Abstract
The development of therapeutic alternatives to treat leishmaniasis has received considerable attention. The present study aimed to investigate the efficacy of the Agaricus blazei Murill water extract (AbM) to treat BALB/c mice infected with Leishmania amazonensis. First, a dose–titration curve was performed. The most well-defined concentration able to induce the most effective results in the infected animals, considering a daily administration of the product, was that of 100 mg kg−1 day−1. In this context, the AbM was administered orally, beginning on day 0 up to 20 days postinfection. Additional animals were treated with amphotericin B (AmpB, 5 mg kg−1 day−1) by peritoneal route for the same period of time, while the control group received distilled water. The animals were evaluated at 14 weeks post-infection, at which time the parasitological and immunological parameters were analyzed. Mice treated with the AbM presented a 60 % reduction in the inflammation of infected footpads as compared to untreated control-infected mice. Moreover, in the treated mice, as compared to the untreated controls, approximately 60 and 66 % reductions could be observed in the parasite burdens of the footpad and draining lymph nodes, respectively. In addition, no parasites could be detected in the spleen of treated mice at week 14 postinfection. These treated animals produced significantly higher levels of interferon gamma (IFN-γ) and nitric oxide (NO), higher levels of parasite-specific IgG2a isotype antibodies, and lower levels of interleukin (IL)-4, and IL-10 in the spleen and lymph node cell cultures than did the controls. Differences could be observed by comparing animals treated with AbM to those treated with AmpB, as indicated by a significant reduction in tissue parasitism, higher levels of IFN-γ and NO, and lower levels of IL-4 and IL-10, as well as by a decreased hepatic toxicity. In conclusion, the present study’s data show that the A. blazei Murill water extract presents a high potential for the treatment of leishmaniasis, although additional studies on mice, as well as on other mammal hosts, are warranted in an attempt to determine this extract’s true efficacy as compared to other known therapeutic products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is a group of vector-transmitted diseases that are endemic in 88 tropical and subtropical countries. Many geographic regions are endemic for multiple Leishmania species. This is the case in South America, where the disease is caused by at least eight different species of Leishmania (WHO 2000). American tegumentary leishmaniasis (ATL) includes a variety of forms that are commonly referred to by their clinical and pathologic features: cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL), and diffuse cutaneous leishmaniasis (DCL). Leishmania amazonensis is associated with all clinical forms of ATL (Afonso and Scott 1993; Grimaldi and Tesh 1993) as well as with visceral leishmaniasis (VL) in humans (Barral et al. 1991).
Historically, the treatment of leishmaniasis has been based on the use of pentavalent antimonials. The parenteral administration of these compounds is still the first choice therapy; however, increased parasite resistance and side effects, such as arthralgias, myalgias, pancreatitis, leukopenia, and cardiotoxicity, are important problems experienced by patients (Berman 2003; Croft and Coombs 2003; Oliveira et al. 2011). Liposomal amphotericin B (AmpB) is considered effective, though these formulations are very expensive (Mondal et al. 2010). Results from clinical trials of oral miltefosine treatment are encouraging; however, therapies using miltefosine are expensive, are linked to potential toxicity, and should not be given to pregnant or to childbearing age women (Kedzierski et al. 2009). Therefore, the development of alternative therapeutic strategies to treat leishmaniasis has become a high priority (Frézard and Demicheli 2009). Over the past few decades, major emphasis has been given to the identification of new formulations for both oral and topical treatments of the disease (Berman 2005; Croft and Coombs 2003). In this context, new treatment routes, as compared to parenteral administration, represent an interesting approach and offer several advantages, including improved safety, better compliance, and a lesser pain than that produced by needle-use administration (Aguiar et al. 2009; Croft and Olliaro 2011).
Agaricus blazei Murill is a commonly found mushroom in Brazil, and its use has been associated with folk medicine in the treatment of some diseases like leukemia, cancer, and arterial hypertension (Kim et al. 2005; Talcott et al. 2007; Kim et al. 2009). Compounds such as β-d-glucans, glycoproteins, saponins, tannins, polysaccharides, steroids, ergosterol, and fatty acids have been detected in this mushroom, which have been shown to activate the host’s immune response in different in vitro experiments (Sorimachi et al. 2001; Bernardshaw et al. 2005; Forland et al. 2010). Recently, the present study’s group showed that the A. blazei Murill water extract presents an effective in vitro antileishmanial activity against different Leishmania species, including L. amazonensis (Valadares et al. 2011). In addition to the elimination of parasites in the infected macrophages, the water extract showed no cytotoxic effects in either murine macrophages or human red blood cells.
The present study investigated the efficacy of the A. blazei Murill water extract (AbM) in the treatment of BALB/c mice that had been experimentally infected by L. amazonensis, a highly susceptible mouse model. The extract was administered orally in BALB/c mice. Its capability of treating the infected animals was compared to that obtained through parenteral treatment using AmpB, by examining parasitological and immunological parameters.
Materials and methods
Mice
The Committee on the Ethical Handling of Research Animals from Federal University of Minas Gerais (UFMG) approved all the animal handling methods and procedures (code 056/2010). Female BALB/c mice (8 weeks age) were obtained from the breeding facilities of the Department of Biochemistry and Immunology, Institute of Biological Sciences, UFMG, and were maintained under specific pathogen-free conditions.
Parasites and antigen preparation
Leishmania amazonensis (IFLA/BR/1967/PH-8) was grown at 24 °C in Schneider’s medium (Sigma, St. Louis, MO, USA), supplemented with 20 % heat-inactivated fetal bovine serum (FBS, Sigma), 20 mM l-glutamine, 200 U/mL penicillin, and 100 μg/mL streptomycin, at pH 7.4. The soluble L. amazonensis antigenic extract (SLA) was prepared from 1 × 1010 stationary-phase promastigotes, as previously described by Coelho et al. (2003). Parasites were kindly provided by Dr Maria Norma Melo (Department of Parasitology, ICB, UFMG).
Agaricus blazei Murill water extract
The AbM was prepared by macerating 50 g of fresh mushrooms in 50 mL of sterile Milli-Q water added to a protease inhibitor cocktail (Sigma, catalog P8340) using a Waring–Blendor homogenizer for 1 h at 4 °C. Next, the mixture was centrifuged at 10,000×g for 30 min at 4 °C (LC5C model, Sorval). The supernatant was then collected, sterilized by being passing through a 0.22-μm membrane, and stored at −80 °C until use.
Infection and treatment regimens
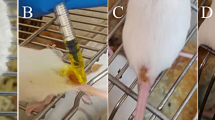
BALB/c mice (n = 12 per group) were infected in the right hind footpad with 2 × 105 stationary-phase promastigotes of L. amazonensis. Animals were divided into three groups, according to the regimens of treatment, each receiving the treatment once a day, beginning at day 0 of infection and followed up until 20 days postinfection: (1) treatment with the A. blazei Murill water extract (AbM, 100 mg kg−1 of body weight/day) by oral route, (2) treatment with amphotericin B deoxycholate (AmpB, 5 mg kg−1 day−1, Sigma, catalog A9528) by parenteral route, and (3) administration of distilled water by oral route. All animals were maintained in abstinence of food for 3 h pretreatment and 1 h posttreatment.
Cutaneous lesion development
The course of the disease was monitored weekly by measuring the footpad thickness with an electronic caliper (799-6/150 model, Starrett®, Brazil) and expressed as the increase in thickness of the infected hind footpad, as compared to the uninfected left footpad. Mice were evaluated for lesion development for 14 weeks.
Estimation of parasite load
The mice (n = 6 per group) were euthanized at 10 and 14 weeks postinfection, when the infected footpads, spleen, and draining lymph node (dLN) were collected for parasite quantitation by means of a limiting dilution assay, as described by Vieira et al. (1996). Briefly, the organs were weighed and homogenized, using a glass tissue grinder in sterile phosphate-buffered saline (PBS). Tissue debris was removed by centrifugation at 150×g, and cells were concentrated by centrifugation at 2,000×g. The pellet was resuspended in 1 mL of Schneider’s insect medium supplemented with 20 % FBS. Two hundred twenty microliters of the resuspension was plated onto 96-well flat-bottom microtiter plates (Nunc, Nunclon®, Roskilde, Denmark) and diluted in log-fold serial dilutions in supplemented Schneider’s culture medium with a 100–10−24 dilution. Each sample was plated in triplicate and read 7–10 days after the beginning of the culture at 24 °C. Pipette tips were discarded after each dilution to avoid carrying adhered parasites from one well to another. Results are expressed as the negative log of the titer (i.e., the dilution corresponding to the last positive well) adjusted per microgram of tissue.
Cytokine analysis
To measure IFN-γ, IL-4, and IL-10, the spleen and dLN cultures (5 × 106 cells, each one) were collected at week 10 postinfection and stimulated with SLA L. amazonensis (50 μg/mL−1), in duplicate, on 24-well, flat-bottomed plates (Nunc) for 48 h at 37 °C, 5 % CO2. The IFN-γ, IL-4, and IL-10 levels were determined in the culture supernatants by commercial kits (catalogue nos. 555138, 555232, and 555252 to IFN-γ, IL-4, and IL-10, respectively; BD OptEIA™ Pharmingen), according to manufacturer’s instructions.
Nitric oxide production
To view the macrophage activation via nitric oxide (NO) production, the spleen and dLN (5 × 106 cells, each) were stimulated with SLA L. amazonensis (50 μg/mL−1) for 48 h at 37 °C, 5 % CO2. Following incubation, 50 μL of culture supernatants was mixed with an equal volume of Griess reagent (Sigma). After an incubation of 30 min at room temperature, the nitrite concentration was calculated using a standard curve of known concentration.
Serum levels of alanine transaminase and aspartate transaminase
Serum samples from the mice were collected to determine alanine transaminase (ALT) and aspartate transaminase (AST) levels. The dosages were performed using commercial kits (catalogs K049 and K048 for ALT and AST, respectively, Bioclin Quibasa Ltda., Brazil), according to manufacturer’s instructions.
Analysis of humoral responses
SLA L. amazonensis-specific IgG, IgG1, and IgG2a antibodies were measured by ELISA (Coelho et al. 2003). Briefly, 96-well plates (Falcon model) were sensitized with SLA L. amazonensis (1 μg/well) for 18 h at 4 °C. Next, the plates were washed five times with PBS 1×/Tween 20 0.05 %, and the wells were blocked with a PBS 1×/bovine serum albumin 10 %/Tween 20 0.05 % solution, for 2 h at 37 °C. The plates were then washed seven more times under the same conditions, and serum samples (1:100 diluted) were added, in duplicate, for 1 h at 37 °C. After, the plates were washed seven times; specific peroxidase-labeled antibodies for mouse IgG, IgG1, and IgG2a isotypes (Sigma) were added separately (1:5,000 diluted), and incubation occurred for 1 h at 37 °C. Next, the plates were again washed seven times, at which time H2O2 and o-phenylenediamine were added for the development of reactions that occurred for 30 min in the dark and were stopped by the addition of 20 μL H2O2 2 N. Optical densities were read at 492 nm in an ELISA microplate spectrophotometer (LAB-660 model, LGC Biotechnology).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism software (version 5.0 for Windows). The differences among the diverse groups were evaluated using the one-way ANOVA analysis, followed by Bonferroni’s posttest for multiple comparisons. Differences were considered significant when P < 0.05. Data shown are representative of two different experiments, which presented similar results.
Results
In vivo efficacy of the Agaricus blazei Murill water extract to protect against L. amazonensis
In this study, an oral treatment employing an A. blazei Murill water extract (AbM) was applied in BALB/c mice experimentally infected with L. amazonensis. In previous in vivo experiments developed by the present study’s group (data not shown), a dose–titration curve was performed to determine the best concentration of AbM able to induce the most effective results in treating the infected mice. It could be observed that the concentration of 100 mg kg−1 day−1, administered once a day up to 20 days postinfection, produced the most reliable results concerning the animal treatments. Therefore, in the present study, AbM was administered orally at this concentration from day 0 up to 20 days postinfection. As a result, a significant reduction in the average lesion sizes could be observed in the animals treated with either AbM or AmpB after the ninth week of infection, as compared to the control mice (Fig. 1). Animals from both treatment groups displayed similar results in the swelling of the footpad, while controls showed a significant increase in their infected footpads. The protective response was sustained up to 14 weeks postinfection, when the mice treated with AbM or AmpB were still able to control the increase in lesion size.
Footpad swelling in mice infected with L. amazonensis and treated with A. blazei Murill or amphotericin B. BALB/c mice (n = 8 per group) were infected in the right hind footpad with 2 × 105 stationary-phase promastigotes of L. amazonensis. From day 0 of infection up to 20 days postinfection, animals were treated orally with the A. blazei Murill water extract (AbM, 100 mg kg−1 day−1), while another group received amphotericin B (AmpB, 5 mg kg−1 day−1) by parenteral route for the same period of time. An additional group received distilled water by oral route. All animals received treatments once a day. Lines indicate the mean ± standard deviation (SD) of the lesion size (in millimeters) of the infected animals. Lesion development in the infected animals was monitored weekly up to week 14 postinfection. Significant differences between the AbM and AmpB groups and the control group are indicated in the graph (“a” for AbM and control groups, and “b” for AmpB and control groups)
To evaluate the parasite burden in the animals, the infected footpads, spleen, and dLN were collected at weeks 10 and 14 postinfection. Both treatments, i.e. with AbM and AmpB, as compared to the control mice, resulted in significant reductions in the parasite load, in nearly all evaluated organs, not including the dLN at week 14 postinfection, when results between the control and AmpB groups displayed no significant difference between them (Fig. 2). However, animals treated with AbM, as compared to those treated with AmpB, displayed better results in reducing the parasite load, including a complete elimination of parasites in the spleen of the animals at week 10 postinfection, which was maintained until week 14 of the treatment follow-up.
Parasite burden in the infected animals treated with A. blazei Murill or amphotericin B. BALB/c mice (n = 8 per group) were infected in the right hind footpad with 2 × 105 stationary-phase promastigotes of L. amazonensis and were killed at weeks 10 and 14 postinfection. The parasite load was then determined in the infected footpads, spleen, and dLN. Bars indicate the mean ± standard deviation (SD) of the groups. Significant differences among the AbM, AmpB, and control groups are indicated in the graphs. N.D. Not detected
SLA-specific cellular and humoral responses elicited against L. amazonensis
To evaluate if the treatment altered immunological parameters associated with the resistance and/or susceptibility of BALB/c mice infected with L. amazonensis, the production of IFN-γ, IL-4, and IL-10 in the spleen and dLN culture supernatants was evaluated at week 10 postinfection. As shown in Fig. 3, spleen cells from mice treated with AbM produced significantly higher levels of SLA-specific IFN-γ than those secreted by spleen cells of animals treated with AmpB or control mice. Spleen cells were also incubated alone (nonstimulated, background control) and produced very low levels of all evaluated cytokines (data not shown).
Cytokine levels in the infected animals treated with A. blazei Murill or amphotericin B. Single-cell suspensions were obtained from the spleen and dLN of infected and treated mice, 10 weeks post-infection. Cells were stimulated with SLA (50 μg/mL−1) for 48 h at 37 °C in 5 % CO2. IFN-γ, IL-4, and IL-10 levels were measured in culture supernatants by ELISA. Bars indicate the mean ± standard deviation (SD) of the groups. Significant differences among the AbM, AmpB, and control groups are indicated in the graphs
The SLA-specific IL-4 and IL-10 production was also investigated. Spleen cells from AbM-treated mice produced lower levels of IL-4 in relation to the levels detected in the control mice (Fig. 3). In the control group, in both splenic and dLN cultures, as compared to the IFN-γ levels, a higher production of IL-4 could be observed, suggesting a predominance not only in the draining site of infection but also in the systemic level of a Th2 response. In addition, the production of IL-10 by dLN in the AbM group proved to be significantly lower when comapred to the AmpB and control groups (Fig. 3).
The humoral response elicited against L. amazonensis in the groups was investigated in an attempt to determine the global anti-Leishmania antibody response induced after the treatments of the infected animals (Fig. 4). It could be observed that the control mice, as compared to the groups treated with AbM or AmpB, produced higher levels of SLA-specific IgG antibodies, as well as higher levels of IgG1 in relation to the IgG2a isotype. By contrast, treatment with AbM affected the global specific L. amazonensis humoral response, given that these animals produced lower levels of SLA-specific IgG antibodies and higher levels of IgG2a, as compared to the IgG1 isotype, suggesting the predominance of a Th1 immune response in the group that received the treatment with the A. blazei water extract.
SLA-specific antibody responses in the infected animals treated with A. blazei Murill or amphotericin B. Serum samples were obtained from the infected and treated mice, 10 weeks post-infection. Serum samples were tested by ELISA to determine the presence of SLA-specific IgG, IgG1, and IgG2a antibodies. Bars indicate the mean ± standard deviation (SD) of the groups. Significant differences among the AbM, AmpB, and control groups are indicated in the graphs
To determine the influence of the treatments using AbM or AmpB as effector functions of infected macrophages in killing L. amazonensis killing, the nitrite production in the spleen and dLN of the animals was also assayed (Fig. 5). The nitrite production in spleen cell supernatants was significantly higher in the mice treated with AbM, as compared to those treated with AmpB or the control mice. Curiously, when dLN was used to determine the nitrite production, the levels obtained in the treated groups were lower than those obtained using splenic cell cultures.
Nitric oxide production. Spleen and dLN cells (5 × 106) were stimulated with SLA (50 μg/mL−1) for 48 h at 37 °C in 5 % CO2. Following incubation, 50 μL of culture supernatants was mixed with an equal volume of Griess reagent (Sigma). After an incubation of 30 min at room temperature, nitrite levels (μM) were calculated using a standard curve of known concentrations. Bars indicate the mean ± standard deviation (SD) of the groups. Significant differences among the AbM, AmpB, and control groups are indicated in the graphs
The hepatic toxicity of the treatments using AbM or AmpB was also investigated in the animals. Serum samples of the mice were collected and the ALT and AST levels were determined using commercial kits. The data presented in Fig. 6 show that mice treated with AmpB displayed higher levels of ALT and AST when compared to the levels obtained in the animals treated with AbM. Moreover, the levels of ALT and AST in animals treated with AbM were comparable to those detected in noninfected/nontreated mice, suggesting the absence of hepatic toxicity in the AbM-treated mice.
Serum levels of alanine transaminase and aspartate transaminase. Serum samples of naive, control (distilled water), AmpB, or AbM-treated and infected (AbM-I), or AbM-treated (AbM-NI) and non-infected mice were collected to determine the serum levels of alanine transaminase (ALT) and aspartate transaminase (AST) using commercial kits. White (ALT) and black (AST) bars indicate the mean ± standard deviation (SD) of the groups. The single asterisk indicates significant differences in relation to the naive mice; double asterisk indicates significant differences in relation to the control mice, and the number symbol indicates significant differences in relation to the AmpB group
Discussion
The development of new therapeutic strategies and products to treat leishmaniasis has received considerable attention in recent years, including the use of new formulations administered by oral and topical routes (Aguiar et al. 2009, 2010; Croft and Olliaro 2011). The purpose of this study was to analyze whether or not the A. blazei Murill water extract was effective against different Leishmania species in in vitro experiments (Valadares et al. 2011) when administered at day 0 of infection and continuing up to 20 days postinfection, as well as whether or not it was capable of treating BALB/c mice experimentally infected with L. amazonensis.
The experimental model used in this study is extremely susceptible and can be considered a highly stringent model for experimental chemotherapy, given that BALB/c mice infected with L. amazonensis commonly develop a progressive lesion at the inoculation site, followed by ulceration and loss of tissue, which occurs simultaneously with the appearance of visceralization and metastasis at distant sites (Cupolilo et al. 2003). This model is generally refractory to treatment, especially when treatment is administered to developed lesions that have been followed up to check for progressive infections. In this case, the treatments do not commonly lead to significant reductions in parasitism and/or cures, unless additional interventions, such as the association of drugs, are employed (Aguiar et al. 2010). Therefore, in the present study, the treatment with AbM began immediately after infection, and its effect was directly compared to AmpB treatment, a well-known model of treatment for leishmaniasis, before the parasite burden had reached increased levels in the infected animals. In this context, both AbM and AmpB treatments were able to promote a significant reduction in both lesion size and tissue parasitism in the treated and infected animals. However, at approximately 10 weeks postinfection, the AbM treatment, as compared to AmpB therapy, proved to be more effective, leading to a significant reduction in the parasite burden in different sites, including a complete elimination of the parasites in the spleen of the treated and infected animals, which could be observed at 10 and 14 weeks postinfection. This is consistent with previous reports indicating that the A. blazei Murill water extract presents an effective in vitro antileishmanial activity against different Leishmania species, including L. amazonensis (Valadares et al. 2011).
It was also possible to verify that the treatment with AbM was effective in inducing an elevated Th1 immune response in the infected animals. These mice presented a higher IFN-γ and NO production, associated with the predominance of L. amazonensis-specific IgG2a isotype antibodies. By contrast, control BALB/c mice that had been chronically infected with L. amazonensis displayed high levels of IL-4 or IL-10 by splenic and dLN cell cultures, and a higher production of IgG1 isotype antibodies. These results are in agreement with Padigel et al. (2003), who showed that low levels of IL-10 produced by splenic cultures of BALB/c mice infected with L. amazonensis can favor a higher production of IFN-γ and NO and that low levels of IL-4 are also important to a healing phenotype in the infected animals with L. amazonensis.
Macrophages represent the main infected host cells in leishmaniasis and play a relevant role in the immunological control of intracellular parasitism, through the production of oxygen derivative metabolites (Balaraman et al. 2004). Through the upregulation of NO production within the cells, macrophages can trigger intracellular killing mechanisms of the internalized parasites (Van Assche et al. 2011). Therefore, leishmaniasis treatment efficacy is dependent on synergic interactions between the anti-Leishmania effect of drugs and the immune responses of the animals, in turn leading to the production of IFN-γ and NO. The present study observed that mice treated with AbM produced high levels of NO. In this context, the increased ability of AbM-treated L. amazonensis infected macrophages to produce NO may be associated with the decreased parasite loads observed in these mice. It is also possible that the higher production of IFN-γ and lower levels of IL-4 and IL-10 observed in these animals have limited the multiplication of parasites, thus leading to a reduction in the tissue parasitism and, consequently, for the activation of parasite killing mechanisms of the macrophages. In this context, Sorimachi et al. (2001) reported that the A. blazei mushroom is able to induce a strong immune-stimulating effect based on the NO production in bone marrow-derived macrophages. Bernardshaw et al. (2006) also showed that the AbM is able to induce the of NO and proinflammatory cytokine (IL-1, IL-6, IL-8, and tumor necrosis factor alpha) production, but not of the IL-10, in human monocytes. Similarly, Tang et al. (2009) showed that this mushroom is also effective in inducing the production of high levels of IFN-γ and low levels of IL-4 and IL-10 in spleen cell supernatants of naive BALB/c mice. Altogether, these data indicate that treatment with AbM induced infected BALB/c mice toward a L. amazonensis-specific Th1 immune response that was maintained during infection, allowing these animals to prevent the development of a more severe disease, as was also observed in the control mice.
Prior reports have associated mushroom immune properties with polysaccharide fractions. Similarly, β-glucans fungi have also been reported to activate leukocytes and to increase the phagocytic activity of these cells, monocytes, and granulocytes; the production of reactive oxygen intermediates; as well as the production of inflammatory mediators, such as cytokines and chemokines (Sorimachi et al. 2001; Bernardshaw et al. 2006, 2007). A chemical characterization was performed to provide additional information concerning the chemical entities in the A. blazei Murill water extract that may well be responsible for the antileishmanial biological effects observed in the present study. The result illustrated the presence of tannins, saponins, glycoproteins, and polysaccharides within AbM.
Finally, no hepatic toxic effect could be observed in the AbM-treated mice. By contrast, in the animals treated with AmpB, significant elevations in the ALT and AST levels could be detected, indicating a possible hepatic damage caused by the use of AmpB in the animals, as previously described by Croft and Coombs (2003). In this context, the present study’s results are in agreement with findings from Hsu et al. (2008) and Wu et al. (2011), whose studies showed that in vivo treatment with A. blazei led to the reduction of ALT and AST levels in rats in models of chronic hepatitis B and hepatic fibrosis, respectively, as well as induced a healing phenotype in the treated and infected animals.
In conclusion, this study’s data show that the A. blazei Murill water extract presents a high potential to be employed in the treatment of leishmaniasis, although additional studies are necessary to determine its efficacy in association with well-known therapeutic products, as well as its efficacy in other mammal models.
References
Afonso LC, Scott P (1993) Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect Immun 61:2952–2959
Aguiar MG, Silva DL, Nunan FA, Nunan EA, Fernandes AP, Ferreira LAM (2009) Combined topical paromomycin and oral miltefosine treatment of mice experimentally infected with Leishmania (Leishmania) major leads to reduction in both lesion size and systemic parasite burdens. J Antimicrob Chemother 64:1234–1240
Aguiar MG, Pereira AMM, Fernandes AP, Ferreira LAM (2010) Reductions in skin and systemic parasite burdens as a combined effect of topical paromomycin and oral miltefosine treatment of mice experimentally infected with Leishmania (Leishmania) amazonensis. Antimicrob Agents Chemother 54:4699–4704
Balaraman S, Tewary P, Singh VK, Madhubala R (2004) Leishmania donovani induces interferon regulatory factor in murine macrophages: a host defense response. Biochem Biophys Res Commun 317:639–647
Barral A, Pedral-Sampaio D, Grimaldi G Jr, Momen H, McMahon-Pratt D, Ribeiro-de-Jesus A, Almeida R, Badaró R, Barral-Netto M, Carvalho EM, Johnson WD Jr (1991) Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. AmJTrop Med Hyg 44:536–546
Berman J (2003) Current treatment approaches to leishmaniasis. Curr Opin Infect Dis 16:397–401
Berman F (2005) Clinical status of agents being developed for leishmaniasis. Expert Opin Invest Drugs 14:1337–1346
Bernardshaw S, Hetland G, Ellertsen LK, Tryggestad AM, Johnson E (2005) An extract of the medicinal mushroom Agaricus blazei Murill differentially stimulates production of pro-inflammatory cytokines in human monocytes and human vein endothelial cells in vitro. Inflammation 29:147–153
Bernardshaw S, Hetland G, Ellertsen LK, Tryggestad AMA, Johnson E (2006) An extract of the medicinal mushroom Agaricus blazei Murill differentially stimulates production of pro-inflammatory cytokines in human monocytes and human vein endothelial cells in vitro. Inflammation 29:147–153
Bernardshaw S, Lyberg T, Hetland G, Johnson E (2007) Effect of an extract of the mushroom Agaricus blazei Murill on expression of adhesion molecules and production of reactive oxygen species in monocytes and granulocytes in human whole blood ex vivo. APMIS 115:719–725
Coelho EAF, Tavares CAP, Carvalho FAA, Chaves KF, Teixeira KN, Rodrigues RC, Matlashewski G, Gazzinelli RT, Fernandes AP (2003) Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect Immun 71:3988–3994
Croft SL, Coombs GH (2003) Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol 19:502–508
Croft SL, Olliaro P (2011) Leishmaniasis chemotherapy-challenges and opportunities. Clin Microbiol Infect 17:1478–1483
Cupolilo SM, Souza CS, Abreu-Silva AL, Calabrese KS, Goncalves da Costa SC (2003) Biological behavior of Leishmania (L.) amazonensis isolated from a human diffuse cutaneous leishmaniasis in inbred strains of mice. Histol Histopathol 18:1059–1065
Forland DT, Johnson E, Tryggestad AM, Lyberg T, Hetland G (2010) An extract based on the medicinal mushroom Agaricus blazei Murill stimulates monocyte-derived dendritic cells to cytokine and chemokine production in vitro. Cytokine 49:245–250
Frézard F, Demicheli C (2009) New delivery strategies for the old pentavalent antimonial drugs. Expert Opin Drug Deliv 7:1343–1358
Grimaldi G Jr, Tesh RB (1993) Leishmaniases of the New World: current concepts and implications for future research. Clin Microbiol Rev 6:230–250
Hsu CH, Hwang KC, Chiang YH, Chou P (2008) The mushroom Agaricus blazei Murill extract normalizes liver function in patients with chronic hepatitis B. J Altern Complement Med 14:299–301
Kedzierski L, Sakthianandeswaren A, Curtis JM, Andrews PC, Junk PC, Kedzierska K (2009) Leishmaniasis: current treatment and prospects for new drugs and vaccines. Curr Med Chem 16:599–614
Kim YW, Kim KH, Choi HJ, Lee DS (2005) Anti-diabetic activity of beta-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol Lett 27:483–487
Kim CF, Jiang JJ, Leung KN, Fung KP, Lau CB (2009) Inhibitory effects of Agaricus blazei Murill extracts on human myeloid leukemia cells. J Ethnopharmacol 122:320–326
Mondal S, Bhattacharya P, Rahaman M, Ali N, Goswami RP (2010) A curative immune profile one week after treatment of Indian kala-azar patients predicts success with a short-course liposomal amphotericin B therapy. PLoS Negl Trop Dis 27:e764
Oliveira LF, Schubach AO, Martins MM, Passos SL, Oliveira RV, Marzochi MC, Andrade CA (2011) Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop 118:87–96
Padigel UM, Alexander J, Farrell JP (2003) The role of interleukin-10 in susceptibility of BALB/c mice to infection with Leishmania mexicana and Leishmania amazonensis. J Immunol 171:3705–3710
Sorimachi K, Akimoto K, Ikehara Y, Inafuku K, Okubo A, Yamazaki S (2001) Secretion TNF-α, IL-12 and nitric oxide by macrophages activated with Agaricus blazei Murill fractions in vitro. Cell Struct Funct 26:103–108
Talcott JA, Clark JA, Lee IP (2007) Measuring perceived effects of drinking an extract of basidiomycetes Agaricus blazei Murill: a survey of Japanese consumers with cancer. BMC Complement Alternat Med 29:7–32
Tang NY, Yang JS, Lin JP, Hsia TC, Fan MJ, Lin JJ, Weng SW, Ma YS, Lu HF, Shen JJ, Lin JG, Chung JG (2009) Effects of Agaricus blazei Murill extract on immune responses in normal BALB/c mice. In Vivo 23:761–766
Valadares DG, Duarte MC, Oliveira JS, Chávez-Fumagalli MA, Martins VT, Costa LE, Leite JPV, Santoro MM, Régis WCB, Tavares CAP, Coelho EAF (2011) Leishmanicidal activity of the Agaricus blazei Murill in different Leishmania species. Parasitol Int 60:357–363
Van Assche T, Deschacht M, Da Luz RA, Maes L, Cos P (2011) Leishmania-macrophage interactions: insights into the redox biology. Free Radic Biol Med 51:337–351
Vieira LQ, Goldschmidt M, Nashleanas M, Pfeffer K, Mak T, Scott P (1996) Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J Immunol 157:827–835
World Health Organization (2000) The disease and its impact. http://who.int/emc/diseases/leish/index.html
Wu MF, Hsu YM, Tang MC, Chen HC, Chung JG, Lu HF, Lin JP, Tang NY, Yeh C, Yeh MY (2011) Agaricus blazei Murill extract abrogates CCl4-induced liver injury in rats. In Vivo 25:35–40
Acknowledgments
This work was supported by grants from Pró-Reitoria de Pesquisa from UFMG (Edital 08/2011), FAPEMIG (CBB-APQ-00496-11, CBB-APQ-02364-08, and CBB-APQ-00496-11), CNPq (APQ-472090/2011-9), Instituto Nacional de Ciência e Tecnologia em Nanobiofarmacêutica (INCT NANO-BIOFAR), and Instituto Nacional de Ciência e Tecnologia em Vacinas (INCT-V), CNPq. DGV, APF, and EAFC are grant recipient of CNPq, while MACF is a grant recipient of CAPES. This study was in part supported in Spain by grants from Ministerio de Ciencia e Innovación FIS/PI1100095.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valadares, D.G., Duarte, M.C., Ramírez, L. et al. Therapeutic efficacy induced by the oral administration of Agaricus blazei Murill against Leishmania amazonensis . Parasitol Res 111, 1807–1816 (2012). https://doi.org/10.1007/s00436-012-3028-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-3028-1