Abstract
Trichuris sp. individuals were collected from Myocastor coypus from fancy breeder farms in the Czech Republic. Using morphological and biometrical methods, 30 female and 30 male nematodes were identified as Trichuris myocastoris. This paper presents the first molecular description of this species. The ribosomal DNA (rDNA) region, consisting of internal transcribed spacer (ITS)-1, 5.8 gene and ITS-2, was sequenced. Based on an analysis of 651 bp, T. myocastoris was found to be different from any other Trichuris species for which published sequencing of the ITS region is available. The phylogenetic relationships were estimated using the maximum parsimony methods and Bayesian analyses. T. myocastoris was found to be significantly closely related to Trichuris of rodents than those of ruminants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocastor coypus (coypu or nutria) is a South American semi-aquatic rodent with a wide geographical distribution, from central Bolivia and southern Brazil to Tierra del Fuego (Argentina), and as a result of escapes and release from fur farms, feral populations now occur in Europe, Asia and North America (Woods et al. 1992).
In the Czech Republic, nutrias were first bred in 1925, when several animals were imported from Argentina. Within a short time, nutria breeding greatly expanded. Currently, nutrias are increasingly used not only for their fur but also for their high-quality meat.
Nutrias, like other animals, suffer from many diseases as well as many parasitic infections. However, studies on nutria parasites are sporadic and focus mainly on wild nutria from the Americas (Babero and Lee 1961; Rossin et al. 2009; Gayo et al. 2011; Martino et al. 2012). The most common endoparasites of wild nutria are Eimeria spp., Longistriata maldonadoi, Strongyloides myopotami and Trichuris myocastoris (Babero and Lee 1961; Pastuszko 1991; Rossin et al. 2009; Martino et al. 2012); for European nutria, they are mainly coccidia, S. myopotami and T. myocastoris (Zajíček 1955; Hohner 1961; Pastuszko 1991). Pellérdy (1963, 1974) reported the following coccidia in nutria: Eimeria myopotami, Eimeria pellucida, Eimeria coypi, Eimeria seideli and Eimeria nutriae.
T. myocastoris was first described in 1933 by Enigk; Baruš et al. (1975) redescribed this species. On the basis of a detailed study of the morphology and metrical characters of their specimens, which were compared to the original description of T. myocastoris and Trichuris nutria, Baruš et al. (1975) demonstrated that T. myocastoris and T. nutria (Petrov, 1941) are synonyms.
In the Czech Republic, nutrias are widespread and they are often bred on farms for their meat and fur. On these farms, they live in close proximity to various domestic animals (especially small ruminants). It is thus questionable whether Trichuris sp., which are very common in nutria in the Czech Republic, are the same species described by Enigk in 1933 (England) and redescribed by Baruš in 1975 (Azerbaijan). It is also possible that Trichuris sp. originated from another host; Dobrovol’skii (1952) reported that Trichuris ovis had been found in the muskrat. A genetic analysis of Trichuris sp. has yet to be made from a nutria.
The aim of this study was to identify T. myocastoris from different fancy breeder farms in the Czech Republic using morphometrical as well as genetic molecular methods.
Materials and methods
Morphological study
Trichuris sp. individuals were collected from M. coypus (young and adult animals) that originated from three nutria farms in the Czech Republic. A total of 60 worms (30 males and 30 females) were separated and rinsed in a physiological saline solution (0.9 w/v NaCl) in order to remove contaminants. These specimens were used for morphometrical determination.
In order to identify Trichuris species found in the caecum and colon of the hosts, morphological and biometric studies were performed with an Olympus microscope (BX51).
A total of 60 Trichuris individual specimens were identified at the species level according to Enigk (1933) and Baruš et al. (1975).
The following morphometrical features were measured in each specimen: total body length, length of oesophagus, posterior body length, body width at oesophagus end, maximum posterior body width and spicule length.
Molecular study
We analyzed a total of six Trichuris sp. individuals. Genomic DNA from individual worm was extracted using a QIAamp Tissue Kit (Qiagen) according to the manufacturer’s protocol. Genomic DNA was detected using 0.8 % agarose gel electrophoresis and GelRed (Biotium). The ribosomal DNA (rDNA) region, consisting of internal transcribed spacer (ITS)-1, 5.8 gene and ITS-2, was amplified by the polymerase chain reaction (PCR), using a universal pair of primers: forward NC5 5′-GTA GGT GAA CCT GCG GAA GGA TCA TT-3′ and reverse NC2 5′-TTA GTT TCT TTT CCT CCG CT-3′ (Zhu et al. 2000). The PCR reaction consisted of 15.5 μl of Combi PPP Master Mix (Top-Bio), 5 μl of each primer, 5 μl of template DNA and PRC water (Top-Bio), for a total volume of 50 μl. The PCR profile carried out on MJ Mini™ thermocycler (Bio-Rad) started with a 10-min period of initial denaturation at 94 °C, followed by 35 cycles. Each cycle consisted of a denaturation step at 94 °C for 1 min, a primer annealing step at 55 °C for 1 min and an elongation step at 72 °C for 1 min. The PCR was terminated at a final elongation period of 72 °C for 10 min. Products of the PCR were detected using 0.8 % agarose gel electrophoresis with GelRed (Biotium). The PCR products were purified and sequenced by Macrogen Inc., Korea. Each sample was sequenced from both (3′ and 5′) ends of both fragments using the same primers that are used for double-strand PCR amplification.
The raw chromatograms were manually assembled and checked by eye for potential errors using computer software BioEdit 5.0.9 (Hall 1999); the same program was used to align the sequences using the ClustalW algorithm. Sequences were compared with the online GenBank (NCBI) nucleotide database using the BLASTn program.
In addition to our samples, we used sequences of several Trichuris species downloaded from the GenBank database to set the phylogenetic position of our individuals within the genus. Detailed information regarding material used in our molecular study is listed in Table 1.
Our new six sequences of rDNA region consisting of ITS-1, 5.8 gene and ITS-2 were deposited into the GenBank database under the following accession numbers: KM877518–KM877523.
The phylogenetic relationships were estimated using the maximum parsimony (MP) method and Bayesian analysis (BAY). Equal weight was employed for all sites for the MP analysis, which was conducted using PAUP* version 4.0b10 (Swofford 2000). Tree searches were performed using heuristic searches with a stepwise addition of taxa and TBR branch swapping algorithm. Bootstrap analyses (1000 replicates) were used to assess the relative robustness of tree nodes. The Bayesian tree was constructed using MrBayes version 3.0 (Huelsenbeck and Ronquist 2001). Six Monte Carlo Markov chains ran simultaneously for 1,000,000 generations, with a sampling frequency of 100. Likelihood scores reached stability after c. 30,000 generations. The corresponding trees were discarded as a burn-in, and the remaining trees were used to construct a 50 % majority-rule consensus tree. The posterior probabilities were used to indicate branch supports in the final tree.
Results
Morphological study
The following is a brief description of the whipworm body found in nutria in the Czech Republic. This coincides with a description from Azerbaijan (Baruš et al. 1975); however, nematodes in our study were slightly longer (see Table 2).
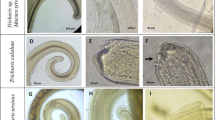
The body of whipworms was white, the anterior part of the body gradually tapers towards the anterior end, and this part of body is distinctly narrower than the oesophageal region. The total male body length ranged from 21.38 to 49.44 mm, with the anterior part measuring 13.01–32.25 mm in length. Small rounded papillae are located on each side of the cloaca. The posterior end of the body is rounded. The spicule is markedly sclerotized, measuring 2.55–4.90 mm in length and 0.024–0.038 mm in width in the middle section and 0.012–0.017 mm in the distal section. Spicule sheath is tubular and covered with spines; however, the distal part of the bulb is covered with spines and the distal tubular part is without spines. The shape of the spicule sheath varies according to the evagination of the spicule, from tubular shape to the bulb (Fig. 1a, b).
The female body is 33.89–61.56 mm in length, and the anterior portion is 20.85–38.43 mm in length. The posterior part of the body measures 12.03–22.69 mm in length and 0.29–0.40 mm in maximum width. The vulva is located at the oesophagus end, and it is in the shape of a transverse slit with slightly frilled margins. It is situated in the centre of a circular cuticular disc, which has a distinctly smooth surface. The vagina extends in a straight line, loops three times and finally connects to the uterus (Fig. 2a, b). The distance from the vulva to the uterus ranged from 1.17 to 1.62 mm. The biometrical parameters of individuals are shown in Table 2.
Molecular study
The final matrix of 19 sequences consisted of 166 bp containing 20 variable characters with 14 parsimony informative sites. Altogether, the analyzed specimens of Trichuris revealed 14 haplotypes. Our six specimens from M. coypus represent six unique haplotypes. Employed methods have recovered trees of very similar topologies with high statistical supports and sorted sequences into well-supported clusters (Fig. 3).
Discussion
The nutria is indigenous to the southern part of South America, and both wild and captive-raised animals are used for fur and meat. Nutria breeding was mainly used to produce fur during the 1990s. Due to recent changes in the world market situation, meat has changed and become the main product of nutria breeding. In the Czech Republic, nutria meat is similarly becoming popular, especially due to its high protein content and low fat and cholesterol content (Tůmová et al. 2013).
T. myocastoris is a common parasite of wild and domestic nutria (Zajíček 1955; Hohner 1961; Babero and Lee 1961; Pastuszko 1991; Rossin et al. 2009; Martino et al. 2012). This parasite was identified by Enigk (1933) and Petrov (1941) and redescribed in detail by Baruš et al. (1975). The fine structures of the cuticle and oesophagus of this species were described by Wright (1968a, b, 1972).
In our study, 60 individuals (young as well-breeding animals) from three localities were described and measured. Morphometrical data of males and females correspond to the description of Trichuris nematodes reported by Baruš (1975) in Azerbaijan. The main diagnostic characteristics in Trichuris spp. are body length and spicule length and width (Špakulová 1994). With respect to our specimens, the spicule length ranged from 2.55 to 4.9 mm. The other authors reported spicule lengths from 2.7 mm (Enigk 1933) to 3.9 mm (Petrov 1941). The Trichuris individuals in this study were slightly longer than those in previous studies. This can be due to the fact that the number of nutria that was dissected in this study was large individuals culled from breeding programs.
Trichuris spp. are common parasites of rodents. Trichuris muris and Trichuris arvicolae are the most commonly described species in rodents. The species designation T. muris (Schrank, 1788) is commonly used to refer to trichurids, which are found in Murinae; T. arvicolae is used to refer to the subfamily Arvicolinae (Feliu et al. 2000).
New Trichuris species (Trichuris pardinasi n. sp., Trichuris bainae n. sp., Trichuris thrichomysi n. sp.) were recently described in rodents in South America (Robles et al. 2006; 2014; Torres et al. 2011), and T. muris and T. arvicolae were redescribed in Europe (Feliu et al. 2000). The genus is also commonly found in African rodents, with at least 12 species described to date (Ribas et al. 2013).
In order to describe a new species of the Trichuris genus nowadays, it is necessary to utilize morphological and molecular methods. This is necessary because many species, such as T. arvicolae and T. muris and females of the species T. ovis and Trichuris discolor, are morphologically indistinguishable (Feliu et al. 2000; Salaba et al. 2013). Conversely, Cutillas et al. (2004) and Oliveros et al. (2000) could find no differences between sequences of T. ovis rDNA and Trichuris globulosa rDNA (internal transcribed spacers ITS1-5.8S-ITS2). Similarly, Trichuris capreoli is considered a synonym of T. globulosa by some authors (Cutillas et al. 2004; Oliveros et al. 2000; Gagarin 1972).
Currently, many Trichuris species are only morphologically defined. T. myocastoris was morphologically determined by Enigk 1933 and in detail by Baruš et al. (1975). This paper presents the first molecular description of T. myocastoris.
The BLASTn program within the GenBank database indicated that Trichuris individuals found in M. coypus did not match any of the genetically defined Trichuris species. Sequences are unique within the phylogenetic tree (Fig. 2), and they are clearly separated from other clusters. In particular, genetic differences between T. myocastoris and two trichurids native to the Czech Republic (T. muris and T. arvicolae) disprove the possibility that nutrias could be infected by local species of rodent trichurids after their translocation to the Czech Republic. Molecular analysis has proved that this is a unique genetic dataset, and it has confirmed that T. myocastoris is a separate species within the genus Trichuris. Trichuris nematodes were isolated from domestic M. coypus. The nematodes were identified using morphological and biometrical methods. Subsequently, genomic DNA was isolated and the ITS1-5.8S-ITS2 segment from ribosomal DNA (RNA) was amplified and sequenced using PCR techniques. Molecular analysis has proved the following: (1) this is a unique genetic dataset, which has confirmed that T. myocastoris is a separate species within the genus Trichuris, and (2) T. myocastoris was found to be significantly more closely related to Trichuris from rodents than to those from ruminants.
References
Babero BB, Lee JW (1961) Studies on the helminths of nutria, Myocastor coypus (Molina), in Louisiana with check list of other worm parasites from this host. J Parasitol 47:378–390
Baruš V, Majumdar G, Mikailov TK (1975) Morphology and taxonomy of Trichocephalus myocastoris (Enigk, 1933). Folia Parasitologica Praga 22:207–213
Callejón R, de Rojas M, Ariza C, Ubeda JM, Guevara DC, Cutillas C (2008) Cytochrome oxidase subunit 1 and mitochondrial 16S rDNA sequences of Trichuris skrjabini (Tricocephalida: Trichuridae). Parasitol Res 104:715–716
Cutillas C, Oliveros R, de Rojas M, Guevara DC (2004) Determination of Trichuris skrjabini by sequencing of the ITS1-5.8S-ITS2 segment of the ribosomal DNA: comparative molecular study of different species of Trichurids. J Parasitol 90(3):648–652
Dobrovol’skii AV (1952) Parazitarnye zabolevaniya u ondatry (Ondatra zibethica L.). Zoologicheskii Zhurnal 31(4):640–642
Enigk K (1933) Einige Nematoden aus der Nutria. Parasitol Res 6(3):326–331
Feliu C, Spakulova M, Casanova JC, Renaud F, Morand S, Hugot JP, Santalla F, Durand P (2000) Genetic and morphological heterogeneity in small rodent whipworms in southwestern Europe: characterization of Trichuris muris and description of Trichuris arvicolae n. sp (Nematoda: Trichuridae). J Parasitol 86(3):442–449
Gagarin VG (1972) Analiz vidogo sostava trihicefalov zvačnyh, zaregistrirovannyh v Sovetskom Spuze. Trudy Vsesojuznogo Instituta Gelmintologii K I Skrjabina 19:39–75
Gayo V, Cuervo P, Rosadilla D, Birriel S, Dell’Oca L, Trelles A, Cuire U, Sierra RMY (2011) Natural Fasciola hepatica infection in nutria (Myocastor coypus) in Uruguay. J Zoo Wildl Med 42(2):354–356
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hohner L (1961) Die Endoparasiten des Sumpfebibers unter besonderer Berücksichtigung der Kokzidien. Zoologischen Institut und Veterinär–Parasitologischen Institut der Karl–Marx Universotät Leipzig. pp. 98–151
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Martino PE, Radman N, Parrado E, Bautista E, Cisterna C, Silvestrini MP, Corba S (2012) Note on the occurrence of parasites of the wild nutria (Myocastor coypus, Molina, 1782). Helminthologia 49(3):164–168
Oliveros R, Cutillas C, de Rojas M, Arias P (2000) Characterization of four species of Trichuris (Nematoda: Enoplida) by their second internal transcribed spacer ribosomal DNA sequence. Parasitol Res 86:1008–1013
Pastuszko J (1991) The examination of intestinal parasitofauna in coypu (Myocastor coypus, Molina 1782) from closed husbandries with particular regard to coccidia. Akademii Rolniczej we Wrocławiu. p. 41
Pellérdy LP (1963) Catalouge of Eimeriidea (Protozoa, Sporozoa). Akademiai Kiadó Budapest. p. 122
Pellérdy LP (1974) Coccidia and coccidiosis. Verlag Paul Parey, Berlin, p 959
Petrov AM (1941) Glistnye bolezni pushnykh zverey. Mosqow. pp. 1–227
Ribas A, Lopez S, Makundi RH, Leirs H, de Bellocq JG (2013) Trichuris spp. (Nematoda: Trichuridae) from two rodents, Mastomys natalensis and Gerbilliscus vicinus in Tanzania. J Parasitol 99(5):868–875
Robles MD, Navone GT, Notarnicola J (2006) A new species of Trichuris (Nematoda: Trichuridae) from Phyllotini rodents in Argentina. J Parasitol 92(1):100–104
Robles MD, Cutillas C, Panei CJ, Callejón R (2014) Morphological and molecular characterization of a new Trichuris species (Nematoda- Trichuridae), and phylogenetic relationships of Trichuris species of cricetid rodents from Argentina. PLoS One 11(9):1–11
Rossin MA, Varela G, Timi JT (2009) Strongyloides myopotami in ctenomyid rodents: transition from semi-aquatic to subterranean life cycle. Acta Parasitologica 54(3):257–262
Salaba O, Rylková K, Vadlejch J, Petrtýl M, Scháňková Š, Brožová A, Jankovská I, Jebavý L, Langrová I (2013) The first determination of Trichuris sp. from roe deer by amplification and sequenation of the ITS1-5.8S-ITS2 segment of ribosomal DNA. Parasitol Res 112(3):955–960
Torres EJL, Nascimento APF, Menezes AO, Garcia J, dos Santos MAJ, Maldonado A, Miranda K, Lanfredi RM, de Souza W (2011) A new species of Trichuris from Thrichomys apereoides (Rodentia: Echimyidae) in Brazil: morphological and histological studies. Vet Parasitol 176(2-3):226–235
Tůmová E, Chodová D, Hrstka Z (2013) Hodnocení masné užitkovosti nutrií. Certifikovaná metodika ČZU v Praze, p. 27
Woods CA, Contreras L, Willner-Chapman G, Whidden HP (1992) Myocastor coypus. Mamm Species 398:1–8
Zajíček D (1955) Parazitární invaze u mladých nutrií. Sborník Vysoké školy zemědělské a lesnické v Brně, řada B, Spisy fakulty veterinární 3–4(224):95–104
Zhu X, Gasser RB, Jacobs DE, Hung G-C, Chilton NB (2000) Relationships among some ascaridoid nematodes based on ribosomal DNA sequence data. Parasitol Res 86:738–744
Acknowledgments
This study has been funded by the Ministry of Agriculture of the Czech Republic (Nutria Genetic Resources Programme) and the Grant of National Agency for Agricultural Research of the Czech Republic, v.v.i. CIGA No. 20152021. We would also like to thank Mr. Brian Kavalir for his proofreading services.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rylková, K., Tůmová, E., Brožová, A. et al. Genetic and morphological characterization of Trichuris myocastoris found in Myocastor coypus in the Czech Republic. Parasitol Res 114, 3969–3975 (2015). https://doi.org/10.1007/s00436-015-4623-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4623-8