Abstract
Mosquitoes transmit serious human diseases, causing millions of deaths every year. The use of synthetic insecticides to control vector mosquitoes has caused physiological resistance and adverse environmental effects in addition to high operational cost. Insecticides of synthesized natural products for vector control have been a priority in this area. In the present study, silver nanoparticles (AgNPs) synthesized using Cassia roxburghii plant leaf extract against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus were determined. Larvae were exposed to varying concentrations of synthesized AgNPs (12, 24, 36, 48, and 60 μg/mL) and aqueous leaf extracts (60, 120, 180, 240, and 300 μg/mL) for 24 h. The synthesized AgNPs were characterized by UV–Vis spectrum, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), with energy-dispersive X-ray spectroscopy analysis (EDX), transmission electron microscopy, and X-ray diffraction analysis (XRD). Compare to aqueous extracted synthesized AgNPs showed extensive mortality rate against An. stephensi, Ae. aegypti, and C. quinquefasciatus with the LC50 and LC90 values that were 26.35, 28.67, 31.27 and 48.81, 53.24, and 58.11 μg/mL, respectively. No mortality was observed in the control. This is the first report on mosquito larvicidal activity of plant-synthesized nanoparticles. Thus, the use of C. roxburghii to synthesize silver nanoparticles is a rapid, eco-friendly, and a single-step approach, and the AgNPs formed can be potential mosquito larvicidal agents. Therefore, this study proves that C. roxburghii is a potential bioresource for stable, reproducible nanoparticle synthesis (AgNPs) and also can be used as an efficient mosquito control agent. This is the first report on the larvicidal activity of the plant extract and AgNPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mosquito vectors of human disease are responsible for the transmission of pathogens of malaria, yellow fever, chikungunya, dengue, lymphatic filariasis, and encephalitis. Interruption of disease transmission by killing or preventing infected mosquitoes from biting human beings is one of the approaches for the control of mosquito-borne diseases. Mosquitoes also cause allergic responses in humans that include local skin and systemic reactions such as angioedema (Peng et al. 1999). Aedes aegypti, a vector of dengue, is widely distributed in tropical and subtropical zones. Dengue fever incidence has increased fourfold since 1970, and nearly half the world’s population is now at risk. In 1990, almost 30 % of the world population, 1.5 billion people lived in regions where the estimated risk of dengue transmission was greater than 50 % (Hales et al. 2002). An outbreak of chikungunya virus infection emerged in the southwest Indian Ocean islands in 2005, spread out to India, and resulted in an ongoing outbreak that has involved >1.5 million patients, including travelers who have visited these areas (Taubitz et al. 2007). Anopheles stephensi are major malaria vectors in India. With an annual incidence of 300–500 million clinically manifested cases and a death toll of 1.1–2.7 million, malaria is still one of the most important communicable diseases. Currently, about 40 % of the world’s population lives in areas where malaria is endemic (Wernsdorfer and Wernsdorfer 2003). Culex quinquefasciatus, a vector of lymphatic filariasis, is widely distributed in tropical zones with around 120 million people infected worldwide and 44 million people having common chronic manifestation (Bernhard et al. 2003).
The synthesized nanoparticles, which are less likely to cause ecological damage, have been identified as potential replacement of synthetic chemical insecticides, hence the need to use green synthesized silver nanoparticles for the control of disease vectors. Plants and microbes are currently used for nanoparticle synthesis. The use of plants for synthesize of nanoparticles is rapid, low cost, eco-friendly, and a single-step method for biosynthesis process (Huang et al. 2007). It has been reported that medicinally valuable angiosperms have the greatest potential for synthesis of metallic nanoparticles with respect to quality and quantity (Song and Kim 2009). Among the various known synthesis methods, plant-mediated nanoparticle synthesis is preferred as it is cost-effective, environmentally friendly, and safe for human therapeutic use (Kumar and Yadav 2009). Biological control or control through natural derivatives deserves favor over control by synthetic chemicals as they are more target-specific, relatively nontoxic, easily biodegradable, and less expensive, and even can provide effective and environmentally friendly solution of controlling the population of these harmful vectors. But, those factors cannot nullify the usefulness of chemical insecticides, especially at the time of outbreak when rapid control of vectors is indispensable. At this point, application of different nanoparticles (NPs) synthesized by green chemical route opens up new scope of research to find its utility in mosquitocidal activity expecting that these nanoparticles should be more susceptible to mosquito body system due to its biogenic nature as well as less harmful to the environment due to minimal dosage. AgNPs may be released into the environment from discharges at the point of production, from erosion of engineered materials in household products (antibacterial coatings and silver-impregnated water filters), and from washing or disposal of silver-containing products (Benn and Westerhoff 2008).
The larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Ae. aegypti and An. stephensi have been observed (Salunkhe et al. 2011). Nelumbo nucifera synthesized silver nanoparticles using aqueous leaf extract against the larvae of Anopheles subpictus and C. quinquefasciatus have been observed (Santhoshkumar et al. 2011). The larvicidal activity of synthesized silver nanoparticles (AgNPs) utilizing aqueous extract from Eclipta prostrata, a member of the Asteraceae, has been investigated against fourth instar larvae of filariasis vector, C. quinquefasciatus, and malaria vector, An. subpictus (Rajkumar and Rahuman 2011). The larvicidal efficacy of the crude leaf extracts of Ficus benghalensis, with three different solvents like methanol, benzene, and acetone, was tested against the early second, third, and fourth instar larvae of C. quinquefasciatus, Ae. aegypti, and An. stephensi (Govindarajan 2010a). The leaf extract of Acalypha indica with different solvents—benzene, chloroform, ethyl acetate, and methanol—has been tested for larvicidal, ovicidal activity, and oviposition attractancy against An. stephensi (Govindarajan et al. 2008a). The larvicidal and repellent properties of essential oils are from various parts of four plant species Cymbopogon citratus, Cinnamomum zeylanicum, Rosmarinus officinalis, and Zingiber officinale against Culex tritaeniorhynchus and An. subpictus (Govindarajan 2011a). Focus on use of nanomaterials for benefit of human health, energy, defense, catalysis, agriculture, and environment is increasing at a rapid pace. Recent advances in this field, particularly the ability to prepare highly ordered nanoparticles of different sizes and shapes, have led to the development of new biocidal agents (Jones et al. 2008). Larvicidal activity of silver nanoparticle synthesized using various plant extracts and fungi against mosquito vector is well documented (Veerekumar et al. 2013, 2014; Muthukumaran et al. 2015). Cassia roxburghii was found in most parts of central and southern India, often along the roads for shade. Cassia roxburghii leaves are used for ring worm infections (Swarbrick and Boylan 2002). The presence of Anthraquinone glycosides and Chrysophanol, Physcion, Rhein, and Roxburghinol has been reported earlier (Duggal and Misra 1982). Cassia species are well known in folk medicine for their laxative and purgative uses (Rastogi and Mehrotra 2002). They are also used for treating skin diseases such as ring worm, scabies, eczema, and wounds (Mohanty and Das 2006). However, there was no scientific evidence justifying the mosquito larvicidal activity of Cassia roxburghii. In view of this, a laboratory study was carried out to assess the effectiveness of AgNPs synthesized with the help of C. roxburghii against the larvae of An. stephensi, Ae. aegypti, and C. quinquefasciatus mosquitoes.
Materials and methods
Collection of materials
Fresh leaves of C. roxburghii (Family: Fabaceae) (Fig. 1) were collected from Kodiyakarai, Tamil Nadu, India, and the taxonomic identification was made by Dr. V. Vengatesalu, Professor, Department of Botany, Annamalai University, Annamalai Nagar, Tamil Nadu, India. The voucher specimen was numbered and kept in our research laboratory for further reference. Silver nitrate was obtained from Qualigens Fine Chemicals, Mumbai, India.
Preparation of plant extracts
The leaves (C. roxburghii) were dried in shade and ground to fine powder in an electric grinder. Aqueous extract was prepared by mixing 50 g of dried leaf powder with 500 mL of water (boiled and cooled distilled water) with constant stirring on a magnetic stirrer (Veerekumar et al. 2013). The suspension of dried leaf powder in water was left for 3 h, filtered through Whatman no. 1 filter paper, and the filtrate was stored in amber-colored air-tight bottle at 10 °C temperature until use.
Mosquitoes
The laboratory-bred pathogen-free strains of mosquitoes were reared in the vector control laboratory, Department of Zoology, Annamalai University. At the time of adult feeding, these mosquitoes were 3–4 days old after emergences (maintained on raisins and water) and were starved for 12 h before feeding. Each time, 500 mosquitoes per cage were fed on blood using a feeding unit fitted with parafilm as membrane for 4 h. Ae. aegypti feeding was done from 12 noon to 4.00 p.m. and An. stephensi and C. quinquefasciatus were fed during 6.00 p.m. to 10.00 p.m. A membrane feeder with the bottom end fitted with parafilm was placed with 2.0 ml of the blood sample (obtained from a slaughter house by collecting in a heparinized vial and stored at 4 °C) and kept over a netted cage of mosquitoes. The blood was stirred continuously using an automated stirring device, and a constant temperature of 37 °C was maintained using a water jacket circulating system. After feeding, the fully engorged females were separated and maintained on raisins. Mosquitoes were held at 28 ± 2 °C, 70–85 % relative humidity, with a photo period of 12-h light and 12-h dark.
Synthesis of silver nanoparticles
The fresh leaves of C. roxburghii broth solution was prepared by taking 10 g of thoroughly washed and finely cut leaves in a 300-mL Erlenmeyer flask along with 100 mL of sterilized double-distilled water and then boiling the mixture for 5 min be used within 1 week. The filtrate was treated with aqueous 1 mM AgNO3 (21.2 mg of AgNO3 powder in 125 mL Milli-Q water) solution in an Erlenmeyer flask and incubated at room temperature. Eighty-eight-milliliter aqueous solution of 1 mM of silver nitrate was reduced using 12 mL of leaf extract at room temperature for 10 min, resulting in a brown-yellow solution indicating the formation of AgNPs (Veerekumar et al. 2014).
Characterization of the synthesized AgNPs
Synthesis of AgNP solution with leaf extract may be easily observed by UV–Vis spectroscopy. The bio-reduction of the Ag ions in solutions was monitored by periodic sampling of aliquots (1 mL) of the aqueous component after 20 times dilution and measuring the UV–Vis spectra of the solution. UV–Vis spectra of these aliquots were monitored as a function of time of reaction on a Shimadzu 1601 spectrophotometer in the 300–800-nm range operated at a resolution of 1 nm. Further, the reaction mixture was subjected to centrifugation at 60,000×g for 40 min; the resulting pellet was dissolved in deionized water and filtered through Millipore filter (0.45 μm). An aliquot of this filtrate containing silver nanoparticles was used for Fourier transform infrared (FTIR) and transmission electron microscopy (TEM) analysis. For electron microscopic studies, 25 μL of sample was sputter-coated on a copper stub, and the images of the nanoparticles were studied using scanning electron microscopy (SEM; JEOL, model JFC-1600), and TEM (JEOL, model 1200EX) measurements were operated at an accelerating voltage of 120 kV and later with an XDL 3000 powder. FTIR spectra of the samples were measured using PerkinElmer Spectrum One instrument in the diffuse reflectance mode at a resolution of 4 cm−1 in KBr pellets. An aliquot of this filtrate containing silver nanoparticles was used for X-ray diffraction (XRD) analysis.
Larvicidal activity
Larvicidal activity of the aqueous crude extract and AgNPs from C. roxburghii was evaluated according to WHO protocol (2005). Based on the wide range and narrow range tests, aqueous crude extract was tested at 60, 120,180, 240, and 300 μg/mL concentrations, and AgNPs was tested at 12, 24, 36, 48, and 60 μg/mL concentrations. Twenty numbers of late third instar larvae were introduced into a 500-mL glass beaker containing 249 mL of dechlorinated water, and 1 mL of desired concentrations of leaf extract and silver nanoparticles was added. For each concentration, five replicates were performed, for a total of 100 larvae. Larval mortality was recorded at 24 h after exposure, during which no food was given to the larvae. Each test included a set control groups (silver nitrate and distilled water) with five replicates for each individual concentration. The lethal concentrations (LC50 and LC90) were calculated by probit analysis (Finney 1971).
Statistical analysis
The average larval mortality data were subjected to probit analysis for calculating LC50, LC90, and other statistics at 95 % confidence limits of upper confidence limit and lower confidence limit, and chi-squared values were calculated using the Statistical Package of Social Sciences 12.0 software. Results with p < 0.05 were considered to be statistically significant.
Results
Larvicidal activity of aqueous extract and synthesized AgNPs
The results of larvicidal activity of C. roxburghii aqueous leaf extract and synthesized AgNPs against late third instar An. stephensi, Ae. aegypti, and C. quinquefasciatus were noted and presented in Tables 1 and 2. Considerable mortality was evident after the treatment of C. roxburghii for all three important vector mosquitoes. The LC50 and LC90 values of C. roxburghii aqueous leaf extract appeared to be effective against An. stephensi (LC50 133.69 μg/mL and LC90 243.59 μg/mL), followed by Ae. aegypti (LC50 149.68 μg/mL and LC90 274.63 μg/mL), and C. quinquefasciatus (LC50 163.48 μg/mL and LC90 293.43 μg/mL). Most considerable mortality was evident after the treatment of silver nanoparticles. AgNPs against the vector mosquitoes of An. stephensi, Ae. aegypti, and C. quinquefasciatus had the following LC50 and LC90 values: An. stephensi had LC50 and LC90 values of 26.35 and 48.81 μg/mL, Ae. aegypti had LC50 and LC90 values of 28.67 and 53.24 μg/mL, and C. quinquefasciatus had LC50 and LC90 values of 31.27 and 58.11 μg/mL. The control showed nil mortality in the concurrent assay. χ 2 value was significant at p < 0.05 level.
Characterization of silver nanoparticles
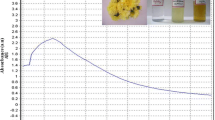
Color change was noted by visual observation in the C. roxburghii leaf extracts when incubated with AgNO3 solution. C. roxburghii leaf extract without AgNO3 did not show any change in color. The color of the extract changed to light brown within an hour, and later, it changed to dark brown during a 6-h incubation period after which no significant change occurred (Fig. 2a, b). The absorption spectrum of C. roxburghii leaf extracts at different wavelengths ranging from 300 to 800 nm revealed a peak at 460 nm (Fig. 2c). FTIR analysis of the purified nanoparticles showed the presence of bands due to O–H group Stretch hydrogen bonded (3222.44), C-H Stretch Alkenes (2922.23), C-H Stretch Alkanes (2853.35), C (triple bond) N Stretch (2209.12), C–C Stretch Aromatics (1593.43), C–H stretch Aromatics (1514.24), N–O Stretch Nitro (1384.18), C–O Stretch Acid (1240.74), C–H alkyl halides (1167.81), C–O Stretch Alcohol (1102.98), and C– C1 Stretch alkyl halides (833.39) (Fig. 3). SEM micrographs of the synthesized AgNPs of C.roxburghii magnified at ×60,000 and measured at 500 nm are shown in Fig. 4a. The triangular, pentagonal, and hexagonal structures are clear. EDX proves the chemical purity of the synthesized AgNPs (Fig. 4b). The electron microscopic study of the nanoparticles using TEM revealed that the nano-Ag predominates with spherical, triangle, truncated triangles, and decahedral morphologies ranging from 57 to 95 nm with an average size of 32 nm (Fig. 5). XRD analysis (Fig. 6) showed intense peaks at 2θ values of 38.13°, 44.32°, 49.12°, 64.53°, and 77.38° corresponding to (461), (103), (10), (99), and (91) Bragg’s reflection based on the face-centered cubic structure of silver nanoparticles.
Discussion
Mosquito-borne diseases are one of the most public health problems in the developing countries. Many approaches have been developed to control mosquito menace. One such approach to prevent mosquito-borne disease is by killing mosquito at larval stage. Management of this disease vector using synthetic chemicals has failed because of insecticide resistance, vector resurgence, and environmental pollution (Wondji et al. 2009). In the present study to assess the larvicidal properties of C. roxburghii leaf extract and synthesized AgNPs against An. stephensi, Ae. aegypti, and C. quinquefasciatus. This result is also comparable to earlier reports of Priyadarshini et al. (2012) who observed that the highest larval mortality was found in the synthesized AgNPs against the first to fourth instar larvae and pupae with LC50 values of 10.14, 16.82, 21.51, and 27.89 ppm, respectively, LC90 values of 31.98, 50.38, 60.09, and 69.94 ppm, respectively, and LC50 and LC90 values of pupae of 34.52 and 79.76 ppm, respectively. Govindarajan (2010b) reported that the larvicidal activity of the crude extract of Sida acuta against three important mosquitoes with LC50 values ranges between 38 and 48 mg/L. The crude extract had strong repellent action against three species of mosquitoes as it provided 100 % protection against An. stephensi for 180 min followed by Ae. aegypti (150 min) and C. quinquefasciatus (120 min), respectively. The methanol extract of Cassia fistula exhibited LC50 values of 17.97 and 20.57 mg/L, An. stephensi and C. quinquefasciatus, respectively (Govindarajan et al. 2008b). The maximum efficacy was observed in crude aqueous and synthesized AgNPs against C. quinquefasciatus (LC50 27.49 and 4.56 mg/L; LC90 70.38 and 13.14 mg/L) and against An. subpictus (LC50 27.85 and 5.14 mg/L; LC90 71.45 and 25.68 mg/L), respectively. A biological method has been used to synthesize stable silver nanoparticles that were tested as mosquito larvicides against Ae. aegypti, An. stephensi, and C. quinquefasciatus (Arjunan et al. 2012).
Biosynthesized silver nanoparticles using fungus Cochliobolus lunatus was used for the control of Ae. aegypti and An. stephensi and reported that the nanoparticles are significantly effective against second, third, and fourth instar larvae of Ae. aegypti (LC50 1.29, 1.48, and 1.58; LC90 3.08, 3.33, and 3.41 ppm) and against An. stephensi (LC50 1.17, 1.30, and 1.41; LC90 2.99, 3.13, and 3.29 ppm) (Salunkhe et al. 2011). Sathyavathi et al. (2010) was in good agreement with the results of present study with the diffraction peaks at 44.50, 52.20, and 76.7 corresponding to the (111), (200), and (220) facets of the face-centered cubic crystal structure. Senthil Nathan et al. (2008) reported that fourth instar larvae of An. stephensi are highly sensitive to the ethyl acetate extract of the leaves of Dysoxylum malabaricum. Miao et al. (2010) demonstrated, for the first time, that silver-engineered nanoparticles can be taken in and accumulated inside the Ochromonas danica cells, where they exerted their toxic effects. Ecotoxicity study was determined by Ag NPs in 48-h effective concentration 50 (EC50) values for Daphnia magna of suspensions of 60 and 300 nm Ag NPs that were 1.0 (95 % confidence interval (CI) = 0.1–1.3) and 1.4 mg Ag/L (95 % CI = 0.3–2.1), respectively. Earthworms (Eisenia fetida) were exposed to AgNO3 (94.21 mg/kg) and Ag NPs (727.6 mg/kg) with similar size ranges coated with either polyvinylpyrrolidone (hydrophilic) or oleic acid (amphiphilic) 773.3 mg/kg during a standard subchronic reproduction toxicity test (Shoults-Wilson et al. 2010). Marimuthu et al. (2011) reported the larvicidal effect of aqueous crude leaf extracts, Ag NPs, and synthesized Ag NPs using Mimosa pudica showed highest mortality in synthesized Ag NPs against the larvae of An. subpictus (LC50=8.89, 11.82, and 0.69 ppm) and against the larvae of C. quinquefasciatus (LC50 = 9.51, 13.65, and 1.10 ppm), respectively.
The larvicidal efficacy of benzene, hexane, ethyl acetate, methanol, and chloroform leaf extract of Cardiospermum halicacabum against C. quinquefasciatus and Ae. aegypti. The LC50 values were 174.24, 193.31, 183.36, 150.44, and 154.95 ppm, and 182.51, 200.02, 192.31, 156.80, and 164.54 ppm, respectively (Govindarajan 2011b). Tiwary et al. (2007) observed the larvicidal activity of linalool-rich essential of Zanthoxylum armatum against different mosquito species viz., C. quinquefasciatus (LC50 49 ppm), Ae. aegypti (LC50 54 ppm), and An. stephensi (LC50 58 ppm). Cheng et al. (2003) examined plant EOs against Ae. aegypti larvae with LC50 values ranging from 36.0 to 86.8 μg/mL. Cavalcanti et al. (2004) reported that the larvicidal activity of EOs from Brazilian plants with LC50 values ranging from 60 to 69 μg/mL against Ae. aegypti larvae. Rahuman et al. (2000) also found that nhexadecanoic acid in Feronia limonia dried leaves was effective against fourth-instar larvae of C. quinquefasciatus, An. stephensi, and Ae. aegypti with LC50 values of 129.24, 79.58, and 57.23 μg/mL, respectively. The EO from the leaves of C. anisata exhibited significant larvicidal activity, with 24-h LC50 values of 140.96, 130.19, and 119.59 ppm, respectively (Govindarajan 2010c). The highest larvicidal activity was observed in the EO from Z. officinale against C. tritaeniorhynchus and An. subpictus with the LC50 and LC90 values as 98.83 and 57.98 ppm, and 186.55 and 104.23 ppm, respectively (Govindarajan 2011a). The larvicidal activity of crude benzene and ethyl acetate extracts of leaf of Ervatamia coronaria and Caesalpinia pulcherrima against An. stephensi, Ae. aegypti, and C. quinquefasciatus. The highest larval mortality was found in benzene extract of E. coronaria against the larvae of An. stephensi, Ae. aegypti, and C. quinquefasciatus with the LC50 and LC90 values were 79.08, 89.59, 96.15 ppm, and 150.47, 166.04, 174.10 ppm, respectively (Govindarajan et al. 2011). Amer and Mehlhorn (2006) examined 41 plant extracts and 11 oil mixtures against the Ae. aegypti, An. stephensi, and C. quinquefasciatus using the skin of human volunteers to find out the protection time and repellency. The five most effective oils were those of Litsea (Litsea cubeba), Cajeput (Melaleuca leucadendron), Niaouli (Melaleuca quinquenervia), Violet (Viola odorata), and Catnip (Nepeta cataria), which induced a protection time of 8 h at the maximum and a 100 % repellency against all three species.
The maximum efficacy in the aqueous extract of M. paradisiaca against the larvae of hematophagous Haemaphysalis bispinosa, Hippobosca maculata, the larvae of An. stephensi, and C. tritaeniorhynchus with LC50 values of 28.96, 31.02, 26.32, and 20.10 mg/mL, respectively (Jayaseelan et al. 2011), was observed. The higher mortality rates at lower doses are comparable with earlier reports of AgNPs produced by plant N. nucifera leaf extracts (LC50 = 0.69 ppm, LC90 = 2.15 ppm) against An. subpictus and C. quinquefasciatus (LC50 = 1.10 ppm, LC90 = 3.59 ppm) (Santhoshkumar et al. 2011). The LC50 and LC90 values of Cassia tora leaf extracts against adulticidal activity of hexane, chloroform benzene, acetone, and methanol (C. quinquefasciatus, Ae. aegypti, and An. stephensi) were the following: For C. quinquefasciatus, LC50 values were 338.81, 315.73, 296.13, 279.23, and 261.03 ppm and LC90 values were 575.77, 539.31, 513.99, 497.06, and 476.03 ppm; for Ae. aegypti, LC50 values were 329.82, 307.3, and 252.03 ppm and LC90 values were 563.24, 528.33, 496.92, 477.61, and 448.05 ppm; and for An. stephensi, LC50 values were 317.28, 300.30, 277.51, 263.35, and 251.43 ppm and LC90 values were 538.22, 512.90, 483.78, 461.08, and 430.70 ppm, respectively (Amerasan et al. 2012).
Essential oils obtained from aromatic plants like Eucalyptus (E. sideroxylon, E.globulus ssp globulus, and E. globulus ssp maidenii) were good and safe alternatives due to their low toxicity to mammals and easy biodegradability with knockdown time 50 % (KT50) values of 24.75, 27.73, and 31.39 min (Toloza et al. 2010). Subarani et al. (2013) reported that the Vinca rosea-synthesized silver nanoparticles did not exhibit any noticeable toxicity on Poecilia reticulata after 24, 48, and 72 h of exposure. These results suggest that the synthesized AgNPs have the potential to be used as an ideal eco-friendly approach for the control of the Ae. aegypti larvae. Bansal et al. (2009) reported that the 24-h LC50 values as observed for aqueous extracts for green unripe and yellow ripe fruits were 112.7, 498.2, and 846.3 mg/L and 104.7, 267.7, and 832.2 mg/L for An. stephensi, Ae. aegypti, and Cx. quinquefasciatus, respectively. In conclusion, the synthesis of AgNPs with plant leaves shows a “green approach” that can be used as an effective reducing agent for the synthesis of silver nanoparticles. This biological reduction of metal would be a boon for the development of clean, nontoxic, and environmentally acceptable metal nanoparticles; the formed silver nanoparticles are hydrophilic in nature, disperse uniformly in water, are highly stable, and had significant mosquito larvicidal activity against An. stephensi, Ae. aegypti, and Cx. quinquefasciatus. This is the first report on the mosquito larvicidal activity of synthesized nanoparticles from C. roxburghii.
References
Amer A, Mehlhorn H (2006) Repellency effect of forty-one essential oils against Aedes, Anopheles and Culex mosquitoes. Parasitol Res 99:478–490
Amerasan D, Murugan K, Kovendan K, Mahesh Kumar P, Panneerselvam C, Subramaniam J, John William S, Hwang JS (2012) Adulticidal and repellent properties of Cassia tora Linn. (Family: Caesalpinaceae) against Culex quinquefasciatus, Aedes aegypti, and Anopheles stephensi. Parasitol Res 111(5):1953–1964
Arjunan NK, Murugan K, Rejeeth C, Madhiyazhagan P, Barnard DR (2012) Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vector-Borne Zoonotic Dis 12(3):262–268
Bansal SK, Singh KV, Kumar S (2009) Larvicidal activity of the extracts from different parts of the plant Solanum xanthocarpum against important mosquito vectors in the arid region. J Environ Biol 30(2):221–226
Benn T, Westerhoff P (2008) Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 42:4133–4139
Bernhard L, Bernhard P, Magnussen P (2003) Management of patients with lymphoedema caused by filariasis in North–eastern Tanzania: alternative approaches. Physiotherapy 89:743–749
Cavalcanti ESB, Morais SM, Lima MAA, Santana EWP (2004) Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Mem Inst Oswaldo Cruz 99:541–544
Cheng SS, Chang HT, Chang ST, Tsai KH, Chen WJ (2003) Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour Technol 89:99–102
Duggal JK, Misra K (1982) Anthraquinones of Cassia marginata seeds. Planta Med 45(5):48–50
Finney DJ (1971) Probit analysis, vol 551. Cambridge University Press, London, pp 68–72
Govindarajan M (2010a) Larvicidal and repellent activities of Sida acuta Burm. F. (family: Malvaceae) against three important vector mosquitoes. Asian Pac J Trop Med 3(9):691–695
Govindarajan M (2010b) Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (willd.) Hook. F. Benth (Rutaceae) against three mosquito species. Asian Pac J Trop Med 3:874–877
Govindarajan M (2010c) Larvicidal efficacy of Ficus benghalensis L. plant leaf extracts against Culex quinquefasciatus Say, Aedes aegypti L. and Anopheles stephensi L. (Diptera: Culicidae). Eur Rev Med Pharmacol Sci 14(2):107–111
Govindarajan M (2011a) Mosquito larvicidal and ovicidal activity of Cardiospermum halicacabum Linn. (Family: Sapindaceae) Leaf extract against Culex quinquefasciatus (say.) and Aedes aegypti (Linn.) (Diptera: Culicidae). Eur Rev Med Pharmacol Sci 15(7):787–794
Govindarajan M (2011b) Larvicidal and repellent properties of some essential oils against Culex tritaeniorhynchus Giles and Anopheles subpictus Grassi (Diptera: Culicidae). Asian Pac J Trop Med 4(2):106–111
Govindarajan M, Jebanesan A, Pushpanathan T (2008a) Larvicidal and ovicidal activity of Cassia fistula Linn. Leaf extract against filarial and malarial vector mosquitoes. Parasitol Res 102:289–292
Govindarajan M, Jebanesan A, Pushpanathan T, Samidurai K (2008b) Studies on effect of Acalypha indica L. (Euphorbiaceae) leaf extracts on the malarial vector, Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res 103(3):691–695
Govindarajan M, Mathivanan T, Elumalai K, Krishnappa K, Anandan A (2011) Mosquito larvicidal, ovicidal and repellent properties of botanical extracts against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 109:353–367
Hales S, Wet ND, Maindonald J, Woodward A (2002) Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet 360:830–834
Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N, Hong J, Chen C (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 18:105104
Jayaseelan C, Rahuman AA, Rajakumar G, Kirthi AV, Santhoshkumar T, Marimuthu S, Bagavan A, Kamaraj C, Zahir AA, Elango G (2011) Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heartleaf moonseed plant, Tinospora cordifolia Miers. Parasitol Res 109(1):185–194
Jones N, Ray B, Ranjit KT, Manna AC (2008) Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 279:71–76
Kumar V, Yadav SK (2009) Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol 84:151–157
Marimuthu S, Rahuman AA, Rajakumar G, Santhoshkumar T, Kirthi AV, Jayaseelan C, Bagavan A, Zahir AA, Elango G, Kamaraj C (2011) Evaluation of green synthesized silver nanoparticles against parasites. Parasitol Res 108(6):1541–1549
Miao AJ, Luo Z, Chen CS, Chin WC, Santschi PH, Quigg A (2010) Intracellular uptake: a possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica. PLoS One 5:15196
Mohanty ABD, Das (2006) Interspecific Genetic Diversity in 15 Species of Cassia L. Evident by Chromosome and 4C Nuclear DNA Analysis. J Bio sci 6(4):664–670
Muthukumaran U, Govindarajan M, Rajeswary M, Hoti SL (2015) Synthesis and characterization of silver nanoparticles using Gmelina asiatica leaf extract against filariasis, dengue, and malaria vector mosquitoes. Parasitol Res 114(5):1817–27
Peng Z, Yang J, Wang H, Simons FER (1999) Production and characterization of monoclonal antibodies to two new mosquito Aedes aegypti salivary proteins. Insect Biochem Mol Biol 29:909–914
Priyadarshini K, Murugan K, Panneerselvam C, Ponarulselvam S, Hwang J-S, Nicoletti M (2012) Biolarvicidal and pupicidal potential of silver nanoparticles synthesized using Euphorbia hirta against Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res 111(3):997–1006
Rahuman AA, Gopalarkrishnan G, Saleem G, Arumrgam S, Himalayan B (2000) Effect of Feronia limonia on mosquito larvae. Fitoterapia 71:553–555
Rajkumar G, Rahuman AA (2011) Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vector. Acta Trop 196–203
Rastogi P, Mehrotra BN (2002) Compendium of Indian medicinal plants (volume 5).178: 4S
Salunkhe RB, Patil SV, Patil CD, Salunke BK (2011) Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res 109:823–831
Santhoshkumar T, Rahuman AA, Rajakumar G, Marimuthu S, Bagavan A, Jayaseelan C, Zahir AA, Elango G, Kamaraj C (2011) Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res 108:693–702
Sathyavathi R, Balamurali Krishna M, Venugopal Rao S, Saritha R, Narayana Rao D (2010) Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Adv Sci Lett 3:1–6
Senthil Nathan S, Hisham A, Jayakumar G (2008) Larvicidal and growth inhibition of the malaria vector Anopheles stephensi by triterpenes from Dysoxylum malabaricum and Dysoxylum beddomei. Fitoterapia 79(2):106–111
Shoults-Wilson WA, Reinsch BC, Tsyusko OV, Bertsch PM, Lowry GV, Unrine JM (2010) Effect of silver nanoparticle surface coating on bioaccumulation and reproductive toxicity in earthworms (Eisenia fetida). Nanotoxicology. doi:10.3109/17435390.2010.537382
Song JY, Kim BS (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32:79–84
Subarani S, Sabhanayakam S, Kamaraj C (2013) Studies on the impact of biosynthesized silver nanoparticles (AgNPs) in relation to malaria and filariasis vector control against Anopheles stephensi Liston and Culex quinquefasciatus Say (Diptera:Culicidae). Parasitol Res 112:487–499
Swarbrick J, Boylan JC (2002) Plants as drugs-Encyclopaedia of Pharmaceutical Technology Marcel Dekker Inc, New York (2nd edition) 2214–2216
Taubitz W, Cramer JP, Kapaun A, Pfeffer M, Drosten C, Dobler G et al (2007) Chikungunya fever in travelers: clinical presentation and course. Clin Infect Dis 45:508
Tiwary M, Naik SN, Tewaryb DK, Mittalc PK, Yadavc S (2007) Chemical composition and larvicidal activities of the essential oil of Zanthoxylum armatum DC (Rutaceae) against three mosquito vectors. J Vect Born Dis 44:198–204
Toloza AC, Lucía A, Zerba E, Masuh H, Picollo MI (2010) Eucalyptus essential oil toxicity against permethrin-resistant Pediculus humanus capitis (Phthiraptera: Pediculidae). Parasitol Res 106(2):409–414
Veerekumar K, Govindarajan M, Rajeswary M (2013) Green synthesis of silver nanoparticles using Sida acuta (Malvaceae) leaf extract against Culex quinquefasciatus, Aedes aegypti and Anopheles stephensi (Diptera: Culicidae). Parasitol Res 112(12):4073–4085
Veerekumar K, Govindarajan M, Rajeswary M (2014) Low-cost and ecofriendly green synthesis of silver nanoparticles using Feronia elephantum (Rutaceae) against Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti (Diptera: Culicidae). Parasitol Res 113:1775–1785
Wernsdorfer G, Wernsdorfer WH (2003) Malaria at the turn from the 2nd to the 3rd millennium. Wien Klin Wochenschr 115:2–9
Wondji CS, Irving H, Morgan J, Lobo NF, Collins FH, Hunt RH (2009) Two duplicated P450 genes are associated with pyrethroid resistance in Anopheles funestus, a major malaria vector. Genome Res 19:452–459
World Health Organization (2005) Guidelines for laboratory and field testing of mosquito larvicides. Communicable disease control, prevention and eradication, WHO pesticide evaluation scheme. WHO, Geneva, WHO/CDS/WHOPES/GCDPP/1.3
Acknowledgments
The authors would like to thank Professor and Head of the Department of Zoology, Annamalai University for the laboratory facilities provided. The authors would also like to acknowledge the cooperation of staff members of the VCRC (ICMR), Pondicherry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muthukumaran, U., Govindarajan, M. & Rajeswary, M. Green synthesis of silver nanoparticles from Cassia roxburghii—a most potent power for mosquito control. Parasitol Res 114, 4385–4395 (2015). https://doi.org/10.1007/s00436-015-4677-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4677-7