. guanduensis with 19.0 versus 25.3 μm for H. melini n. sp., and still the two polar capsules of H. guanduensis are unequal in size, while they are equal in H. melini n. sp.
Compared with Henneguya species which infect Siluriformes fish from other regions of the world (Sarkar 1985; Landsberg 1987; Kostoïngue et al. 2001; Reed et al. 2003; Molnár et al. 2006; Iwanowicz et al. 2008; Griffin et al. 2008; Rabie et al. 2009; Morsy et al. 2012), H. melini n. sp. resembles those of Henneguya suprabranchiae Landsberg, 1987, and Henneguya gurleyi, Kudo, 1919, which infect the freshwater catfish Clarias lazera and Ameiurus nebulosus, respectively (Landsberg 1987; Iwanowicz et al. 2008). However, a small number of noticeable morphologic differences were observed, such as spore tail length (25.3 μm for H. melini n. sp.; 41.1 μm for H. gurlei and 29.0 μm for H. suprabranchiae) and the number of turns of the polar filaments (five to six turns in H. melini n. sp., 10 to 11 turns in H. suprabranchiae and nine turns in H. gurlei). Other differences may also be observed in the width of the spores (4.7 μm for H. melini n. sp., 3.0 μm for H. suprabranchiae, and 5.4 μm for H. gurlei).
The morphology of H. melini n. sp. also was compared with Henneguya species that have been reported infecting gills of not Siluriformes fishes from Amazon Basin. The species described in this paper has a total size similar to that of Henneguya aequidens Videira et al., 2015, and Henneguya striolata Casal et al., 1997. However, H. aequidens has longer tail (27 ± 0.6 μm), is greater in width (6 ± 0.8 μm), and the polar capsules are smaller in length (3 ± 0.3 μm) (Videira et al., 2015). In the same way, H. striolata has more number of filament turns (13 to14) and the polar capsules are smaller in width (1.1–1.3 μm) (Casal et al., 1997). Regarding the body width of spore, polar capsule width, or length and shape, H. melini n. sp. resembles Henneguya astyanax Vita et al., 2003, Henneguya friderici Casal et al., 2003), Henneguya amazonica Rocha et al., 1992, Henneguya adherens Azevedo and Matos, 1995, Henneguya malabarica Azevedo and Matos, 1996, and Henneguya schizodon Eiras et al., 2004. Nevertheless, it has been noticed that the parasite was readily distinguishable by lower number of polar filament turns (five to 6 for H. melini n. sp.; 8–9 for H. astyanax, 7–8 for H. friderici, 6–7 for H. malabarica, and 8–10 for H. schizodon). Differences may also be observed in the length of the tail of the spores (25.3 μm for H. melini n. sp.; 18.5 μm for H. adherens, 41.7 μm for H. amazonica, 32.6 μm for H. astyanax, 19.1 μm for H. friderici, and 16.3 μm for H. schizodon). In the same way, considering the shape and size of myxospores and polar capsules, number of polar filament turns, host, and tissue affinity, H. melini n. sp. differs from all other Henneguya spp. parasites of freshwater fish found out of the Amazon basin, at least, in one of those characteristics (Eiras 2002; Eiras and Adriano 2012).
Ultrastructural studies on the plasmodial wall of species of the Henneguya genus are of major important for understanding host–parasite interactions and to show the characteristic features of this important group of parasites (Current and Janovy 1976; Hallett and Diamant 2001; El-Mansy and Bashtar 2002; Abdel-Ghaffar et al. 2008). The plasmodial wall of H. melini n. sp. is bordered by a single membrane and presents pinocytotic channels in the ectoplasma zone of the plasmodium, which are clearly involved in nutrient acquisition, to supporting the developmental forms inside the plasmodia (Hallett and Diamant 2001; El-Mansy and Bashtar 2002; Naldoni et al. 2014). Sporogenesis of H. melini n. sp. has many similarities with other previously described Henenguya species (Ali 1999; Casal et al. 2003; Abdel-Ghaffar et al. 2008; Naldoni et al. 2011); however, there was only a thin layer of the plasmodia with young developmental stages and mature spores viewed near the peripheric area, characterizing asynchronic development, but with slow development. The plasmodia were surrounded by connective tissue capsule; with fibroblast and collagen fibers, slow layers disposed transversal and longitudinally, as observed in other studies (Adriano et al. 2005; Matos et al. 2005; Sitjà-Bobadilla 2008; Iwanowicz et al. 2008).
The new species has been characterized only on morphological/morphometric, host, and geographic area data; we recognize the importance of molecular data in the descriptions of new myxosporeans taxa. However, some difficulties were encountered to standardizing PCR reactions to this species, which, added to the few samples obtained and limitations to accessing new samples of the same regions, making it impossible to provide sequencing data of H. melini n. sp. So, future molecular and phylogenetic studies are highly recommended, since they will permit stronger taxonomic comparison, as well as show the phylogenetic position of this species in the evolutionary context of myxosporeans.
In the present study, the prevalence and intensity of H. melini n. sp. were relatively low, with only 16.7 % of the specimens of C. melini being infected and with an intensity of infection ranging from one up to three plasmodia. This finding is in agreement with other Henneguya spp. from South American Siluriformes from natural environment. Martins et al. (2004) reported a prevalence of 13.4 % for Henneguya sp. parasite of Pimelodus maculatus, Naldoni et al. (2011), who reported for Henneguya eirasi a prevalence of 17.1 % in specimens of P. corrucans and P. fasciatum, and Carriero et al. (2013), who reported a prevalence of 16 and 19 % for Henneguya maculosus in P. reticulatum and P. corrucans. However, the results in the present study are in contrast with those described by Naldoni et al. (2009), who reported a high prevalence, which ranged of 75 up to 100 % for Henneguya Pseudoplatystoma infecting the farmed pintado hybrid (P. corrucans × P. fasciatum).
In our study, no disease symptoms were observed in the infected fish specimens. However, several species of the family Myxobolidae have showed to induce pathogeny in their hosts (Molnár 1998; El-Mansy and Bashtar 2002; Mohammed et al. 2002; Adriano et al. 2005; Feist and Longshaw 2006; Ali et al. 2007; Naldoni et al. 2009). Thus, as C. melini is commonly used in aquariums, there is a need of constant monitoring of the fish for diagnosis and timely control of infections by myxosporean to better prevent future disease outbreaks.
References
Abdallah VD, Azevedo RK, Luque JL, Bomfim TCB (2007) Two new species of Henneguya Thélohan, 1892 (Myxozoa, Myxobolidae), parasitic on the gills of Hoplosternum littorale (Callichthyidae) and Cyphocharax gilbert (Curimatidae) from the Guandu River, State of Rio de Janeiro, Brazil. Parasitol Latinoam 62:35–41
Abdel-Ghaffar F, Abdel-Baki AS, Bayoumy EM, Bashtar AR, Qurieshy SA, MorseyKS AA, Mehlhorn H (2008) Light and electron microscopic study on Henneguya suprabranchiae Landsberg, 1987 (Myxozoa: Myxosporea) infecting Oreochromis niloticus, a new host record. Parasitol Res 103:609–617
Adriano EA, Arana S, Cordeiro NS (2005) An ultrastructural and histopathological study of Henneguya pellucida n. sp. (Myxosporea: Myxobolidae) infecting Piaractus mesopotamicus (Characidae) cultivated in Brazil. Parasite 12(3):221–227
Adriano EA, Carriero MM, Maia AAM, Silva MRM, Naldoni J, Ceccarelli OS, Arana S (2012) Phylogenetic and host − parasite relationship analysis of Henneguya multiplasmodialis n. sp. infecting Pseudoplatystoma spp. in Brazilian Pantanal wetland. Vet Parasitol 185:110–120
Ali MA (1999) Henneguya ghaffari sp. n. (Myxozoa: Myxosporea), infecting the Nile perch Lates niloticus (Teleostei: Centropomidae). Dis Aquat Org 38:225–230
Ali MA, Abdel-Baki AS, Sakran TH, Entzeroth R, Abdel-Ghaffar F (2007) Myxobolus lubati n. sp. (Myxosporea: Myxobolidae), a new parasite of haffara seabream Rhabdosargus haffara (Forsskal, 1775), Red Sea, Egypt: a light and transmission electron microscopy. Parasitol Res 100:819–827
Azevedo C, Matos E (1995) Henneguya adherens n. sp. (Myxozoa, Myxosporea), parasite of the Amazon fish, Acestrorhynchus falcatus. J Eukaryot Microbiol 42:515–518
Barassa B, Adriano EA, Cordeiro NS, Ceccarelli PS (2012) Morphology and host-parasite interaction of Henneguya azevedoi n. sp., parasite of gills of Leporinus obtusidens from Mogi-Guaçu River, Brazil. Parasitol Res 110:887–894
Casal G, Matos E, Azevedo C (1997) Some ultrastructural aspects of Henneguya striolata sp. nov. (Myxozoa, Myxosporea), a parasite of the Amazonian fish Serrasalmus striolatus. Parasitol Res 83(1):93–95
Casal G, Matos E, Azevedo C (2003) Light and electron microscopic study of the myxosporean, Henneguya friderici n. sp. from the Amazonian teleostean fish, Leporinus friderici. Parasitology 126:313–319
Carriero MM, Adriano EA, Silva MRM, Ceccarelli PS, Maia AAM (2013) Molecular phylogeny of the Myxobolus and Henneguya genera with several new South American species. PLoS ONE 8:e73713
Current WL, Janovy J Jr (1976) Ultrastructure of interlamellar Henneguya exilis in the channel catfish. J Parasitol 62:975–981
Dyková I, Lom J (1978) Histopathological changes in fish gills infected with myxosporidian parasites of the genus Henneguya. J Fish Biol 12:197–202
Eiras JC (2002) Synopsis of the species of the genus Henneguya Thelohan, 1892 (Myxozoa: Myxosporea: Myxobolidae. Syst Parasitol 52(1):43–54
Eiras JC, Takemoto RM, Pavanelli GC (2009) Henneguya corruscans n. sp. (Myxozoa, Myxosporea, Myxobolidae), a parasite of Pseudoplatystoma corruscans (Osteichthyes, Pimelodidae) from the Parana River, Brazil: a morphological and morphometric study. Vet Parasitol 159:154–158
Eiras JC, Adriano EA (2012) Checklist of the species of the genus Henneguya Thélohan, 1892 (Myxozoa, Myxosporea, Myxobolidae) described between 2002 and 2012. Syst Parasitol 83(2):95–104
El-Mansy AIE, Bashtar AR (2002) Histopathological and ultrastructural studies of Henneguya suprabranchiae Landsberg, 1987 (Myxosporea: Myxobolidae) parasitizing the suprabranchial organ of the freshwater catfish Clarias gariepinus Burchell, 1822 in Egypt. Parasitol Res 88:617–626
Feist SW, Longshaw M (2006) Phylum Myxozoa. In: Woo PTK (ed) Fish diseases and disorders: Protozoan and Metazoan infections, 2nd edn. CAB International, UK, pp 230–296
Feijó MM, Arana S, Ceccarelli PS, Adriano EA (2008) Light and scanning electron microscopy of Henneguya arapaima n. sp. (Myxozoa: Myxobolidae) and histology of infected sites in pirarucu (Arapaima gigas: Pisces: Arapaimidae) from the Araguaia River, Brazil. Vet Parasitol 157:59–64
Froese R, Pauly D (2011) FishBase. World Wide Web electronic publication. Version (02/2011) www.fishbase.org (accessed 10 May 2015)
Griffin MJ, Pote LM, Wise DJ, Greenway TE, Mauel MJ, Camus AC (2008) A novel Henneguya species from channel catfish described by morphological, histological, and molecular characterization. J Aquat Anim Health 20:127–135
Hallett SL, Diamant A (2001) Ultrastructure and small-subunit ribosomal DNA sequence of Henneguya lesteri n. sp. (Myxosporea), a parasite of sand whiting Sillago analis (Sillaginidae) from the coast of Queensland, Australia. Dis Aquat Org 46:197–212
Iwanowicz LR, Iwanowicz DD, Pote LM, Blazer VS, Schill WB (2008) Morphology and 18S rDNA of Henneguya gurlei (Myxosporea) from Ameiurus nebulosus (Siluriformes) in North Carolina. J Parasitol 94:46–57
Kostoïngue B, Diebakate C, Faye N, Toguebaye S (2001) Presence of Myxosporidea (Myxozoa: Myxosporea) of the genus Henneguya Thélohan, 1892 in freshwater fishes from Chad (Central Africa). Acta Protozool 40:117–123
Landsberg JH (1987) Myxosporean parasites of the catfish, Clarias lazera (Valenciennes). Syst Parasitol 9:73–81
Lom J, Arthur JR (1989) A guideline for the preparation of species descriptions in Myxosporea. J Fish Dis 12:151–156
Martins ML, Onaka EM, Bozzo FR, Fenerick JJ (2004) Henneguya sp. (Myxozoa: Myxobolidae) in Pimelodus maculatus (Osteichthyes: Siluridae) from Volta Grande reservoir, Minas Gerais, Brazil. B Inst Pesca 30:1–7
Matos E, Tajdari J, Azevedo C (2005) Ultrastructural studies of Henneguya rhamdia n. sp. (Myxozoa) a parasite from the Amazon teleost fish, Rhamdia quelen (Pimelodidae). J Eukaryot Microbiol 52:532–537
Mathews PD, Silva MRM, Maia AMA, Adriano EA (2015) Ultrastructure and ssrRNA sequencing of Myxidium amazonense n. sp. a myxosporean parasite of Corydoras melini from the Rio Negro river, Amazonas state, Brazil. Parasitol Res 114:4675–4683
Mathews PD, Maia AMA, Adriano EA (2016) Morphological and ultrastructural aspects of Myxobolus niger n. sp. (Myxozoa) gill parasite of Corydoras melini (Siluriformes: Callichthyidae) from Brazilian Amazon. Acta Trop 158:214–219
Mohammed AA, AL-Rasheid KA, Sakran T, Abdel-Baki AA, Abdel-Ghaffar FA (2002) Some species of the genus Myxobolus (Myxozoa: Myxosporea) infecting freshwater fish of the River Nile, Egypt, and the impact on their hosts. Parasitol Res 88:9–15
Molnár K (1998) Taxonomic problems, seasonality and histopathology of Henneguya creplini (Myxosporea) infection of the pikeperch Stizostedion lucioperca in Lake Balaton. Folia Parasitol 45:261–269
Molnár K, Székely C, Mohamed K, Shaharom-Harrison F (2006) Myxozoan pathogens in cultured Malaysian fishes. I. Myxozoan infections of the sutchi catfish Pangasius hypophthalmus in freshwater cage cultures. Dis Aquat Org 68:209–218
Morsy K, Abdel-Ghaffar F, Bashtar AR, Mehlhorn H, Al Quraishy S, Abdel-Gaber R (2012) Morphology and small subunit ribosomal DNA sequence of Henneguya suprabranchiae (Myxozoa), a parasite of the catfish Clarias gariepinus (Clariidae) from the River Nile, Egypt. Parasitol Res 111:1423–35
Naldoni J, Arana S, Maia AAM, Ceccarelli PS, Tavares LER, Borges FA, Pozo CF, Adriano EA (2009) Henneguya pseudoplatystoma n. sp. causing reduction in epithelial area of gills in the farmed pintado, a South American catfish: histopathology and ultrastructure. Vet Parasitol 166:52–59
Naldoni J, Arana S, Maia AAM, Silva MRM et al (2011) Host–parasite–environment relationship, morphology and molecular analysis of Henneguya eirasi n. sp. parasite of two wild Pseudoplatystoma ssp. in Pantanal Wetland, Brazil. Vet Parasitol 177:247–255
Naldoni J, Maia AAM, Da Silva MRM, Adriano EA (2014) Henneguya cuniculator sp. nov., a parasite of spotted sorubim Pseudoplatystoma corruscans in the São Francisco Basin, Brazil. Dis Aquat Org 107:211–221
Pote LM, Hanson LA, Shivaji R (2000) Small subunit ribosomal RNA sequences link the cause of proliferative gill disease in channel catfish to Henneguya n. sp. (Myxozoa: Myxosporea). J Aquat Anim Health 12:230–240
Rabie SA, Mohammed NI, Hussein AA, Hussein NM (2009) The infection of freshwater fishes with three species of Henneguya in Qena, Upper Egypt. Egypt Acad J Biol Sci 1:11–19
Reed CC, Basson L, Van As LL (2003) Myxozoans infecting the sharptooth catfish, Clarias gariepinus in the Okavango River and Delta, Botswana, including descriptions of two new species, Henneguya samochimensis sp. n. and Myxobolus gariepinus sp. n. Folia Parasitol 50:183–189
Reis RE (1998) Systematic, Biogeography, and the fossil record of the Callichthyidae: a review of the available data. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS (eds) Phylogeny and Classification of Neotropical Fishes. Edipucrs, Porto Alegre, Brasil, pp 351–362
Rocha E, Matos E, Azevedo C (1992) Henneguya amazonica n. sp. (Myxozoa, Myxobolidae), parasitizing the gills of Crenicichla lepidota Heckel, 1840 (Teleostei, Cichlidae) from Amazon River. Eur J Protistol 28(3):273–278
Sarkar NK (1985) Myxosporidan Henneguya mystusia sp. n. (Myxozoa: Myxosporea) from the gill of a freshwater teleost fish Mystus sp. Acta Protozool 241:55–58
Sitjà-Bobadilla A (2008) Fish immune response to myxozoan parasites. Parasite 15:420–425
Videira M, Velasco M, Azevedo R, Silva R, Gonçalves E, Matos P, Matos E (2015) Morphological aspects of Henneguya aequidens n. sp. (Myxozoa: Myxobolidae) in Aequidens plagiozonatus Kullander, 1984 (Teleostei: Cichlidae) in the Amazon region, Brazil. Parasitol Res 114:1159–1162
Vita P, Corral L, Matos E, Azevedo C (2003) Ultrastructural aspects of the myxosporean Henneguya astyanax n. sp. (Myxozoa: Myxobolidae), a parasite of the Amazonian teleost Astyanax keithi (Characidae). Dis Aquat Org 53:55–60
Acknowledgments
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP (Proc. No. 2013/21374-6-Adriano E. A.). Mathews P.D. was supported by a doctoral scholarship from FAPESP (Proc. No. 2013/14656-5). Adriano E.A. received a research productivity grant from the Brazilian Fostering Agency CNPq (Proc. No. 305630/2013-0). CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) provides support to the Doctoral Program in Animal Biology of UNICAMP. The authors thank Dr. Geraldo Bernardino, secretary of Fishing of the Amazonas State, for help in the logistic of the field works and Dr. Christopher Berger for reviewing the language of the text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathews, P.D., Maia, A.A. & Adriano, E.A. Henneguya melini n. sp. (Myxosporea: Myxobolidae), a parasite of Corydoras melini (Teleostei: Siluriformes) in the Amazon region: morphological and ultrastructural aspects. Parasitol Res 115, 3599–3604 (2016). https://doi.org/10.1007/s00436-016-5125-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5125-z