Abstract
In general, the knowledge on parasites infecting Antarctic birds is scarce. The present study intends to extend the knowledge on gastrointestinal parasites of Emperor Penguins (Aptenodytes forsteri) at the Atka Bay, Antarctica. Fecal samples of 50 individual Emperor Penguins were collected at the Atka Bay and analyzed using the sodium-acetate-formaldehyde (SAF) method for the identification of intestinal helminth eggs and/or protozoan parasite stages. In addition, coproantigen ELISAs were performed to detect Cryptosporidium and Giardia infections. Overall, 13 out of 50 penguins proved parasitized (26 %). The following stages of gastrointestinal parasites were identified: One Capillaria sp. egg, Tetrabothrius spp. eggs, Diphyllobothrium spp. eggs, and proglottids of the cestode Parorchites zederi. The recorded Capillaria infection represents a new host record for Emperor Penguins. All coproantigen ELISAs for the detection of Cryptosporidium spp. and Giardia spp. were negative. This paper provides current data on parasites of the Emperor Penguin, a protected endemic species of the Antarctica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Only nine out of 156 currently recognized avian families are specialized as seabirds such as the penguins (Speniscidae) (Lauckner 1985). The Emperor Penguin, Aptenodytes forsteri, is the most southerly situated penguin species on earth and a top endemic predator in the marine environment of the Antarctic region (Cherel and Kooyman 1998). The population size of the Atka Bay Emperor Penguin colony was composed of approximately 9,600 animals, estimated via satellite pictures by the British Antarctic Survey (BAS) (Fretwell et al. 2012). Moreover, the Atka Bay colony currently belongs to the ten largest colonies of Emperor Penguins of the Antarctic region. In general, birds of the genus Aptenodytes are the heaviest penguins (up to 40 kg) and the deepest divers (250 m, some deep dives up to 564 m) among seabirds (Kooyman et al. 1992; Kooyman and Kooyman 1995; Wienecke et al. 2007). Emperor Penguins (A. forsteri) feed on fish (mainly the Antarctic silverfish Pleuragramma antarcticum), squids (Psychroteuthis glacialis and Alluroteuthis antarcticus), and crustaceans (such as Euphausiacea superba), the proportions of which vary with the geographical location (Cherel and Kooyman 1998).
In general, knowledge on endoparasites of Antarctic birds is scarce (Vidal et al. 2012). So far, most reports focused on helminth infections of marine mammals whilst parasitoses of marine birds, particularly from polar regions, have been neglected in the last years (Lauckner 1985). Furthermore, ecological consequences of wide-spread infections in Antarctic bird populations have received few attentions, although a single outbreak may decimate animal populations (Barbosa and Palacios 2009). Since the Antarctic ecosystem is not beyond the risk of pathogens and/or parasites due to the geographical isolation and climatic conditions, Barbosa and Palacios (2009) voted for more research in this area, which is claimed necessary to establish general patterns of spatial and temporal variation in pathogens and parasites. Since infections with gastrointestinal parasites mainly depend on foraging habits (Hoberg 1996), modifications in host diet owing to climate change or anthropogenic impacts, such as fishing overexploitation, lead to changes in the occurrence of isolated parasites found in Antarctic penguins (Vidal et al. 2012). Especially, the issue of global warming (Steig et al. 2009), which may lead to increased survival of pathogens and potential outbreaks within such ecosystems (Barbosa and Palacios 2009), is believed to influence and possibly change the occurrence and diversity of prey organisms which in turn leads to changes in parasitoses prevalences. So far, studies on penguin parasites mainly focused on Chinstrap Penguins (Pygoscelis antarcticus, Palacios et al. 2012; Vidal et al. 2012), Gentoo Penguins (Pygoscelis papua, Fredes et al. 2007; Diaz et al. 2013), and on the closely related King Penguins (A. patagonicus, Mawson 1953; Prudhoe 1969; Jones 1988; Fonteneau et al. 2011), for reviews see Barbosa and Palacios (2009) and Clark and Kerry (2000). Some penguin species are known to harbor the anisakid ‘sealworm’ Contracaecum spp. (Mawson 1953; Fredes et al. 2007). However, information on gastrointestinal parasites of Emperor Penguins (A. forsteri) are scarce and are restricted to antarctic expeditions long time ago reporting on infections with two cestode species, Parorchites zederi and Tetrabothrius wrighti (Baird 1853; Leiper and Atkinson 1914, 1915; Fuhrmann 1921; Johnston 1937; Prudhoe 1969).
The aim of the current study was to extend the knowledge of intestinal parasite infections of Emperor Penguins (A. forsteri). This study represents the first analysis of intestinal parasites of Antarctic Emperor Penguins for more than 40 years and the first molecular characterization of P. zederi.
Material and methods
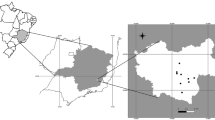
Fecal samples of 50 individual Emperor Penguins (A. forsteri) were collected (Fig. 1) at the Atka Bay, Weddell Sea, East Antarctica (coordinates 70° 36.664′ S–70° 37.064′ S; 008° 7.709′ W–008° 8.769′ W) (Fig. 2) in 2012 during the German Antarctica expedition of the Alfred Wegener Institute for Polar and Marine Research (Bremerhaven, Germany), under the compliance with statutory laws of the German Federal Environment Agency. Collected fecal samples were fixed in 70 % ethanol for conservation reasons. At the Institute for Parasitology, Justus Liebig University, Giessen, Germany, samples were analyzed at the coprological diagnostic laboratory unit using the sodium-acetate-formaldehyde (SAF) technique for identification of intestinal helminth eggs and/or protozoan parasite stages. In addition, coproantigen ELISAs (Giardia/Cryptosporidium, Thermo Scientific, Oxoid ProSpecTTM) were performed to detect Cryptosporidium spp. and Giardia spp. infections.
Shed cestode proglottids found in fecal material were stained in lactic acid-carmine (Blajin and Rukhadze 1929), differentiated in acid 70 % ethanol, dehydrated, mounted in Eukitt, and thereafter analyzed by light microscopy or alternatively cleared in lactophenol and examined without further treatment.
For the molecular characterization of the shed proglottids, DNA was extracted applying the DNeasy blood and tissue kit (Qiagen, Hilden, Germany) according to the tissue protocol. The near complete 18S ribosomal gene was amplified by PCR with universal forward and reverse primers: NC18SF-1 (5′-AAAGATTAAGCCATGCA-3′; Chilton et al. 2006) and WormB (5′- CTTGTTACGACTTTTACTTCC-3′; Littlewood and Olson 2001). The obtained amplicon was purified by agarose gel electrophoresis, isolated, and directly sequenced with the flanking primers and primer #652 (5′-GCAGCCGCGGTAATTCCAGCTC-3′; Nadler et al. 2007) hybridizing to the internal region. The new sequence was submitted to GenBank (accession number KF705621). Sequence analysis for taxonomic classification was performed by BLAST search against the nucleotide collection database (nr/nt). Selected sequences of high score were aligned with MAFFT 7 (Katoh and Standley 2013) using the L-INS-i method and compared using MEGA6 (Tamura et al. 2013).
Results
Endoparasites in fecal samples
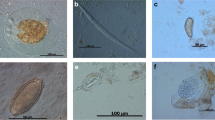
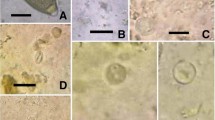
Parasitological calculations (prevalence in %) were made according to Bush et al. (1997). Thirteen out of 50 examined penguins proved parasitized (26 %). Overall, infections with four species of gastrointestinal parasites were diagnosed (illustrations of parasite stages are depicted in Figs. 3 and 4): Capillaria sp. egg (Nematoda) (2.0 % prevalence, 57 μm length/28 μm width), Tetrabothrius spp. eggs (Cestoda) (24 % prevalence, egg closed 73–74 μm length/48 μm width; egg open 41.0–41.5 μm length/48 μm width), Diphyllobothrium sp. eggs (2.0 % prevalence, 55–59 μm length/37–39 μm width), and cestode proglottids (2.0 % prevalence, eggs 51–52 μm length/37 μm width). It is worth noting that the diagnosis of Capillaria eggs in fecal samples represents a new parasite record for Emperor Penguins.
(1–4) Parorchites zederi. 1 Proglottids wider than long. Genital pores irregularly alternating with prominent everted genital atrium, inset close-up view showing protruding cirrus (Scale bar 250 μm). 2–3 Detail view of postmature proglottids from P. zederi (Scale bars 500 μm). Gp genital pore, Ov ovary, T testes, U uterus, and Vg vitelline gland. 4 Isolated egg capsules from gravid proglottids containing one single egg with an oncosphere (Scale bar 20 μm)
Neither protozoan parasites nor acantocephalan eggs was detected by microscopical examination of the SAF-concentrated fecal samples. Furthermore, all samples proved negative for Cryptosporidium spp. and Giardia spp. by coproantigen-ELISAs.
Morphological and molecular characterization of Parorchites zederi
The fecal sample from one penguin contained a strobila (Fig. 4 (1)) of postmature and gravid proglottids. The proglottids exhibited the following characteristics: much wider than long (4,700 × 500 μm), craspedote, each with single genitalia set. The genital pores lay lateral, alternating irregularly in consecutive segments. Proglottids of the present specimen had large genital atriums which are everted and appeared as prominent, funnel-shaped, muscular bulbs, some of which showing a protruding cirrus (close-up view Fig. 4 (1)). The ovary was situated anterior and next to the genital pore (anteroporal), irregularly lobed, and loose in tissue structure; vitellarium, small and compact, kidney-shaped, posterior to ovary. The uterus in the postmature proglottids (Fig. 4 (1–4)) was reticulate and with parenchymatous egg capsules in mature proglottids. These capsules measured 150 × 100 μm containing one egg (monovular) of 40 × 50 μm with the oncophere surrounded by a thick egg membrane (Fig. 4 (4)). Testes were numerous (∼50–100) constituting a transverse band, as wide as the proglottide (Fig. 4 (3)). The cirrus sac was very small.
Given these features and the microscopical image, the strobila and proglottids were consistent with P. zederi Baird 1853 (Dilepididae) following the key of cestode guide books (Schmidt 1986; Kahlil et al. 1994). This identification was verified by comparison with the more detailed descriptions and drawings of P. zederi by Johnston (1937), Cielecka et al. (1992), and Georgiev et al. (1996), of those the last two authors isolated this cestode from Pygoscelis spp. penguins in the South Shetlands.
In addition, we performed a first molecular characterization of P. zederi. We amplified and sequenced a near complete 1,966 bp fragment of the small subunit ribosomal RNA (rRNA) gene by PCR using universal primers. BLAST search and phylogenetic analyses with this new sequence were in accordance with the morphological classification to the family Dilepididae. So far, P. zederi is known as the only member of this order within the pelagic system (Hoberg 2005).
Discussion
Overall, a low number of parasite species were found in Emperor Penguins (A. forsteri), which is in accordance to the previous studies on penguin parasite fauna. In general, the here found prevalences were rather low. Highest prevalences were recorded for the cestode Tetrabothrius spp. of which further species identification based on the morphology of early egg stages was not possible. Representatives of the genus Tetrabothrius had already been recorded in Emperor Penguins (Prudhoe 1969). The complete life cycle of those cestodes is to date still unclear and further investigations are needed. It is known that plerocercoid larval stages of penguin cestodes use a variety of prey fishes/crustaceans (Williams 1995; Hoberg 2005; Vidal et al. 2012; Diaz et al. 2013) as intermediate hosts and birds as final hosts (Fredes et al. 2007); therefore, it can be expected that the cestode Tetrabothrius also have either prey fishes or crustaceans as intermediate hosts in the Antarctic region. On the other hand, the second cestode species found in this study, P. zederi, is a well known parasite of A. forsteri collected by several Antarctic expeditions. This cestode is widely distributed among other Antarctic penguins (Hoberg 2005; Barbosa and Palacios 2009; Vidal et al. 2012) due to a broad oceanic distribution of euphausiids, such as the pelagic crustacean E. superba, which serves as main intermediate hosts for this cestodes (Williams 1995; Hoberg 2005; Vidal et al. 2012; Diaz et al. 2013) and is known as prey organism for Emperor Penguins (Cherel and Kooyman 1998). Despite of an analysis of two large samples of Antarctic krill, Cielecka et al. (1992) did not find any Parorchites stages in krill. However, it was isolated from the intestine of crab and leopard seals. Georgiev et al. (1996) supposed that P. zederi is a complex of species because Cielecka et al. (1992) reported considerable differences in the size of the rostellar hooks from Parorchites isolated from different host species. The SSU rRNA gene sequence of the specimen from A. forsteri might contribute to solve this issue in future experiments. Here, detected P. zederi and Tetrabothrius eggs in Emperor Penguins (A. forsteri) can be distinguished from other parasitic eggs by their characteristic morphology features such as size, egg shell, form, content, and form of operculum, but the contrast is true for other helminth eggs occurring in seabirds such as digenean eggs. To the best of our current knowledge, we here describe the first record of Capillaria spp. eggs in Emperor Penguins (A. forsteri). The genus Capillaria includes a diversity of widespread nematode parasite species, which can have different vertebrates as final hosts, such as fishes, birds, and mammals (Anderson 2002). More than 300 different species of Capillaria have been described so far worldwide, many of them occurring in wildlife (Anderson 2002). For some marine Capillaria species, the life cycle is fortunately known. The final host shed non-embryonated eggs into the marine environment. These Capillaria eggs embryonate thereafter and become infectious. Some species will include fishes as intermediate hosts and these infected fishes can become prey organisms for the penguins, which become infected after consumption and within the final host ingested larva will develop after two molts into adult nematodes and the life cycle is completed. The species C. convoluta has previously been recorded from marine seabirds, such as the Southern Giant Petrel (Macronectes giganteus) (Mawson 1953) but until now never been described in penguins (for review see Barbosa and Palacios 2009). The low occurrence of Capillaria in Emperor Penguins (A. forsteri) could be explained by the strict dependency of the life cycle of this nematode species on the aquatic environment and the stenophagic and pelagic diet of Emperor Penguins (see Vidal et al. 2012). However, we can not entirely rule out the possibility of a false-positive finding through intestinal passage or accidental contamination by feces from the Southern Giant Petrel or South Polar Skua.
Furthermore, the low parasite richness observed in our current study, especially for trematodes, could be related to the very low temperatures observed in the Antarctic environment, which might inhibit survival of larval parasitic stages (Lauckner 1985). Despite hostile environmental and climate conditions, it was possible to isolate four different parasite species. Some other related studies on penguin parasites have also shown similar diversities. Vidal et al. (2012) likewise observed four helminth parasite species: two cestodes (Tetrabothrius pauliani and P. zederi), one nematode (Stegophorus macronetes), and one (Corynosoma sp.) in Chinstrap Penguins.
Diaz et al. (2013) also revealed four parasite species in Gentoo Penguins: one cestode (P. zederi), two nematodes (Stegophorus macronectes and Tetrameres wetzeli), and one acanthocephalan species (Corynosoma shackeltoni). Fredes et al. (2007) detected in total two helminth parasite species, the cestode Tetrabothrius sp. and the ascarid nematode Contracaecum spp. Hoberg (1996) stated that the core parasite composition in pelagic birds consists of cestodes, mainly Tetrabothriidae, but with the present study we could clearly expand the spectrum of endoparasites occurring in Emperor Penguins (A. forsteri). Within the present study, the nematode Capillaria sp. could be identified as one new host record for A. forsteri. The isolated cestoda eggs, Tetrabothrius spp., were already recorded for Emperor Penguins (Prudhoe 1969) as well as P. zederi (Baird 1853). Minor changes/differences within the parasite composition of Emperor Penguins could be detected within this study. Unfortunately, it was not possible to identify all isolated parasite eggs, at least, on genus level and therefore it is difficult to draw conclusions on the life cycles of all isolated parasites. Changes within the gastrointestinal parasite composition in marine environments are directly linked to the surrounding invertebrate and vertebrate communities. These communities are on their part depended from climate and/or anthropogenic impacts (MacKenzie et al. 1995; Dzikowski et al. 2003; Marcogliese 2003, 2005; Hudson et al. 2006; Hechinger et al. 2007). Therefore, marine parasites with complex multiple-host life cycles (Hechinger et al. 2007) are known to be sensitive bioindicators of aquatic ecosystem health (Overstreet 1997; Dzikowski et al. 2003). They require unaffected environmental conditions to get access to the full range of potential parasite intermediate hosts, whereas monoxenous parasite species (single-host) may persist in highly perturbed, extreme environments (Dzikowski et al. 2003; Hechinger et al. 2007). Under natural conditions, marine hosts can accumulate the highest possible parasite load (endoparasites). Within impacted habitats, this endoparasite load will decrease, whereas the ectoparasite load will increase (MacKenzie et al. 1995; Diamant et al. 1999; Dzikowski et al. 2003; Marcogliese 2003, 2005; Hudson et al. 2006; Hechinger et al. 2007).
The parasitological information presented in this study can be used for conservation purposes of pelagic bird species in the Antarctica. Over time, possible ecological long-term changes could be expected and we therefore call for further investigations on parasitosis occurring in the fragile ecological environment of the Antarctica.
References
Anderson RC (2002) Nematode parasites of vertebrates, their development and transmission. CABI Publishing, 2nd edn. 650 pp
Baird W (1853) Descriptions of some new species of entozoa from the collection of the British Museum. Proc Zool Soc London 21:18–25
Barbosa A, Palacios MJ (2009) Health of Antarctic birds: a review of their parasites, pathogens and diseases. Polar Biol 32:1095–1115
Blajin, Rukhadze (1929) On a method for staining flukes and tapeworm segments as whole microscopical preparations. J Trop Med Hyg 33:342–345
Bush O, Lafferty AD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Cherel Y, Kooyman GL (1998) Food of emperor penguins (Aptenodytes forsteri) in the Western Ross Sea, Antarctica. Mar Biol 130:335–344
Chilton NB, Huby-Chilton F, Gasser RB, Beveridge I (2006) The evolutionary origins of nematodes within the order Strongylida are related to predilection sites within hosts. Mol Phylogenet Evol 40:118–128
Cielecka D, Wojciechowska A, Zdzitowiecki K (1992) Cestodes from penguins on King George Island (South Shetlands, Antarctica). Acta Parasitol 37:65–72
Clark J, Kerry K (2000) Diseases and parasites of penguins. Penguin Conserv 13:5–24
Diamant A, Banet A, Paperna I, von Westernhagen H, Broeg K, Kruener G, Koerting W, Zander S (1999) The use of fish metabolic, pathological and parasitological indices in pollution monitoring II. The Red Sea and Mediterranean. Helgoland Mar Res 53:195–208
Diaz JI, Fusaro B, Longarzo L, Coria NR, Vidal V, Jerez S, Ortiz J, Barbosa A (2013) Gastrointestinal helminthes of Gentoo penguins (Pygoscelis papua) from Stranger Point, 25 de Mayo/King George Island, Antarctica. Parasitol Res 112:1877–1881
Dzikowski R, Paperna I, Diamant A (2003) Use of fish parasite species richness indices in analyzing anthropogenically impacted coastal marine ecosystems. Helgoland Mar Res 57:220–227
Fonteneau F, Geiger S, Marion L, Le Maho Y, Robin JP, Kinsella JM (2011) Gastrointestinal helminths of King penguins (Aptenodytes patagonicus) at Crozet Archipelago. Polar Biol 34:1249–1252
Fredes F, Madariaga C, Raffo E, Valencia J, Herrera M, Godoy C, Alcaíno H (2007) Gastrointestinal parasite fauna of gentoo penguins (Pygoscelis papua) from the Península Munita, Bahía Paraíso, Antarctica. Antarct Sci 19:93–94
Fretwell PT, Larue MA, Morin P, Kooyman GL, Wienecke B, Ratcliffe N, Fox AJ, Fleming AH, Porter C, Trathan PN (2012) An emperor penguin population estimate: the first global, synoptic survey of a species from space. PLoS One 7:e33751
Fuhrmann O (1921) Die Cestoden der Deutschen Südpolar Expedition 1901–1903. In: Drygalski E (ed) Deutsche Südpolar Expedition 1901–1903, 16 Zoologie 8:467–542
Georgiev BB, Vasileva GP, Chipev NH, Dimitrova ZM (1996) Cestodes of seabirds at Livingston Island, South Shetlands. Bulgarian Antarctic Res Life Sciences 111–127
Hechinger RF, Lafferty KD, Huspeni TC, Andrew JB, Armand MK (2007) Can parasites be indicators of free-living diversity? Relationships between species richness and the abundance of larval trematodes and of local benthos and fishes. Oecologia 151:82–92
Hoberg EP (1996) Faunal diversity among avian parasite assemblages: the interaction of history, ecology and biogeography. Bull Scand Soc Parasitol 6:65–89
Hoberg EP (2005) Economic, environmental and medical importance: Marine birds and their helminth parasites. In: Rohde K (ed) Marine parasitology. CSIRO, Sydney, pp 414–421
Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol 21:381–385
Johnston TH (1937) Cestoda. In: Johnston TH (ed) Australasian Antarctic expedition 1911–14, Sci Rep, Ser C Zoology and Botany 10:5–74
Jones HI (1988) Notes on parasites in penguins (Spheniscidae) and petrels (Procellariidae) in the Antarctic and sub-Antarctic. J Wildl Dis 24:166–167
Kahlil LF, Jones A, Bray RA (1994) Keys to the cestode parasites of vertebrates. CAB International, Wallingford
Katoh K, Standley EM (2013) MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol 30:772–780
Kooyman GL, Kooyman TG (1995) Diving behavior of emperor penguins nurturing chicks at Coulman Island, Antarctica. Condor 97:536–549
Kooyman GL, Cherel Y, Le Maho Y, Croxall JP, Thorson PH, Ridoux V, Kooyman CA (1992) Diving behavior and energetics during foraging cycles in king penguins. Ecol Monogr 62:143–463
Lauckner G (1985) Diseases of Aves (Marine Birds). In: Kinne O (ed) Diseases of marine animals, vol IV, Part 2, Reptilia, Aves. Mammalia. Biologische Anstalt Helgoland, Hamburg, pp 805–847
Leiper RT, Atkinson EL (1914) Helminthes of the British Antarctic Expedition, 1910–1913. Proc Zool Soc London 222–226
Leiper RT, Atkinson EL (1915) Parasitic worms with a note on free-living nematoda. Brit Antartic (‘Terra Nova’) Exped 1910. Nat Hist Rep Zool 11:19–60
Littlewood DTJ, Olson PD (2001) Small subunit rDNA and the Platyhelminthes: Signal, noise, conflict and compromise. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor & Francis, London, pp 262–278
MacKenzie K, Williams HH, Williams B, McVicar AH, Siddall R (1995) Parasites as indicators of water quality and the potential use of helminth transmission in marine pollution studies. Adv Parasit 35:85–144
Marcogliese DJ (2003) Food webs and biodiversity: are parasites the missing link? J Parasitol 89:106–113
Marcogliese DJ (2005) Parasites of the superorganism: Are they indicators of ecosystem health? Int J Parasitol 35:705–716
Mawson PM (1953) Parasitic nematoda collected by the Australian National Antarctic Research Expedition: Heard Island and Macquarie Island 1948–1951. Parasitology 43:291–297
Nadler SA, Carreno RA, Mejía-Madrid H, Ullberg J, Pagan C, Houston R, Hugot JP (2007) Molecular phylogeny of clade III nematodes reveals multiple origins of tissue parasitism. Parasitology 134:1421–1442
Overstreet RM (1997) Parasitological data as monitors of environmental health. Parassitologia 39:169–175
Palacios MJ, Valera F, Barbosa A (2012) Experimental assessment of the effects of gastrointestinal parasites on offspring quality in chinstrap penguins (Pygoscelis antarctica). Parasitology 139:819–824
Prudhoe S (1969) Cestodes from fish, birds and whales. B.A.N.Z. Antarctic Research Expedition 1929–1931. Rep Ser B 8:171–193
Schmidt GD (1986) CRC handbook of tapeworm identification. CRC Press, Boca Raton
Steig EJ, Schneider DP, Rutherford RD, Mann ME, Comisso JC, Shindell DT (2009) Warming of the Antarctis ice-sheet surface since the 1957 International Geophysical year. Nature 457:459–463
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Vidal V, Ortiz J, Diaz JI, Ruiz de Ybañez MR, Amat MT, Palacios MJ, Benzal J, Valera F, de la Cruz C, Motas M, Barbosa A (2012) Gastrointestinal parasites in Chinstrap Penguins from Deception Island, South Shetlands, Antarctica. Parasitol Res 111:723–727
Wienecke B, Robertson G, Kirkwood R, Lawton K (2007) Extreme dives by free-ranging emperor penguins. Polar Biol 30:133–142
Williams TD (1995) The penguins. Spheniscidae. (Birds Families of the World, No. 2). Oxford University Press, p 328
Acknowledgments
We are thankful to the Alfred Wegener Institute for Polar and Marine Research for logistical support and sample acquisition. Furthermore, we thank the German Federal Environment Agency for the sampling permission. We are grateful to Christine Henrich, Agnes Mohr, and Birgit Reinhardt for technical assistance. Special thanks to Boyko B. Georgiev (Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, Sofia, Bulgaria) and Krzysztof Tomczuk (Faculty of Veterinary Medicine, Lublin, Poland) for providing us with assistive literature.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kleinertz, S., Christmann, S., Silva, L.M.R. et al. Gastrointestinal parasite fauna of Emperor Penguins (Aptenodytes forsteri) at the Atka Bay, Antarctica. Parasitol Res 113, 4133–4139 (2014). https://doi.org/10.1007/s00436-014-4085-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4085-4