Abstract
The kinetics of oocyst shedding and sporulation of two immunologically distinct strains of Eimeria maxima (GS and M6) were compared. Both strains had a prepatent period of approximately 120 h followed by peak oocyst shedding at 144-150 h post inoculation. Mean total oocyst output determined for each strain demonstrated that the fecundity of the M6 strain (12.8 × 103 ± 1.95) of E. maxima was roughly twice that of the GS strain (6.9 × 103 ± 3.33) when inoculated at the rate of 1,000 infective oocysts per bird. The process of oocyst sporulation was followed by repetitive sampling of sporulating oocysts at 26 °C with aeration over a 138 hour period. Sporulation was divided into five morphologically distinguishable stages whose abundance peaked at the following times during sporulation: unsporulated oocysts at 0 h; sporoblast anlagen at 18 h; sporoblasts without sporocyst walls at 22 h; and sporocysts without mature sporozoites at 38 h. The time to 50 % sporulation of E. maxima oocysts observed in the present study was approximately 53 h for both strains and all viable oocysts had completed sporulation by 60 h. In the present study, the prepatent periods, duration of oocyst shedding, and the relative kinetics of sporulation of the GS and M6 strains of E. maxima were found to be virtually identical despite the immunological distinctiveness of these two parasite strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coccidiosis is a diseases caused by obligate protozoan parasites belonging to the genus Eimeria. This disease infects the intestine of a host and reduces the growth efficiency. Coccidiosis is an important disease in poultry and other livestock (Lillehoj and Trout 1993; Shirley et al. 2007; Peek and Landman 2011). Eimeria species have complex life cycles that involve merogony and gametogony in the host, and sporogony in the environment. During sporogony, unsporulated oocysts develop into sporulated oocysts that have four sporocysts each containing two sporozoites (Hammond 1973). As many as seven morphologically distinct stages have been described during the sporulation of Eimeria stiedai (see Durr et al. 1971). For Eimeria maxima, six morphologically distinct stages were described by Tyzzer (1929) as follows: (a) unsporulated oocyst as discharged from intestine; (b) oocyst with band stretching through sporoplasm; (c) oocyst in stage just prior to division (anlagen); (d) oocyst with four sporoblasts; (e) oocyst with immature sporocysts (sporocyst wall present but Stieda body not fully formed); and (f) fully mature oocyst with four mature sporocysts, each with a fully formed Stieda body and containing two fully formed sporozoites possessing refractile bodies.
The Guelph strain of E. maxima (E. maxima GS) was initially isolated from a commercial poultry facility in Ontario, Canada, in 1973; a single-oocyst derived line of this isolate was then selected and has since been passaged at the University of Guelph. The M6 strain of E. maxima (E. maxima M6) was derived initially from a field isolate obtained from litter from a commercial broiler flock in Florida, USA in 1994 (see Martin et al. 1997). The M6 strain was selected by passage through birds that had been previously immunized against E. maxima GS. Single-oocyst derived lines were obtained, and one of these lines was designated E. maxima M6. The GS and M6 strains have been shown to be immunologically distinct and an infection with one strain does not provide cross-protective immunity against the other strain (Beattie et al. 2001). Both strains have been confirmed by a variety of molecular methods to belong to the same species, E. maxima (see Barta et al. 1998; Ogedengbe et al. 2011).
As part of a comprehensive examination of the differences between these two immunologically distinct strains of E. maxima, GS and M6, the pattern of oocyst shedding for each strain was determined and then the kinetics of sporulation using oocysts obtained at the peak of oocyst shedding was characterized in detail.
Materials and methods
Experimental animals and parasites
Day old Barred Rock chicks were obtained from Arkell Research Station (University of Guelph). They were raised in a coccidia-free facility until 28 days of age; experimental infections were initiated in 28-day-old chicks. Animals were provided a 12 h/12 h light–dark cycle and provided feed and water ad lib; experimental procedures were carried out in compliance with the Canadian Council on Animal Care guidelines and have been approved by the University of Guelph's Animal Care Committee.
E. maxima oocysts (strains GS and M6) were propagated separately in chickens raised coccidia-free in the University of Guelph's Central Animal Facility Animal Isolation Unit. Oocysts were purified by salt flotation using standard methods (Ryley et al. 1976). The partially purified oocysts were suspended in 2.5 % potassium dichromate (w/v, aqueous) before incubation on a rotary platform shaker at 26 °C to sporulate for a minimum of 72 h. All parasites for experimental infections were stored at 4 °C for less than 60 days prior to use. Immediately prior to inoculation into experimental birds, oocysts were collected by centrifugation (1,000 × g for 10 min) and then suspended in distilled water; sporulated oocysts were counted and diluted to the appropriate dosage with additional distilled water.
Determining the peak of oocyst shedding
Ten 28-day-old chickens were divided randomly into two groups. Each chicken was placed in a separate wire-floored cage. Chickens were inoculated by oral gavage with 1.0 × 103 oocysts per bird using a 1-cc tuberculin syringe (no attachment fitted). Five of the chickens were inoculated with the M6 strain and the other five chickens were inoculated with GS strain.
Fecal samples were collected continuously throughout days 6–10 post inoculation (PI). A stack of 16 foil sheets that covered the entire cage floor were placed beneath infected birds at the end of day 5 post inoculation. All feces from each bird were collected from 120 to 216 h post inoculation (HPI) in 6-h increments by removing the topmost sheet of foil each 6 h; the foil held all feces that had accumulated over the most recent 6-h period. Contaminants such as food and feathers were removed from the collected fecal samples that were then placed into individual sterile beakers.
The total number of oocysts shed during each 6-h interval was determined using a McMaster counting chamber. Oocysts were allowed to float clear of debris for 5 min before counting using a light microscope fitted with a long focal length 16× objective. The following equation was used to calculate the total number of oocysts in a fecal sample:
Total oocyst output per bird was calculated by summing the counts from all samples from a single bird. Parasite fecundity in each bird was then calculated as the total oocyst output divided by the inoculating dose. Mean total oocyst output and mean parasite fecundity of the GS and M6 strains of E. maxima were compared using Student's t test assuming unequal variances and were considered statistically different if p < 0.05.
Kinetics of oocyst sporulation
Ten 28-day-old chicks were divided into two groups and half of the chickens were infected orally with 2.0 × 104 oocysts of E. maxima GS and the remainder were infected orally with E. maxima M6. Fecal samples were collected for a 6-h period spanning the time of peak oocyst shedding determined previously for each strain.
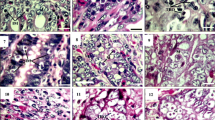
Feces containing oocysts from each strain (GS and M6) were blended with potassium dichromate (2.5 % w/v aqueous) in separate 500-ml Erlenmeyer flasks and then incubated on the same rotary shaker at 26 °C to sporulate. A sample was taken from each flask every 4 h (from 0 to 138 h) to document the morphological changes during sporulation. Five morphologically distinct stages of sporulation could be identified reliably in sporulating oocysts of E. maxima (see Fig. 1). Thus, immediately after collection at each time point, 100 viable oocysts from each fecal sample were examined using an Olympus Provis AX70 microscope (Olympus Canada, Richmond Hill, ON). Each oocyst was assigned to one of five developmental categories based on its morphological appearance: (1) unsporulated; (2) sporoblast anlagen; (3) sporoblasts without sporocyst walls; (4) sporocysts without mature sporozoites; and (5) sporocysts with mature sporozoites.
Oocysts passed through five morphologically identifiable stages during sporulation: a unsporulated oocyst with single spherical zygote within an oocyst wall (0 h sporulation); b sporoblast anlagen formation (18 h sporulation); c and d separate sporoblast but no evidence of sporocyst walls (20–24 h sporulation); e sporocyst walls formed but sporozoites not yet fully formed (38 h sporulation); f fully formed oocyst with mature sporozoites within sporocysts with fully formed Stieda bodies (>60 h sporulation). The kinetics of sporulation through these stages is summarized in Fig. 4
Results
Comparison of oocyst shedding of E. maxima GS and M6 strains
Both strains began shedding unsporulated oocysts during the 120-126 HPI sampling period that corresponds to a prepatent period of about 5 days. For the majority of infected birds, peak oocyst shedding was found to occur during the 144-150 HPI sampling period for both the M6 strain (for three of five birds, Fig. 2) and the GS strain (for four of five birds, Fig. 2). The mean oocyst output for each strain at each 6-h collection period is illustrated in Fig. 3; there was no difference between the two strains with respect to the time PI at which peak oocyst output occurred. Oocysts continued to be shed for the duration of the sampling period, albeit at low numbers from 198 HPI onward. Birds inoculated with 1,000 oocysts of E. maxima M6 shed more oocysts than birds infected with the GS strain in all sampling periods except one. Birds infected with the M6 strain produced significantly more oocysts than birds infected with 1,000 oocysts of E. maxima GS (p < 0.05) during the 144-150 HPI collection period. This sampling period corresponded to the peak of oocyst shedding (5.96 × 106 versus 2.82 × 106 oocysts/bird, respectively). Significantly, more (p < 0.05) oocysts were shed by the E. maxima M6-infected birds during the sampling periods of 192-198, 198-204, and 204-210 HPI. This increased productivity was also reflected in the significantly higher mean total number of oocysts shed by birds infected by E. maxima M6 (p < 0.05) over the course of a single infection (12.76 × 106 oocysts/bird, n = 5) compared with the mean total number of oocysts shed by birds infected by E. maxima GS (6.86 × 106 oocysts/bird, n = 5). The mean fecundity (number of progeny oocysts produced per oocyst inoculated) of the E. maxima M6 (12.8 × 103 ± 1.95) was significantly higher (p < 0.05) than the mean fecundity for E. maxima GS (6.9 × 103 ± 3.33).
Pattern of oocyst shedding by E. maxima GS and M6 in chicks inoculated with 1,000 oocysts/bird at time 0. Total oocyst output during each 6-h collection period for five birds (one line plotted per bird) for E. maxima GS (top) and E. maxima M6 (bottom). The peak of oocyst output was during the 140-150 HPI collection period for both strains
Mean total oocyst output of E. maxima GS and E. maxima M6 in chicks inoculated with 1,000 oocysts/bird at time 0 sampled continuously in 6-h collection periods from 120 to 216 HPI. Columns represent the mean total oocyst output (SD indicated above each bar) from five individual birds inoculated with the indicated parasite strain during each 6-h collection period. Mean total oocyst counts during a single collection period that differ statistically (p < 0.05) between E. maxima GS and E. maxima M6 are indicated by an asterisk
Determination of the kinetics of oocyst sporulation
The sporulation process was divided into five morphologically distinct stages that were then enumerated in a mixture of sporulating oocysts: (1) unsporulated (Fig. 1a); (2) sporoblast anlagen (Fig. 1b); (3) sporoblasts without sporocyst walls (Fig. 1c, d); (4) sporocysts with sporocyst walls but lacking mature sporozoites (Fig. 1e); and (5) sporocysts with mature sporozoites (Fig. 1f).
The kinetics of sporulation of E. maxima M6 at 26 °C is illustrated in Fig. 4. The first group is unsporulated oocysts (at 0 h) that developed to sporoblast anlagen stage at 22 h. The sporoblast anlagen then developed to sporoblast without sporocyst wall at 24 h. As the incubation of oocysts continues, the sporoblast without sporocyst developed to next stage which is sporocysts without mature sporozoites at 38–40 h. The final stage is the sporocysts with mature sporozoites at 60 h and above. The kinetics of GS strain is shown in Fig. 4. It is evident from the two figures that there is no difference in the pace of sporulation between the two strains of E. maxima (M6 and GS) through all of the five enumerated stages (unsporulated, sporoblast anlagen, sporoblasts without sporocyst walls, sporocysts without mature sporozoites, and sporocysts with mature sporozoites).
Kinetics of oocyst sporulation at 26 °C of E. maxima GS (top) and E. maxima M6 (bottom) was followed by determining the proportion of 100 viable oocysts at each of five stages of parasite development during sporulation (see Fig. 1). At the start of sporulation, only unsporulated oocysts were present (dotted lines) but these quickly developed to the sporoblast anlagen form that peaked in abundance at 22 h (dashed lines). Sporoblasts without sporocyst walls appeared shortly thereafter and peaked in abundance at 24 h (hollow dashed lines). Sporocysts possessing walls but without mature sporozoites first appeared at 28 h and peaked in abundance at 44 h (hollow lines). Fully mature oocysts containing sporocysts with mature sporozoites first appeared at 48 h and sporulation was complete by 60 h (solid lines). There were no differences observed between the sporulation kinetics of E. maxima GS and E. maxima M6
Discussion
The process of sporulation of eimeriid coccidia is a complex and necessary feature of all coccidian life cycles; nonetheless, the process of sporulation has been explored infrequently. As part of a comparison of two immunologically distinct strains of E. maxima (GS and M6), the timing of oocyst shedding and kinetics of sporulation were compared between the two strains under the same environmental conditions.
The prepatent period of Eimeria species has been used as one of the distinguishing features to help identify species in a host. The peak of oocyst shedding was at 144–150 HPI (between days 5 and 6 postinoculation) in both strains of E. maxima (GS and M6). In the original description of E. maxima by Tyzzer (1929), chicks receiving large numbers of infective oocysts began to shed small numbers of unsporulated oocysts at the end of 6 days PI (~156 h) and continued shedding oocysts until about 10 days PI; it was clear from Tyzzer's description that in birds given lower doses of oocysts, his strain of E. maxima had a prepatent period of about 7 days (168 h). In contrast, both the GS and M6 strains of E. maxima demonstrated a shorter prepatent period (120–126 h) than Tyzzer's E. maxima strain. The observations on the prepatent period of these E. maxima strains in the present study agree with the 121 h minimum prepatent period listed by Reid and Long (1979) and 123 h determined by Edgar (1955) with their strains of E. maxima. Despite the utility of the prepatent period for helping to identify Eimeria species of domestic fowl obtained from recent field isolates, the prepatent period can be significantly altered by selective passage in a process known as attenuation. Parent lines of E. maxima could have their typical prepatent period of about 120 h reduced to less than 107 h (McDonald et al. 1986). According to these authors, this abbreviated prepatent period is the result of a reduced number of merogonic cycles occurring before gametogony and oocyst formation. Both the M6 and GS strains of E. maxima have been passaged in a laboratory environment for some time with oocyst collection generally performed during day 6 PI; it is possible that such repetitive passaging has selected for a slightly shorter prepatent period than in strains cycling naturally among birds.
Although the timing of oocyst shedding did not differ between the strains, the fecundity of the strains differed significantly. Fecundity, or replicative potential, represents the number of oocysts produced per oocyst inoculated into a suitable and immunologically naïve host (Johnston et al. 2001). Fecundity is calculated by dividing the total number of oocysts shed by a bird by the number of oocysts in the inoculating dose. The number of oocysts produced from chickens that were infected with M6 strain (mean fecundity of 12.8 × 103 ± 1.95) was almost exactly double the number of oocysts that were produced from chickens infected with GS strain (mean fecundity of 6.9 × 103 ± 3.33) with the same dose of oocysts (1.0 × 103 oocysts/bird). Fecundity of various Eimeria species is related inversely to the infectious dose of oocysts provided to birds; larger inoculating doses may produce more oocysts per bird during the course of an infection but the fecundity per oocyst inoculated drops off rapidly (Johnston et al. 2001; Williams 2001). Assuming that the M6 and GS strains of E. maxima had the same infectivity, the observed difference in fecundity between the strains can be explained by a single additional mitotic division during the merogonic portion of the life cycle of the M6 strain compared with the life cycle of the GS strain. Allen et al. (2005) suggested that increased fecundity of an E. maxima strain was associated with increased cross-immunity to other E. maxima strains. Although not examined directly in this study, E. maxima M6 was derived from a strain of coccidia that was somewhat more cross-protective against the GS strain than vice versa (Beattie et al. 2001) which is in agreement with this suggestion by Allen et al. (2005). However, transcriptome analyses of sporulating oocysts of the E. maxima GS and E. maxima M6 strains have uncovered numerous differences in expressed GPI-anchored and other surface antigens (Al-Badri and Barta, unpublished observation). These differences could explain the previously demonstrated strain-specific immune responses against these E. maxima strains (Beattie et al. 2001). Whether or not increasing fecundity, on its own, is sufficient to elicit improved cross-protective immune responses to other strains of E. maxima remains to be determined.
Sporulation of oocysts occurs outside the host in the environment and takes about 2–3 days as the unsporulated oocysts develop into infective, sporulated oocysts. Unsporulated oocysts shed into the environment with feces contain a diploid zygote. Ultrastructural studies demonstrate that diploid oocysts undergo classical meiosis with the two divisions producing four haploid sporoblasts (Fergusson et al. 1978). Following meiosis and sporoblast formation, a single mitotic division within each sporoblast gives rise ultimately to four mature sporocysts each containing two sporozoites (Canning and Anwar 1968; Fergusson et al. 1978; Beesley and Latter 1982; Kinnaird et al. 2004).
The general sporulation process was described by Tyzzer (1929) in which he identified morphologically distinct stages of sporulation of oocysts of Eimeria spp. from gallinaceous birds. These were: (a) oocyst as discharged from intestine; (b) oocyst with band stretching through protoplasmic mass; (c) oocyst in stage just prior to division; (d) oocyst showing division into four cells, or sporoblasts; (e) oocyst, stage showing immature spores with membrane slightly developed; and (f) oocyst, spores fully developed, each with its two sporozoites fully formed. These developmental forms were similar to our recorded developmental stages except that we combined Tyzzer's (1929) stages “a” and “b” into a single “unsporulated” form characterized by a single, spherical sporoplasm within the oocyst. In all other regards, our observations and illustrations agree with Tyzzer's (1929) original description and illustrations of the sporulation of E. maxima.
Norton and Chard (1983) determined the oocyst sporulation times of seven Eimeria species from the domestic fowl. The time at which 50 % of the oocysts were sporulated for each Eimeria species was documented as follows: 11.4 h for Eimeria acervulina; 19.0 h for Eimeria mivati; 19.7 h for Eimeria necatrix; 21.2 h for Eimeria tenella; 24.8 h for Eimeria praecox; 38.1 h for E. maxima; and 38.3 h for Eimeria brunetti. The time for 50 % of E. maxima oocysts to sporulate in the present study (approximately 53 h) was considerably longer than the 38 h that Norton and Chard (1983) documented for their strain of E. maxima. However, sporulation time is sensitive to the temperature at which the oocysts are held during sporulation (Edgar 1954). Sporulation was completed at 26 °C in the present study whereas Norton and Chard (1983) sporulated their oocysts at 29 °C. In addition, sporulation is an aerobic process and consequently sporulation time is affected by available oxygen in the sporulation medium (Marquardt et al. 1960); this is usually addressed by aeration by forcing air through the sporulation medium (Canning and Anwar 1968) or through agitation such as the platform rotator used in the present study. In earlier studies, sporulation was achieved with oocysts stored in dichromate solutions within Petri dishes without agitation or aeration; this makes direct comparisons between trials difficult. In the environment of the poultry house, oocysts must obtain sufficient oxygen for the sporulation process as well. Waldenstedt et al (2001) determined that sporulation of E. maxima was reduced in the samples with the highest moisture content (62 %) and increased under the driest litter conditions studied (16 % moisture content). The authors concluded that drier litter conditions favored sporulation because of the increased availability of oxygen in the driest substrates compared to the limited amount of oxygen available in moister substrates. Finally, the subjective criteria used to designate oocysts as “fully sporulated” morphologically were not necessarily the same in both studies.
In the present study, the fecundity of E. maxima M6 was determined to be approximately twice that of E. maxima GS. However, the prepatent periods, duration of oocyst shedding, and the relative kinetics of sporulation of E. maxima M6 and GS were found to be virtually identical despite the immunological distinctiveness of these two parasite strains.
References
Allen PC, Jenkins MC, Miska KB (2005) Cross protection studies with Eimeria maxima strains. Parasitol Res 97:179–185
Barta JR, Coles BA, Schito ML, Fernando MA, Martin A, Danforth HD (1998) Analysis of infraspecific variation among five strains of Eimeria maxima from North America. Int J Parasitol 28:485–492
Beattie SA, Fernando MA, Barta JR (2001) A comparison of sporozoite transport after homologous and heterologous challenge in chickens immunized with the Guelph strain or the Florida strain of Eimeria maxima. Parasitol Res 87:116–121
Beesley JE, Latter VS (1982) The sporulation of Eimeria tenella as revealed by a novel preparative method. Z Parasitenkd 67:255–260
Canning EU, Anwar M (1968) Studies on meiotic division in coccidial and malarial parasites. J Protozool 15:290–298
Dürr U, Heunert HH, Milthaler B (1971) Sporogony of Eimeria stiedai (Protozoa, Sporozoa). Acta Vet Acad Sci Hung 21:421–432
Edgar SA (1954) Effect of temperature on the sporulation of oocysts of the protozoan, Eimeria tenella. T Am Microsc Soc 73:237–242
Edgar SA (1955) Sporulation of oocysts at specific temperatures and notes on the prepatent period of several species of avian coccidia. J Parasitol 41:214–216
Fergusson DJ, Birch-Anderson A, Hutchison WM, Siim JC (1978) Light and electron microscopy on the oocysts of Eimeria brunetti I. Development of the zygote and formation of the sporoblasts. Acta Pathol Microbiol Scand 86:1–11
Hammond DM (1973) Life cycles and development of coccidia. In: Hammond DM, Long PL (eds) The Coccidia: eimeria, Isospora, Toxoplasma and related genera. University Park Press, Baltimore, pp 45–79
Johnston WT, Shirley MW, Smith AL, Gravenor MB (2001) Modelling host cell availability and the crowding effect in Eimeria infections. Int J Parasitol 31:1070–1081
Kinnaird JH, Bumstead JM, Mann DJ, Ryan R, Shirley MW, Shiels BR, Tomley FM (2004) EtCRK2, a cyclin-dependent kinase gene expressed during the sexual and asexual phases of the Eimeria tenella life cycle. Int J Parasitol 34:683–692
Lillehoj HS, Trout JM (1993) Coccidia: a review of recent advances on immunity and vaccine development. Avian Pathol 22:3–31
Marquardt WC, Senger CM, Seghetti L (1960) The effect of physical and chemical agents on the oocysts of Eimeria zuernii (Protozoa, Coccidia). J Protozool 7:186–189
Martin AG, Danforth HD, Barta JR, Fernando MA (1997) Analysis of immunological cross-protection and sensitivities to anticoccidial drugs among five geographical and temporal strains of Eimeria maxima. Int J Parasitol 27:527–533
McDonald V, Shirley MW, Bellatti MA (1986) Eimeria maxima: characteristics of attenuated lines obtained by selection for precocious development in the chicken. Exp Parasitol 61:192–200
Norton CC, Chard MJ (1983) The oocyst sporulation time of Eimeria species from the fowl. Parasitology 86:193–198
Ogedengbe JD, Hanner RH, Barta JR (2011) DNA barcoding identifies Eimeria species and contributes to the phylogenetics of coccidian parasites (Eimeriorina, Apicomplexa, Alveolata). Int J Parasitol 41:843–850
Peek HW, Landman WJ (2011) Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet Q 31:143–161
Reid WM, Long PL (1979) A diagnostic chart for nine species of fowl coccidia. Univ Ga Coll Agric Res Rep 335:1–24
Ryley JF, Meade R, Hazelhurst J, Robinson TE (1976) Methods in coccidiosis research: separation of oocysts from faeces. Parasitology 73:311–326
Shirley MW, Smith AL, Blake DP (2007) Challenges in the successful control of the avian coccidia. Vaccine 25:5540–5547
Tyzzer EE (1929) Coccidiosis in gallinaceous birds. Am J Hyg 10:269–383
Waldenstedt L, Elwinger K, Lundén A, Thebo P, Uggla A (2001) Sporulation of Eimeria maxima oocysts in litter with different moisture contents. Poult Sci 80:1412–1415
Williams RB (2001) Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int J Parasitol 31:1056–1069
Acknowledgments
The authors thank The Iraqi Ministry of Higher Education and Scientific Research for supporting RAB. The Natural Sciences and Engineering Research Council of Canada and the Ontario Ministry of Agriculture, Food and Rural Affairs are acknowledged for funding this research through grants to JRB. Julie Cobean is thanked for her technical support during this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Badri, R., Barta, J.R. The kinetics of oocyst shedding and sporulation in two immunologically distinct strains of Eimeria maxima, GS and M6. Parasitol Res 111, 1947–1952 (2012). https://doi.org/10.1007/s00436-012-3041-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-3041-4