Abstract
Angiostrongylus vasorum is a parasitic nematode that can cause serious and potentially fatal disease in dogs and other canids. The aim of this study was to determine the intermediate slug species infected in nature by sampling sites in Greater London and Hertfordshire located within a known hyperendemic region. Overall, A. vasorum larvae were recovered from 6/381 slugs (1.6 %) by tissue digestion, and their identity was confirmed by PCR. Infected slugs originated from three different sites in the Greater London area: one in Waltham Forest and two in Bromley. Slugs parasitised by A. vasorum were identified by a combination of external morphological characteristics and molecular techniques and belonged to three different families: the Arionidae, the Milacidae and the Limacidae. This includes two new host records for the parasite: Arion distinctus and Tandonia sowerbyi. This is the first record of A. vasorum in the family Milacidae, indicating that the parasite has a broader intermediate host range than previously recognised.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The parasite Angiostrongylus vasorum (Nematoda: Metastrongyloidea) infects dogs (Chapman et al. 2004; Taubert et al. 2009), foxes (Morgan et al. 2008) and other canids such as wolves (Segovia et al. 2001) and coyotes (Bourque et al. 2005). This nematode is potentially highly pathogenic in dogs and has an indirect life cycle, with development of the first stage larvae (L1) into the infective third stage larvae (L3) taking place within a gastropod host (Morgan et al. 2005). Gastropods become infected by ingesting L1 shed in the definitive host faeces or by direct penetration of larvae through the epidermis. The definitive host is infected by ingestion of L3 within the intermediate host (Morgan et al. 2005) or possibly by ingestion of free L3 in the environment that has been shed in gastropod secretions (Jefferies et al. 2009). Within the definitive host, adult A. vasorum are found in the right ventricle and pulmonary arteries. In dogs, infection can be subclinical or result in a variety of clinical signs, including coughing, dyspnoea, bleeding diathesis, neurological signs, collapse and sudden death (Chapman et al. 2004; Yamakawa et al. 2009).
A. vasorum has a worldwide distribution (Morgan et al. 2005) and, within Europe, is endemic in several countries including distinct areas of Denmark, France, Ireland, Germany and Great Britain (Morgan et al. 2009; Taubert et al. 2009; Schnyder et al. 2013; Kirk et al. 2014). Within these countries, distribution of the parasite is patchy, forming relatively stable hyperendemic ‘hotspots’ (Morgan and Shaw 2010). In Great Britain, autochthonous infection with A. vasorum was first reported from Cornwall and south Wales in the 1980s (Jones et al. 1980; Simpson and Neal 1982; Patteson et al. 1987; Trees 1987) and more recently the south-east of England (Chapman et al. 2004). Over the past 5 years, there has been an increase in reports of cases outside these areas, including infections acquired in northern England and Scotland (Helm et al. 2009; Yamakawa et al. 2009). Current evidence suggests that these reports reflect expansion of the parasite’s geographic range, rather than being merely a consequence of increased disease awareness; apparent range expansion has also been observed in other countries, including Denmark and Germany (Morgan and Shaw 2010).
With these recent changes in parasite distribution, canine angiostrongylosis is set to become a more common and widespread problem, making it increasingly important to gain a detailed understanding of the epidemiology of this disease. Although many species of slugs and snails have been shown to support the development of A. vasorum to the infective L3 stage (Guilhon and Cens 1973), the full range of intermediate host species remains unknown. Detailed knowledge of the epidemiology of canine angiostrongylosis within the intermediate host in natural infection cycles is essential for accurate prediction of transmission risk to dogs. By sampling slugs from the known A. vasorum hotspot in the south-east of England, this study sought to establish which slug species were involved and estimate their larval burden.
Materials and methods
Slug collection and maintenance
Terrestrial slugs were collected from 14 dog walking sites in the south-east of England (Fig. 1). Of these, four were located in Hertfordshire (Royal Veterinary College playing fields, Potters Bar; Parkfield Open Space, Potters Bar; Oakmere Park, Potters Bar; and Welham Green Recreation Ground), and the other 10 were located in Greater London. Of the Greater London sites, one was located in north London (Higham Hill/Walthamstow allotments, Waltham Forest) and nine were located in south London/north-west Kent (Battersea Park, Wandsworth; Beckenham Place Park, Lewisham; and seven sites in Bromley: Elmfield Wood, Whitehall Recreation Ground, Southborough Recreation Ground, Norman Park, Havelock Recreation Ground, Queensmead Recreation Ground and Harvington Estate). All these locations comprised either woodland or flat grassland and were within the region of south-east England which experiences numbers of angiostrongylosis cases in dogs that are significantly above the national average (Schnyder et al. 2013; Kirk et al. 2014). Slugs were collected in June–July 2009 from Hertfordshire and in June–July 2010 and September 2010–March 2011 from Greater London. The above sites were visited at dawn or dusk, and a total of 381 slugs were collected. These were maintained at 18–20 °C in ventilated plastic containers lined with damp tissue and fed a diet of lettuce and cucumber ad libitum.
Map of sampling locations. The outlines of the Greater London Boroughs (solid lines) and the two sampled Hertfordshire Boroughs (dotted lines) are shown: WH Welwyn Hatfield, H Hertsmere, WF Waltham Forest, W Wandsworth, L Lewisham, B Bromley. Sampling sites are marked with circles; filled circles represent locations where Angiostrongylus vasorum-infected slugs were collected. Map of Great Britain and Ireland showing the location of the Boroughs shown on the larger map (inset; shaded area)
Parasite isolation by tissue digestion
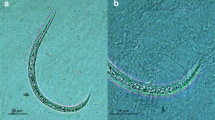
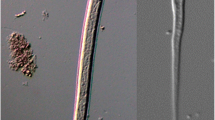
The live mass of each slug was recorded before each individual was decapitated and cut into left and right halves along the sagittal plane. One half was stored at −20 °C, and the other was examined for the presence of nematode larvae by tissue digestion, according to the method described by Ferdushy et al. (2009), with the following modifications. The slug tissue was incubated in artificial digestion fluid consisting of 0.7 % pepsin (Sigma-Aldrich, Gillingham, UK) and 0.5 % hydrochloric acid for 75 min. The resulting solution was centrifuged at 200×g for 2 min, and the supernatant was discarded. The pellet was resuspended in phosphate buffered saline (PBS), strained through a 300-μm aperture sieve, and the material that passed through was screened for nematode larvae using a stereomicroscope (ZoomMaster 65, Prior, Cambridge, UK). Larvae were photographed using an Olympus CX41 microscope (Southend on Sea, Essex, UK) equipped with an Olympus DP20-5 camera.
DNA extraction
DNA was extracted from 25 mg of the stored tissue of all the slugs that were positive for the presence of nematode larvae by tissue digestion. DNA extraction was performed using a DNeasy blood and tissue kit (QIAGEN, Crawley, UK) following a slightly modified protocol, in which the sample was homogenised using a stainless steel bead in a MM300 mixer mill (Retsch GmbH, Haan, Germany) at 30 oscillations per second for 2 min, before overnight incubation with proteinase K at 37 °C. The quantity and quality of extracted DNA were assessed using a Nanodrop ND-1000 (Thermo Scientific, Wilmington, DE, USA).
Molecular identification of A. vasorum
To confirm the presence of A. vasorum larvae, samples were processed using previously described PCR protocols. The entire second ribosomal internal transcribed spacer region (ITS-2) was amplified using the rhabditid primers NC1 and NC2 (Gasser et al. 1993), and a subsection of the ITS-2 region was amplified using the A. vasorum-specific primers AvasF and AvasR (Helm et al. 2009). Thermocycling conditions were optimised using DNA extracted from adult A. vasorum obtained from a dog at post-mortem.
All PCR reactions were performed in a G-Storm GS1 thermal cycler (GRI, Braintree, UK) in a 25-μl reaction volume prepared using either a KAPA2G Robust kit (Kapa Biosystems, Woburn, MA, USA) or a MyTaq HS DNA Polymerase kit (Bioline, London, UK) and a 55 °C annealing temperature, according to the enzyme manufacturer’s instructions. In all experiments, a positive control (either an uninfected slug spiked with A. vasorum or a known infected slug) and a negative control (containing no DNA) were included. Products were visualised on 1.5 % agarose gels stained with SYBR® Safe (Life Technologies, Paisley, UK) according to the manufacturer’s instructions. PCR products were purified using a QIAquick PCR purification kit (QIAGEN) and sent for sequencing to either GATC Biotech (London, UK) or Source BioScience (Cambridge, UK). Sequence analysis was performed using CLC Main Workbench 6 version 6.6.5 (CLC bio, Swansea, UK). These sequences were used to confirm the presence of A. vasorum by comparison to known sequences in the GenBank database.
Identification of slug species
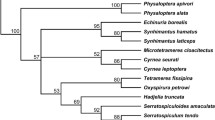
A. vasorum-infected slugs were identified based on external morphological characteristics (Cameron et al. 1983) in combination with DNA sequence data. The mitochondrial genes cytochrome c oxidase subunit I (COI) and 16S rDNA were amplified by PCR using the primers LCO and HCO (Folmer et al. 1994), and 16S-1 and 16S-2 (Barr et al. 2009), respectively. PCR reactions were performed as described above for the amplification of the nematode ITS-2 region but using an annealing temperature of 42 °C for the LCO and HCO primers and 50 °C for the 16S primers. The resulting PCR products were purified, sequenced and analysed as above. The slugs were identified by alignment of all available GenBank sequences from slug species known to occur in Great Britain according to Anderson (2005, updated 2008) and those from this study using Clustal Omega version 1.2.0. (Sievers et al. 2011). Sequence alignments were used to calculate pairwise Kimura-2-Parameter distances and to construct neighbour-joining (NJ) trees with the complete deletion option in MEGA v.5 (Tamura et al. 2011). Branch support was assessed by bootstrapping over 1,000 replicates. The DNA identifications of the slugs were based on the positions of their sequences in the NJ trees relative to the GenBank sequences.
Statistical analysis
All statistical analyses were carried out using R version 3.0.0 (R Foundation for Statistical Computing, Vienna, Austria). To account for multiple statistical testing, p values were adjusted using the standard Bonferroni correction.
Results
Nematode larvae were found in nine of the 381 slugs sampled. Using morphological characteristics, six of these were determined to be A. vasorum (Fig. 2). This was confirmed by PCR, using the A. vasorum-specific Avas primers, which yielded a product with all six samples identified morphologically as A. vasorum, but not with the other three samples. In contrast, all nine samples yielded a PCR product when amplified using the NC primers, confirming that nematode DNA was present and that the lack of product with the Avas primers was not due to uneven distribution of larvae within the slugs. With both primer sets, dilution of DNA by 10-fold gave optimal results, suggesting the presence of PCR inhibitors. Using blastn (Zhang et al. 2000), all sequences obtained using the NC primers were assigned to A. vasorum using a cut-off expect (E) value of 1.62e-79 or lower. The % similarity ranged from 97.2 to 100 % (A. vasorum, GU045375).
The overall prevalence of infection with A. vasorum was 1.6 %. Positive slugs were found at three of the sampled sites (Fig. 1; Table 1): Elmfield Wood (1/31; 3.2 %), Higham Hill/Walthamstow allotments (1/60; 1.7 %) and Queensmead Recreation Ground (4/48; 8.3 %). The difference in prevalence between the different sites was not statistically significant (Fisher’s exact test, p = 0.33). A. vasorum larvae were recovered from specimens collected both during summer and winter (Table 1). The three slugs that were positive for other nematode species all came from the same site in south London (Havelock Recreation Ground).
The A. vasorum-infected slugs were identified as Arion distinctus (n = 1; family Arionidae), Arion ater aggregate (n = 2; family Arionidae), Tandonia sowerbyi (n = 2; family Milacidae) and a species in the family Limacidae (n = 1). The number of larvae recovered per half slug ranged from two in A. distinctus to 23 in A. ater aggregate (Table 1). Larval burden tended to be higher in heavier slugs, but this was not statistically significant (Spearman’s rank correlation coefficient = 0.77, p = 0.10; Fig. 3). Slug mass varied significantly between different collection sites (Kruskal-Wallis test, p = <0.001 after Bonferroni correction), making it difficult to determine whether there is an association between slug mass and infection status overall. However, for the single site from which multiple infected slugs were recovered (Queensmead Recreation Ground), A. vasorum-positive specimens were significantly heavier than uninfected specimens (Mann-Whitney U test, p = 0.024 after Bonferroni correction; Fig. 4).
Discussion
This study has confirmed the presence of A. vasorum larvae in the tissues of at least four different species of terrestrial slug from three different families, A. distinctus, A. ater aggregate, T. sowerbyi and a limacid species. The A. ater aggregate consists of three species (A. ater, Arion rufus and Arion vulgaris = Arion lusitanicus) that cannot be accurately differentiated based on external morphology alone (Cameron et al. 1983). Furthermore, their identification is complicated by the fact that they may hybridise (Roth et al. 2012; Dreijers et al. 2013), and current COI and 16S rDNA sequences on GenBank do not allow unequivocal separation of the species. Since we used tissue digestion to retrieve larval nematodes, more accurate identification based on internal morphology was not possible. The COI sequence from the single specimen identified as belonging to the family Limacidae clustered most closely with sequences from Lehmannia valentiana (Accession numbers JX117876; AM259711; AM259710). However, there are relatively few COI sequences available for this family on GenBank with some British species not represented. Even fewer 16S rDNA sequences are available, covering only two of the eight species found in Great Britain (Anderson 2005). We have therefore refrained from classifying this slug to genus or species level.
The broad range of intermediate host species found in this study is consistent with previous studies describing both experimental (Guilhon and Cens 1973) and natural infection (Ferdushy et al. 2009; Jefferies et al. 2009) and is consistent with the wide intermediate host range found in other species of the genus, such as A. cantonensis (Eckert and Lämmler 1972; Yousif and Lämmler 1975). The fact that species in the A. ater aggregate can support the development of A. vasorum to the infective L3 stage is well recognised (Guilhon and Cens 1973; Ferdushy et al. 2009; Jefferies et al. 2009). Infection of species in the Arion hortensis aggregate (consisting of A. hortensis, A. distinctus and Arion owenii) has also been previously reported from Wales (Jefferies et al. 2009). However, in our study, we could further identify the slug species involved as A. distinctus, based on the COI and 16S rDNA sequences. This is the most common and widespread species in the complex (Davies 1977, 1979; De Winter 1984; Backeljau and Van Beeck 1986). To our knowledge, this is the first report of A. vasorum infection in this species as distinguished from A. hortensis. Records of A. vasorum in ‘A. hortensis’ prior to 1979, such as those reported by Guilhon and Cens (1973), cannot be used as accurate host records since A. hortensis was not formally separated from A. distinctus until 1977 and 1979 (Davies 1977, 1979). Furthermore, it is very difficult to distinguish these two species by their external morphology, and their identification should always be confirmed by anatomical data (De Winter 1984; Backeljau and Van Beeck 1986) or molecular markers (Mc Donnell et al. 2008; Barr et al. 2009; Soroka and Skujiene 2011). In addition to A. distinctus, we have confirmed A. vasorum infection in two T. sowerbyi specimens, which represent the first published report of infection within the family Milacidae. Both T. sowerbyi and A. distinctus tend to be highly synanthropic and are therefore common in man-made habitats such as gardens, parklands and fields (Pfleger and Chatfield 1988). Thus, these slug species are likely to be important for transmission of angiostrongylosis in urban as well as suburban and agricultural areas.
The prevalence of 1.6 % in the current study is substantially lower than determined for the hyperendemic region in Wales by Jefferies et al. (2009) who identified infection in 20 of 47 (42.6 %) slugs by real-time PCR. This difference may at least in part be due to the different methods used. In our study, we have opted to use tissue digestion and microscopy in combination with PCR to avoid false positives. While this approach may have resulted in our calculated prevalence slightly underestimating the true prevalence, using solely PCR to screen a large volume of samples can be prone to false positives (Borst et al. 2004) and may overestimate prevalence. However, another study using tissue digestion to determine prevalence of A. vasorum in a region of Denmark found an overall prevalence of 9 %, reaching 26 % at one sampling location, confirming that local prevalence can be very high in certain localities (Ferdushy et al. 2009).
A major advantage of tissue digestion is that it allows determination of larval burden in individual slugs. Larval burden of the intermediate host is likely to be an important factor determining the outcome of infection in dogs, since the severity of clinical signs appears to be proportional to the number of L3 ingested (Schnyder et al. 2010) and the ingestion of a single large dose of infective larvae has been associated with sudden death (Bolt et al. 1994). Ferdushy et al. (2009) found that while most (82 %) infected slugs harboured a larval burden below 10, a small proportion (14 %) may carry more than 100 larvae; this pattern is typical of parasite aggregation within a host population (Shaw et al. 1998). In the current study, larval burdens never exceeded 23 larvae per half slug. The absence of high burdens may be due to the relatively low number of positive slugs sampled. Alternatively, this could reflect the overall lower prevalence of infection. This would result in lower exposure of the definitive host and in turn reduced opportunity for infection of slugs. Although there was no significant association between larval burden and slug mass, there was a trend for larger individuals to yield a greater number of larvae. A similar trend was observed by Ferdushy et al. (2009). Furthermore, a large epidemiological survey on the related species A. cantonensis reported that larval burden in one species of snail intermediate host was more than three times higher in heavier individuals, when compared to small- and medium-sized individuals (Chen et al. 2011).
Previous studies on A. vasorum have confirmed infection of slugs during the summer (Jefferies et al. 2009) and autumn (Ferdushy et al. 2009). In the current study, slugs infected with A. vasorum were collected both in the summer and winter, indicating that there may be an infection risk to dogs throughout the year. However, further work is required to elucidate the precise seasonal pattern of infection in gastropods.
Conclusion
With reports of canine angiostrongylosis outside the previously recognised hyperendemic regions, an understanding of the epidemiology of this disease is becoming increasingly important. This study has found A. vasorum infection in at least four different slug species, two of which were new host records. Furthermore, we have identified infection in three different families of slugs, including the first published record of infection in the family Milacidae, thereby adding to the growing list of intermediate host species. It is not known whether these new intermediate host records represent a recent host-parasite association or a long-standing association that has not previously been detected. However, if the former is true, it might help to explain the apparent ease with which infection has spread to previously non-endemic regions.
References
Anderson R (2005) An annotated list of the non-marine Mollusca of Britain and Ireland. J Conchol 38:607–637
Backeljau T, Van Beeck M (1986) Epiphallus anatomy in the Arion hortensis species aggregate (Mollusca, Pulmonata). Zool Scr 15:61–68. doi:10.1111/j.1463-6409.1986.tb00209.x
Barr NB, Cook A, Elder P, Molongoski J, Prasher D, Robinson DG (2009) Application of a DNA barcode using the 16S rRNA gene to diagnose pest Arion species in the USA. J Molluscan Stud 75:187–191. doi:10.1093/mollus/eyn047
Bolt G, Monrad J, Koch J, Jensen AL (1994) Canine angiostrongylosis: a review. Vet Rec 135:447–452. doi:10.1136/vr.135.19.447
Borst A, Box ATA, Fluit AC (2004) False-positive results and contamination in nucleic acid amplification assays: suggestions for a prevent and destroy strategy. Eur J Clin Microbiol Infect Dis 23:289–299. doi:10.1007/s10096-004-1100-1
Bourque A, Whitney H, Conboy G (2005) Angiostrongylus vasorum infection in a coyote (Canis latrans) from Newfoundland and Labrador, Canada. J Wildl Dis 41:816–819. doi:10.7589/0090-3558-41.4.816
Cameron RAD, Eversham B, Jackson N (1983) A field key to the slugs of the British Isles. Field Stud 5:807–824
Chapman PS, Boag AK, Guitian J, Boswood A (2004) Angiostrongylus vasorum infection in 23 dogs (1999–2002). J Small Anim Pract 45:435–440. doi:10.1111/j.1748-5827.2004.tb00261.x
Chen D, Zhang Y, Shen H, Wei Y, Huang D, Tan Q, Lan X, Li Q, Chen Z, Li Z (2011) Epidemiological survey of Angiostrongylus cantonensis in the west-central region of Guangdong Province, China. Parasitol Res 109:305–314. doi:10.1007/s00436-011-2255-1
Davies SM (1977) The Arion hortensis complex, with notes on A. intermedius Normand (Pulmonata: Arionidae). J Conchol 29:173–187
Davies SM (1979) Segregates of the Arion hortensis complex (Pulmonata: Arionidae), with the description of a new species, Arion owenii. J Conchol 30:123–127
De Winter AJ (1984) The Arion hortensis complex (Pulmonata: Arionidae): designation of types, descriptions, and distributional patterns, with special reference to the Netherlands. Zool Meded 59:1–17
Dreijers E, Reise H, Hutchinson JMC (2013) Mating of the slugs Arion lusitanicus auct. non Mabille and A. rufus (L.): different genitalia and mating behaviours are incomplete barriers to interspecific sperm exchange. J Molluscan Stud 79:51–63. doi:10.1093/mollus/eys033
Eckert J, Lämmler G (1972) Angiostrongylose bei Mensch und Tier. Z Parasitenkd 39:303–322. doi:10.1007/BF00329093
Ferdushy T, Kapel CMO, Webster P, Al-Sabi MNS, Grønvold J (2009) The occurrence of Angiostrongylus vasorum in terrestrial slugs from forests and parks in the Copenhagen area, Denmark. J Helminthol 83:379–383. doi:10.1017/S0022149X09377706
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Gasser RB, Chilton NB, Hoste H, Beveridge I (1993) Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Res 21:2525–2526
Guilhon J, Cens B (1973) Angiostrongylus vasorum (Baillet, 1866). Etude biologique et morphologique. Ann Parasitol Hum Comp 48:567–596
Helm J, Gilleard JS, Jackson M, Redman E, Bell R (2009) A case of canine Angiostrongylus vasorum in Scotland confirmed by PCR and sequence analysis. J Small Anim Pract 50:255–259. doi:10.1111/j.1748-5827.2009.00741.x
Jefferies R, Morgan ER, Shaw SE (2009) A SYBR green real-time PCR assay for the detection of the nematode Angiostrongylus vasorum in definitive and intermediate hosts. Vet Parasitol 166:112–118. doi:10.1016/j.vetpar.2009.07.042
Jones GW, Neal C, Turner GR (1980) Angiostrongylus vasorum infection in dogs in Cornwall. Vet Rec 106:83
Kirk L, Limon G, Guitian J, Hermosilla C, Fox M (2014) Angiostrongylus vasorum in United Kingdom mainland—a nationwide postal questionnaire survey of veterinary practices. Vet Rec 175:118. doi:10.1136/vr.102196
Mc Donnell RJ, Paine TD, Stouthamer R, Gormally MJ, Harwood JD (2008) Molecular and morphological evidence for the occurrence of two new species of invasive slugs in Kentucky, Arion intermedius Normand and Arion hortensis Férussac (Arionidae: Stylommatophora). J Ky Acad Sci 69:117–123. doi:10.3101/1098-7096-69.2.117
Morgan E, Shaw S (2010) Angiostrongylus vasorum infection in dogs: continuing spread and developments in diagnosis and treatment. J Small Anim Pract 51:616–621. doi:10.1111/j.1748-5827.2010.01000.x
Morgan ER, Shaw SE, Brennan SF, De Waal TD, Jones BR, Mulcahy G (2005) Angiostrongylus vasorum: a real heartbreaker. Trends Parasitol 21:49–51. doi:10.1016/j.pt.2004.11.006
Morgan ER, Tomlinson A, Hunter S, Nichols T, Roberts E, Fox MT, Taylor MA (2008) Angiostrongylus vasorum and Eucoleus aerophilus in foxes (Vulpes vulpes) in Great Britain. Vet Parasitol 154:48–57. doi:10.1016/j.vetpar.2008.02.030
Morgan ER, Jefferies R, Krajewski M, Ward P, Shaw SE (2009) Canine pulmonary angiostrongylosis: the influence of climate on parasite distribution. Parasitol Int 58:406–410. doi:10.1016/j.parint.2009.08.003
Patteson MW, Wotton PR, Lucke VM, Wright AI, Gibbs C (1987) Angiostrongylus vasorum in a terrier. Vet Rec 120:349
Pfleger V, Chatfield J (1988) A guide to the snails of Britain and Europe. Hamlyn, London
Roth S, Hatteland BA, Solhøy T (2012) Some notes on reproductive biology and mating behaviour of Arion vulgaris Moquin-Tandon 1855 in Norway including a mating experiment with a hybrid of Arion rufus (Linnaeus 1758) x ater (Linnaeus 1758). J Conchol 41:249–257
Schnyder M, Fahrion A, Riond B, Ossent P, Webster P, Kranjc A, Glaus T, Deplazes P (2010) Clinical, laboratory and pathological findings in dogs experimentally infected with Angiostrongylus vasorum. Parasitol Res 107:1471–1480. doi:10.1007/s00436-010-2021-9
Schnyder M, Schaper R, Bilbrough G, Morgan ER, Deplazes P (2013) Seroepidemiological survey for canine angiostrongylosis in dogs from Germany and the UK using combined detection of Angiostrongylus vasorum antigen and specific antibodies. Parasitology 140:1442–1450. doi:10.1017/S0031182013001091
Segovia JM, Torres J, Miquel J, Llaneza L, Feliu C (2001) Helminths in the wolf, Canis lupus, from north-western Spain. J Helminthol 75:183–192. doi:10.1079/JOH200152
Shaw DJ, Grenfell BT, Dobson AP (1998) Patterns of macroparasite aggregation in wildlife host populations. Parasitology 117:597–608. doi:10.1017/s0031182098003448
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi:10.1038/msb.2011.75
Simpson VR, Neal C (1982) Angiostrongylus vasorum infection in dogs and slugs. Vet Rec 111:303–304
Soroka M, Skujiene G (2011) Species identification of slugs of genus Arion Férussac, 1819 (Mollusca, Pulmonata) on the basis of genetics studies. Ekologija 57:70–80. doi:10.6001/ekologija.v57i2.1887
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Taubert A, Pantchev N, Vrhovec MG, Bauer C, Hermosilla C (2009) Lungworm infections (Angiostrongylus vasorum, Crenosoma vulpis, Aelurostrongylus abstrusus) in dogs and cats in Germany and Denmark in 2003-2007. Vet Parasitol 159:175–180. doi:10.1016/j.vetpar.2008.10.005
Trees AJ (1987) Angiostrongylus vasorum in dogs in Wales. Vet Rec 120:424
Yamakawa Y, McGarry JW, Denk D, Dukes-McEwan J, Macdonald N, Mas A, McConnell F, Tatton B, Valentine EG, Wayne J, Williams JM, Hetzel U (2009) Emerging canine angiostrongylosis in northern England: five fatal cases. Vet Rec 164:149–152. doi:10.1136/vr.164.5.149
Yousif F, Lämmler G (1975) The suitability of several aquatic snails as intermediate hosts for Angiostrongylus cantonensis. Z Parasitenkd 47:203–210. doi:10.1007/BF00418203
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7:203–214. doi:10.1089/10665270050081478
Acknowledgments
We would like to thank Emma Wanostrocht for sample collection and The Royal Veterinary College for financial support (Royal Veterinary College manuscript number 715).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zainab Patel and A. Christina Gill contributed equally to this work.
Rights and permissions
About this article
Cite this article
Patel, Z., Gill, A.C., Fox, M.T. et al. Molecular identification of novel intermediate host species of Angiostrongylus vasorum in Greater London. Parasitol Res 113, 4363–4369 (2014). https://doi.org/10.1007/s00436-014-4111-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4111-6