Abstract
The spread of organic farming and the development of resistance to anthelmintics by parasites, especially in small ruminants, have necessitated the search for alternative methods of nematode control. Biological control using nematophagous fungi is one option; however, few studies have been conducted with native strains. The present study was divided into two phases. In the first phase, we aimed to isolate, identify, and assess the in vitro predatory activity of nematophagous fungi that had been isolated on Trichostrongylidae third-instar larvae. In the second phase, the isolate with superior predatory activity in vitro was molecularly characterized, and its morphological plasticity was observed using scanning electron microscopy (SEM) on Haemonchus third-instar larvae. Of the 56 soil samples from different regions of Paraná State, Brazil, 57 fungal strains were recovered, of which four exhibited predatory activity. Two pure isolates were obtained: the CED and LIN strains. After demonstrating 96.35 % predatory activity for the CED strain, this strain was selected and characterized using molecular criteria by sequencing the rDNA internal transcribed spacer and was identified as Arthrobotrys conoides (GenBank ID: JN191309). Morphological patterns in this strain during the interaction between the fungus and the nematode were revealed by SEM, in which two extensions of the infection bulb that was used to pierce the nematode's cuticle were clearly visible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies conducted in three major sheep-raising countries (Australia, South Africa, and Uruguay) have classified nematodes as being responsible for most of the damage to this animal species (Waller 2006). In Brazil, this scenario is no different. Among the barriers to controlling these diseases, one of the most significant is the high level of the resistance of gastrointestinal helminths to different compounds, which has been reported since the 1990s (Echevarria et al. 1996; Thomaz-Soccol et al. 1996, 2004; Waller and Faedo 1996). To resolve this problem, new methods for controlling gastrointestinal helminths in ruminants have been studied, with an emphasis on biological control using nematophagous fungi (Waller 2006; Ketzis et al. 2006), which raises expectations for their use in integrated pest management (Waller et al. 2004; Larsen 2006; Maingi et al. 2006).
Research has been conducted on the fungus Duddingtonia flagrans (Fernandez et al. 1999; Faedo et al. 2000; Chandrawathani et al. 2004; Epe et al. 2009; Cruz et al. 2011); however, few studies have isolated and utilized native strains (Manueli et al. 1999; Sanyal 2000), which is crucial because fungi adapt easily to regional climatic conditions.
Therefore, isolating and characterizing fungal strains with predatory activity is essential for further research. One of the steps in selecting strains of interest involves characterizing the interaction between fungi and nematodes (Mendonza-de-Gives et al. 1999), and scanning electron microscopy (SEM) is a tool that shows this interaction between predator and prey in addition to the predatory mechanisms used (Nordbring-Hertz et al. 1989).
In this study, we aimed to select a native nematophagous fungal strain with the potential for use in biological control programs in ruminants, and to this end, this study was divided into two phases. In the first phase, we sought to isolate, identify, and assess the in vitro predatory activity of the isolated strains on Trichostrongylidae third-instar larvae. In the second phase, after selecting the best strain based on the results from the in vitro assay, we characterized that strain using molecular markers and characterized its morphological plasticity by SEM during predation on Haemonchus sp.
Materials and methods
Phase one
Soil samples
A total of 56 soil samples were collected between August and November 2006 from different regions of Paraná State, including the west-central, north-central, south-central, south-eastern, western and south-western regions, incorporating agricultural, livestock, integrated crop-livestock, and environmentally protected areas. The samples were collected at a depth of 0–5 cm, placed into labeled plastic bags, and stored at 4 °C until the fungi were isolated.
Free-living nematode larvae used in isolation
The free-living nematode larvae of Panagrellus sp. that were used to isolate the fungi were maintained in Petri dishes with a medium containing oatmeal that was mixed and moistened with distilled water (Heintz 1978). The nematodes were extracted from the culture medium by immersing small quantities of oats in a Baermann funnel and were collected in hemolysis tubes after 6–8 h of decanting. Next, the larvae were washed seven times with distilled water and recovered after centrifugation at 72.67×g for 10 min. The supernatant was discarded after each centrifugation. Subsequently, the larvae were immersed in a solution containing 0.05 % streptomycin and chloramphenicol for 6 h. Following this step, the washing process was repeated, and the total number of larvae was estimated by counting five 20-μL aliquots under a light microscope at 10× magnification (Araújo et al. 1994).
Isolation and identification of nematophagous fungi
Fungi were isolated according to the method described by Duddington (1955) and modified by Santos et al. (1991). The 2-g soil samples were distributed in the center of Petri dishes containing 2 % water–agar medium (WA) with 0.05 % chloramphenicol in duplicate. Then, 1 mL of resuspended Panagrellus sp. (approximately 1,000 larvae) was added, and the Petri dishes were incubated at 26 °C and monitored daily under a stereoscopic microscope for 10 days. After this initial period, the samples were monitored on a weekly basis for 30 days. Once the nematodes were trapped within the fungal structures, fragments of the culture were transferred to Petri dishes containing Potato Dextrose Agar (PDA) [Himédia®, India] medium until pure fungal cultures were obtained (Saumell et al. 1999).
Morphological characterization of fungal isolates
To morphologically characterize the isolates, the samples were cultured in a PDA medium, and the microculture slides were identified and classified based on a key prepared by Cooke and Godfrey (1964) and Philip (2002).
In vitro assay
The in vitro assay was performed in 8-cm Petri dishes containing 2 % WA medium. The experiment was completely randomized using the two isolated strains, CED and LIN, and a control with five replicates each.
Fragments of the culture of each isolate were removed from test tubes containing corn meal agar medium [CMA; Difco® (17 g L−1)], transferred to 8-cm Petri dishes containing 15 mL of PDA medium and incubated at 26 °C and 95 % humidity for 6 days. After this period, approximately 4-mm fragments of each culture were transferred to dishes containing 2 % WA medium and incubated at 26 °C in darkness for 6 days (Campos et al. 2008). On the sixth day, 350 μL of a solution containing approximately 1,000 infective Trichostrongylidae third instar larvae (L3) was added in the following proportions: 64 % Haemonchus sp., 22 % Trichostrongylus sp., and 14 % Strongyloides papillosus.
The control group consisted of Petri dishes containing 2 % WA without fungus to which 350 μL of resuspended 1,000 L3 was added. All of the dishes were incubated again at 26 °C in darkness for 6 days. On the sixth day of the interaction between the fungi and L3, the dishes were removed from the incubator, and the agar was removed from the Petri dishes using a metal spatula. This agar was subjected to the Baermann method for 6 h as described by Araújo et al. (1994), and the larvae were counted and identified as described by Van Wyk et al. (2004).
For larval counting, the contents of the tubes were standardized to 1 mL, and the estimated number of larvae in five 50-μL aliquots was extrapolated to the tube volumes. The predation rate was estimated using the following formula (Braga et al. 2010)
where
- XC:
-
average number of larvae recovered in the control group
- XT:
-
average number of larvae recovered in the treatment group
Phase two
In this phase, the strain that exhibited the greatest predatory activity in the in vitro assay was selected.
Molecular characterization by sequencing the ITS region of rDNA
-
a)
DNA extraction
After 4–5 days of culture on a PDA medium, approximately 1-cm2 fragments of the fungal culture were transferred to 2-mL Eppendorf tubes containing 300 μL of a cetyltrimethylammonium bromide (CTAB) buffer [2 % CTAB (w/v); 1.4 M NaCl; 100 mM Tris–HCl, pH 8.0; 20 mM EDTA; 0.2 % 2-mercaptoethanol (v/v)] and 80 mg of silica gel (2:1, w/w) (Merck, Germany). The cells were manually lysed with a sterile pestle for approximately 5 min. Next, 200 μL of CTAB buffer was added, and the mixture was homogenized and incubated at 65 °C for 10 min. Then, 500 μL of chloroform was added, and the solution was homogenized and centrifuged at 20,500×g for 5 min. The supernatant was transferred to new tubes, and two volumes of cold 96 % ethanol were added. The DNA was precipitated at −20 °C for 30 min and centrifuged again at 12,000×g for 5 min. Subsequently, the pellet was washed with cold 70 % ethanol. The material was dried at room temperature, resuspended in 97.5 μL of TE containing 2.5 μL of 0.02 U μL−1 RNase and incubated at 37 °C for 5 min prior to storage at −20 °C (Gerrits Van Den Ende and De Hoog 1999).
-
b)
Sequencing of the ITS segments of rDNA
The rDNA ITS was amplified using the primers V9G and LS266 (Gerrits Van Den Ende and De Hoog 1999) and sequenced with the primers ITS5 and ITS4 (White et al. 1990). The amplicons were purified using the GFX PCR DNA purification kit (GE Healthcare, U.K.). Sequencing was performed on an ABI 3130 automated sequencer. The sequences were edited and aligned using the Staden sequence analysis package v1.6.0 (Staden 1996). Sequence analysis was performed using the sequence alignment software BLASTn with the NCBI database (National Center for Biotechnology Information [http://www.ncbi.nlm.nih.gov/]. Phylogenetic analyses were performed by Mega 4.0.2 (Tamura et al. 2007) using the neighbor-joining method (Saitou and Nei 1987) and the Jukes-Cantor correct distance model (Jukes and Cantor 1969). The nucleotide sequence obtained in this study was deposited in GenBank under accession number JN191309.
Scanning electron microscopy
To observe the interactions between larvae and fungi, the materials were prepared for scanning electron microscopy (SEM) using the techniques described by Nordbring-Hertz (1983) and Campos et al. (2008). Dialysis membranes (Sigma®) were cut to a 6-cm diameter, placed inside Erlenmeyer flasks with distilled water, and autoclaved at 121 °C for 15 min. After autoclaving, six 6-cm diameter Petri dishes containing 2 % WA medium were covered with the membranes. Then, fragments of cultures of the CED strain were transferred to the dishes, and the edges were sealed with 2 % WA medium, thereby preventing the larvae from traveling down to the dialysis membrane (Campos et al. 2008). Subsequently, the plates were incubated at 26 °C in darkness for 4 days (Nordbring-Hertz 1983).
After a 4-day incubation, the Petri dishes were removed from the incubator, and 50 μL of a suspension of approximately 500 Haemonchus sp. third-instar larvae was added. Six control Petri dishes containing 2 % WA without fungi were used to assess the viability of the larvae and contamination with other fungi. During the first 8 h, the dishes were observed hourly under an optical microscope at 10× and 40× magnifications; the next observations were performed at 12, 18, 24, and 36 h post-inoculation. The backs of the Petri dishes were marked with a permanent marker, and the dialysis membranes within the marked areas were excised using a sterile scalpel.
The samples were fixed with 2.5 % glutaraldehyde in 0.05 M phosphate buffer at pH 7.4 for 24 h. Then, they were washed six times in the same buffer and dried in a series of increasing ethanol concentrations of 30 %, 50 %, 60 %, 70 %, 95 %, and 100 % for 10 min each, with the exception of the 100 % concentration, in which the samples were immersed three times for 5 min (Nordbring-Hertz 1983).
The samples were dried to critical point under CO2, coated in gold, and observed on a scanning electron microscope (JEOL-JSM 6360).
Isolation of infective Haemonchus sp. larvae for use in SEM
Sheep feces that were naturally infected with gastrointestinal nematodes predominantly composed of Haemonchus sp. (Cruz et al. 2011) were collected directly from the rectum to perform a coproparasitological assay. After obtaining Trichostrongylidae eggs, coprocultures were prepared with 20 g of feces mixed with sterile vermiculite at a 1:1 ratio (Roberts and O'Sullivan 1950) and were incubated at 28 °C. After 10 days, the infective larvae (L3) were recovered using the Baermann method, washed seven times with distilled water to prevent bacterial contamination, and centrifuged at 72.67×g for 10 min; we discarded the supernatant after each centrifugation. Subsequently, the nematodes were incubated overnight in a solution containing 0.05 % streptomycin sulfate and 0.05 % chloramphenicol. The washing process was repeated as previously described (Araújo et al. 1994). For larval counting, the contents of the tubes were standardized to 1 mL, and the estimated number of larvae in five 50-μL aliquots was extrapolated to the tube volumes.
These procedures are in agreement with the ethics committee and animal Universidade Estadual do Centro Oeste do Paraná—Brazil.
Results
Phase one—isolation, identification, and in vitro testing
Of the 56 soil samples collected from different regions of Paraná State, Brazil, 57 fungi were isolated. Of these, four isolates exhibited predatory activity against Panagrellus sp. in 2 % WA culture medium, although only two pure isolates were obtained; these were the CED and LIN strains.
Morphological identification using the microculturing technique revealed that the CED strain possessed conidia that ranged from 18.6 to 25.3 μm in length and were approximately 9.7 μm in width, and they were divided by a septum. The basal cell was at least two times smaller than the distal cell. The traps that formed were three-dimensional, and each ring was approximately 22.6 μm in diameter, which was suggestive of either Arthrobotrys conoides or Arthrobotrys oligospora.
The other strain, called LIN, identified as Arthrobotrys musiformis by the morphological characteristics of the conidia, which exhibited an elongated ellipsoidal to musiform shape, was bicellular with an inframedial septum and formed traps consisting of two-dimensional adhesive networks.
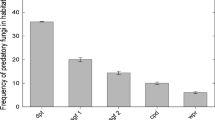
When assessing the in vitro predatory activity against Trichostrongylidae larvae in the Petri dishes, the CED strain exhibited 96.35 % efficacy, whereas the LIN strain exhibited 85.75 % efficacy (Table 1). Therefore, the CED strain was selected for the second phase of this study.
Phase two—molecular characterization and SEM
Because the classical methods of species identification did not lead to conclusive results, the ITS regions of rDNA were sequenced. The 601-bp sequence that was obtained was aligned and submitted to GenBank for comparison with other sequences. The nucleotide sequence obtained in this study was deposited in GenBank under accession number JN191309.
The molecular identification suggests that the CED isolate belongs to the species A. conoides (AY773455.1, with 97 % similarity). We used 15 strains of Arthrobotrys sp. deposited in GenBank with similarities ranging from 90 % to 100 % to the CED isolate for the phylogenetic analyses in this study (Table 2).
The tree based on the rDNA ITS sequencing was built by neighbor joining and by applying the Jukes-Cantor correct distance model implemented in Mega 4.0.2 with 1,000 rapid bootstrap inferences. According to the clustering, two major groups were obtained with high bootstrap values. The phylogenetic analysis showed that the CED isolate was closer to A. conoides than to A. oligospora (Fig. 1).
Using SEM, the fungal morphological plasticity was monitored at different predation stages, from trap formation to adhesion, capture and digestion and culminating in the death of the nematode, thereby confirming this isolate's predatory activity.
Trap formation was observed at 7 h after the addition of larvae. At 12 h, three-dimensional adhesive structures were observed (Fig. 2a) that trapped the third-instar larvae of Haemonchus sp. (Fig. 2b). Furthermore, two extensions in the infection bulb (Fig. 2c, d) were used to pierce the nematode's cuticle. In addition to the mechanical pressure exerted by the fungus (Fig. 2e) during penetration, it was also possible to observe the nematode's cuticle after penetration, suggesting the activity of hydrolytic enzymes that solubilize the cuticle (Figs. 2f and 3h).
SEM of the trap formation process and interaction between the nematophagous fungus, A. conoides, and L3 of Haemonchus sp. at the dialysis membrane surface. a Formation of three-dimensional networks 12 h after inoculation with nematode larvae. b Infective larvae of Haemonchus sp. captured after 12 h of interaction between the nematode and the fungus. c L3 captured and immobilized in three-dimensional networks. d Magnification of the area highlighted in panel c showing the extension of the infection bulb. e Hyphal pressure point on the nematode's cuticle; arrow indicates the pressure point. f Hyphal penetration site (white arrow) on the nematode's cuticle
SEM of the trap formation process and interaction between the nematophagous fungus, A. conoides, and L3 of Haemonchus sp. at the dialysis membrane surface. g Trapped larvae. h Magnification of the area highlighted in panel g showing the presence of a large number of NHB (black arrow, panel h). Note the site of penetration by the fungus and the hydrolysis of the nematode's cuticle (white arrow, panel h). i and j Larvae trapped in three-dimensional networks at various points on the body. l After 36 h, the turgor of the L3 is decreased. m Magnification of the area highlighted in panel l showing the presence of hyphae on the larva's body (white arrow), suggesting the digestion of the larva
Figure 3g, h shows a large quantity of bacilli at the bulb's penetration site.
Throughout the process, the three-dimensional networks increased in addition to the imprisonment of the nematode at various points on its body, which became completely immobilized and overtaken by hyphal growth (Fig. 3i, j). After 18 h, many larvae were dead, whereas others were “exhausted”, i.e., they moved slowly in the presence of light. Other newly captured worms struggled vigorously, thereby demonstrating the force involved in the processes of larval adhesion and trapping.
At 24 h post-inoculation, most of the trapped L3 exhibited no further movement in the presence of light, and at 36 h, some larvae appeared “shriveled”, i.e., empty, indicating absorption by the fungi (Fig. 3l, m).
Discussion
It is known that season, temperature, and the amount of organic matter in the soil are factors that may correlate with successful strain isolation. However, the greatest difficulty we encountered was in isolating pure strains; often, the trap structures were observed, but the faster growth of other fungi, especially Fusarium and Aspergillus, prevented successful isolation for transplanting. It is possible that the increased pH of the culture medium prevented the development of other microorganisms (Gardner et al. 2000), or it may be that other isolation methods lead to better results (Kelly et al. 2009) .
The two strains isolated in this study, CED and LIN, are from the south-central region of Paraná state, Brazil, a region that is characterized by a mesothermal, humid subtropical climate with no dry season, cool summers, moderate winters, and an average temperature of 14 °C in the coldest month and 20.3 °C in the hottest month from 1976 to 2009 (Iapar 2009). Annual rainfall varies from 1,400 to 2,000 mm, with the lowest rainfall levels in April, May, and August. The altitude of the region is approximately 1,100 m (Maak 1968).
The soil sample from which the CED strain was isolated was collected in late spring (November) from an area of crop-livestock integration where sheep are consigned in the winter. The soil sample from which the LIN strain was isolated was collected in October from a commercial sheep property, which is considered ideal for isolating agents used in the biological control of nematode parasites because there is a higher probability of these agents surviving the passage through the gastrointestinal tract of ruminants (Kelly et al. 2009).
The morphological identification of the LIN strain was conclusive for A. musiformis, but the morphological identification of the CED strain was only suggestive for A. conoides and A. oligospora. According to Cooke and Godfrey (1964), conidia of A. conoides are conical, 19–42 μm in length and 8–15 μm in width and constricted at the septum with a flattened base and larger distal cell. In contrast, the conidia of A. oligospora are ovoid and more globose, have a length of 22–32 μm and a width of 12–20 μm and have a spiked base. According to Philip (2002), A. oligospora conidia are 16–30 μm in length and 8–16 μm in width, with a distal cell that is twice as large, they are flattened at the septum and the traps are 20–59 μm in diameter. Oliveira et al. (2002) conducted morphological and isoenzyme characterization of several nematophagous fungal isolates isolated from various regions of Brazil and found that Brazilian strains of A. conoides had conidia of 31–44 μm in length and 11–14 μm in width, and A. oligospora had conidia of 17–28 μm length and 11–14 μm width, which does not agree with what was proposed by Cooke and Godfrey (1964) and Philip (2002). Therefore, from the references consulted and microscopic observation of the morphological characteristics, the CED strain was suggestive of A. conoides and A. oligospora. Perhaps, the difficulty of identifying this species is related the medium culture used (Singh et al. 2005).
Because this strain exhibited better in vitro predatory activity against third-instar larvae of Trichostrongylidae, it was selected for the second phase of the study. Molecular identification and phylogenetic analysis confirmed that the CED strain was A. conoides. This is the fourth strain that had an ITS region sequence that was deposited in GenBank under JN191309.
The Arthrobotrys genus is distributed worldwide and in Brazil, where it is researched as fungi with predatory activity against nematodes (Dalla Pria et al. 1991; Naves and Campos 1991; Nordbring-Hertz et al. 2002). The fungus can utilize two different carbon sources, one in organic matter as a saprophyte and the other from predatory activity against nematodes, which makes it adaptable to various habitats (Wachira et al. 2009), and thereby produces a smaller impact on the environment when used in biological control programs.
The plasticity of the A. conoides strain JN191309 was verified in predation against the third instar larvae of Haemonchus sp. by SEM. Despite several studies of the predatory mechanisms used by nematophagous fungi, in this study, it was possible to clearly document the extensions in the infection bulb that were used to pierce the nematode's cuticle. Nordbring-Hertz et al. (2006) studied the predatory mechanism using SEM; however, in the present study, the morphological plasticity of the fungus while penetrating the nematode was clearly visualized by SEM.
According to Araújo et al. (2001), the recognition of the larval cuticle by the fungus appears to be mediated by a lectin–carbohydrate interaction. Nordbring-Hertz et al. (2006) suggested that this process may be affected by proteases with high homology to subtilisin PII, which have demonstrated nematotoxic activity, in addition to penetration and digestion. According to Wang et al. (2006), the fungal penetration of the nematode's cuticle is a combination of physical force and hydrolytic enzymes.
The presence of nematophagous fungus helper bacteria (NHB) was previously observed by other authors who reported their association with D. flagrans and Haemonchus contortus larvae (Campos et al. 2008) and with A. conoides and A. musiformis (Graminha et al. 2001). Dupponois et al. (1998) hypothesized that these bacteria are involved in the processes of fungal predation, sporulation, and pathogenicity because they produce substances that act as molecular bridges between the fungus and the nematode.
According to Mankau (1980), the Arthrobotrys genus is as complex as the Penicillium genus; among 50 strains of A. conoides that were isolated, different patterns of sporulation, chlamydospore production, growth, and predation were reported. Therefore, to facilitate its industrial-scale production, a detailed study of the physiology, ecology, and biochemistry of this strain is necessary to assess its predatory activity in the field and to investigate which physical and chemical factors in the soil favor or inhibit its development.
The results obtained in this study show that A. conoides strain JN191309, which was isolated in Brazil, is a promising alternative for use in the control of infective larvae of trichostrongylides.
References
Araújo JV, Santos MA, Ferraz S, Maia AS (1994) Biological control “in vitro” of infective Haemonchus placei larvae by predacious fungi Arthrobotrys musiformis. Arq Bras Med Vet Zoot 46:197–204
Araújo JV, Campos AK, Paiva F, Vieira-Bressan MCR (2001) Efeito antagonista de fungos predadores do gênero Arthrobotrys sobre larvas infectantes de Oesophagosthomum radiatum, Cooperia punctata e Haemonchus placei. (Antagonistic effect of fungal predators from the Arthrobotrys genus on infective larvae of Oesophagosthomum radiatum, Cooperia punctata and Haemonchus placei.). Rev Bras Cienc Vet 8:81–84
Braga FR, Silva AR, Carvalho RO, Araújo JV, Guimarães PGC, Fujiwara RT, Frassy LN (2010) In vitro predatory activity of the fungi Duddingtonia flagrans, Monacrosporium thaumasium, Monacrosporium sinense, Arthrobotrys robusta on Ancylostoma celanicum, third-stage larvae. Vet Microbiol 146:183–186
Campos AK, Araújo JV, Guimarães MP (2008) Interactions between the nematophagous fungus Dunddingtonia flagrans and infective larvae of Haemonchus contortus (Nematoda: Trichostrongyloidea). J Helminth 82:337–341
Chandrawathani P, Jamnah O, Adnan M, Waller PJ, Larsen M, Gillespie AT (2004) Field studies on the biological control of nematode parasites of sheep in the tropics, using the microfungus Duddingtonia flagrans. Vet Parasitol 120:177–187
Cooke RC, Godfrey BES (1964) A key of nematode-destroying fungi Trans. Brit Mycol Soc 47:61–74
Cruz DG, Araújo FB, Molento MB, DaMatta RA, Santos CP (2011) Kinetics of capture and infection of infective larvae of trichostrongylides and free-living nematodes Panagrellus sp. by Duddingtonia flagrans. Parasitol Res 109:1085–1091
Dalla Pria M, Ferraz S, Muchovej JJ (1991) Isolamento e identificação de fungos nematófagos de amostras de solo de diversas regiões do Brasil. (Isolation and identification of nematophagous fungi in soil samples from different regions of Brazil). Nematol Bras 19:170–177
Duddington CL (1955) Notes on the technique of handling predacious fungi. Trans Brit Mycol Soc 38:97–103
Dupponois R, Amadou MB, Matteile T (1998) Effect of some rhizospher bacteria for the biocontrol of nematodes of the genus Meloidogyne with Arthrobotrys oligospora. Fund Appl Nematol 2:157–163
Echevarria FAM, Borba MFS, Pinheiro AC, Waller PJ, Hansen JW (1996) The prevalence of anthelmintic resistance in nematode parasites of sheep in Southern Latin America: Brazil. Vet Parasitol 62:199–206
Epe C, Holst C, Koopmann R, Schnieder T, Larsen M, Von Samson-Himmelstjerna G (2009) Experiences with Duddingtonia flagrans administration to parasitized small ruminants. Vet Parasitol 159:86–90
Faedo M, Larsen M, Thamsborg S (2000) Effect of different times of administration of the nematophagous fungus Duddingtonia flagrans on the transmission of ovine parasitic nematodes on pasture—a plot study. Vet Parasitol 94:55–65
Fernandez AS, Larsen M, Henningsen E, Nansen P, Gronvold J, Bjorn H, Wolstrup J (1999) Effect of Duddingtonia flagrans against Ostertagia ostertagi in cattle grazing at different stocking rates. Parasitol 119:105–111
Gardner K, Wiebe MG, Gillispie AT, Trinci APJ (2000) Production of chlamydospores of the nematode-trapping Duddingtonia flagrans in shake flask culture. Mycol Res 104:205–209
Gerrits Van Den Ende AHG, Hoog GS (1999) Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana. Stud Mycol 43:151–162
Graminha EBN, Maia AS, Santos JM, Candido RC, Silva GS, Costa AJ (2001) Avaliação in vitro da patogenicidade de fungos predadores de nematóides parasitos de animais domesticos (In vitro assessment of pathogenicity of predatory fungi on parasitic nematodes of domestic animals). Sem Cienc Agr 22:11–16
Heintz CE (1978) Assessing the predacity of nematode trapping fungi in vitro. Mycol 70:1086–1100
Iapar (2009) Sistema de monitoramento agroclimático do Paraná. (Agro-climatic monitoring system of Paraná). Availableat: http://www.iapar.br/arquivos/Image/monitoramento/Medias_Historicas/Guarapuava.ht. Accessed on 08/10/2010.
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic, New York, pp 21–132
Kelly P, Good B, Hanharan JP, Fitzpatrick R, Wall TDT (2009) Screening for presence of nematophagous fungi collect from Irish sheep pastures. Vet Parasitol 165(3–4):345–349
Ketzis JK, Vercruysse J, Stromberg BE, Larsen M, Athanasiadou S, Houdijk JGM (2006) Evaluation of efficacy expectations for novel and non-chemical helminth control strategies in ruminants. Vet Parasitol 139:321–335
Larsen M (2006) Biological control of nematodes parasites in sheep. J An Sci 84:133–139
Maak R (1968) Geografia física do Estado do Paraná. Curitiba: Banco de Desenvolvimento do Estado do Paraná (Physical Geography of Paraná State). Development Bank of Paraná State, Curitiba, p 350
Maingi N, Krecek RC, Biljon NV (2006) Control of gastrointestinal nematodes in goats on pastures in South Africa using nematophagous fungi Duddingtonia flagrans and selective anthelmintic treatments. Vet Parasitol 138:328–336
Mankau R (1980) Biological control of nematode pests by natural enemies. Ann Rev Phytopathol 18:415–440
Manueli PR, Waller PJ, Faedo M, Mahommed F (1999) Biological control of nematode parasites of livestock in Fiji: screening of fresh dung of small ruminants for the presence of nematophagous fungi. Vet Parasitol 81:39–45
Mendonza-De-Gives P, Davies KG, Clark SJ, Behnke JM (1999) Predatory behavior of trapping fungi against of mutants of Caenorhabditis elegans and different plant and animal parasitic nematodes. Parasitol 119:95–104
Naves RL, Campos VP (1991) Ocorrência de fungos predadores de nematóides no sul de Minas Gerais e estudo da capacidade predatória e crescimento in vitro de alguns de seus isolados. (Presence of predatory fungi on nematodes in southern Minas Gerais state and the predatory capacity and in vitro growth of their isolates.). Nematol Bras 15:153–162
Nordbring-Hertz B (1983) Dialysis membrane technique for studying microbial interaction. Appl Environ Microbiol 45:399–407
Nordbring-Hertz B, Friman E, Veenhuis M (1989) Hyphal fusion during initial stage of trap formation in Arthrobotrys oligospora. Antonie Leeuwenhoek 55:237–244
Nordbring-Hertz B, Jansson HB, Tundlid, A (2002) Nematophagous fungi. In: Encyclopedia of Life Sciences. Macmillian Publishing Group/www.els.net. pp. 681–690.
Nordbring-Hertz B, Jansson HB, Tunlid A (2006) Nematophagous fungi. In: Encyclopedia of Life Sciences. John Wiley & Sons, Ltd., Chichester. doi:10.1038/npg.els.0004293, http://www.els.net/ pp1-1.
Oliveira RDL, Ferraz S, Alfenas AC, Dias-Arieira CR (2002) Caracterização morfológica e isoenzimática de espécies de Arthrobotrys ocorrentes no Brasil. (Morphological and isoenzyme characterization of Arthrobotrys species in Brazil). Nematol Bras 26:181–197
Philip J (2002) Nematophagous fungi: Guide by Philip Jacobs, BRIC-Version online. Accessed on: June/2008. Available at www.biological-research.com/.
Roberts FHS, O'Sullivan PJ (1950) Methods for egg counts and larval cultures for strongyles infecting tract of cattle. Austr J Agr 1:6–7
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Santos MA, Ferraz S, Muchovej J (1991) Detection and ecology of nematophagous fungi from Brazilian soils. Nematol Bras 15:121–134
Sanyal PK (2000) Screening for Indian isolates of predacious fungi for use in biological control against nematode parasite of ruminants. Vet Res Commun 24:55–62
Saumell CA, Padilha T, Santos C, Roque MVC (1999) Nematophagous fungi in fresh feces off cattle in the Mata region in the Minas Gerais state. Brazil Vet Parasitol 82:217–220
Singh RK, Kumar N, Singh KP (2005) Morphological variations in Conidia of Arthrobotrys oligospora on different media. Mycobiol 33:118–120
Staden R (1996) The Staden sequence analysis package. Mol Biotech 5:233–241
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA 4: molecular evolutionary genetics analysis. Mol Biol Evol 24:1596–1599
Thomaz-Soccol V, Sotomaior C, Souza FP, Castro EA (1996) Occurrence of resistance to anthelmintics in sheep in Paraná State. Brazil Vet Rec 139:421–422
Thomaz-Soccol V, Souza FP, Sotomaior C, Castro EA, Milczewski V, Pessoa MC, Mocelin G (2004) Resistance of gastrointestinal nematodes of anthelmintics in sheep (Ovies aries). Braz Arch Biol Techn 47:41–47
Van Wyk JA, Cabaret J, Michael LM (2004) Morphological identification of nematode larvae of small ruminants and cattle simplified. Vet Parasitol 119:277–306
Wachira P, Mibey R, Okoth S, Kimenju J, Kiarie J (2009) Diversity of nematode destroying fungi in Taita Taveta. Kenya Fungal Ecol 2:60–65
Waller PJ (2006) From discovery to development: current industry perspectives for the development of novel methods of helminth control in livestock. Vet Parasitol 139:1–14
Waller PJ, Faedo M (1996) The prospects for biological control of the free-living stages of nematode parasites of livestock. Internat J Parasitol 26:915–925
Waller PJ, Schwann O, Ljungstro MBL, Rydzik A, Yeates GW (2004) Evaluation of biological control of sheep parasites using Duddingtonia flagrans under commercial farming conditions on the Island of Gotland. Vet Parasitol 126:299–315
Wang RB, Yang JK, Lin C, Zhang Y, Zhang KQ (2006) Purification and characterization of an extracellular serine protease from the nematode-trapping fungus Dactylella shizishanna. Lett Appl Microbiol 42:589–594
White TJ, Bruns T, Lee S, Taylor J (1990) PCR protocols: A guide to methods and applications. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Academic Press, New York, pp 315–322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falbo, M.K., Soccol, V.T., Sandini, I.E. et al. Isolation and characterization of the nematophagous fungus Arthrobotrys conoides . Parasitol Res 112, 177–185 (2013). https://doi.org/10.1007/s00436-012-3123-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-3123-3