Abstract
Avian schistosome Trichobilharzia szidati is a member of the largest genus within the family Schistosomatidae (Trematoda). Population genetic structure of Trichobilharzia spp. schistosomes, causative agents of cercarial dermatitis in humans, has not been studied yet. The knowledge of the genetic structure of trichobilharzian populations is essential for understanding the host–parasite coevolutionary dynamics and epidemiology strategies. Here we examined genetic diversity in three geographically isolated local populations of T. szidati cercariae inhabiting Russia based on nuclear (randomly amplified polymorphic DNA, RAPD) and mt (cox1) markers. We analyzed T. szidati cercariae shed from seven naturally infected snails of Lymnaea stagnalis. Using three random primers, we demonstrated genetic variation among populations, thus posing genetic structure across geographic sites. Moreover, T. szidati cercariae have been genetically structured among hosts (infrapopulations). Molecular variance analysis was performed to test the significance of genetic differentiation within and between local populations. Of total parasitic diversity, 18.8% was partitioned between populations, whereas the higher contribution (48.9%) corresponds to the differences among individual cercariae within infrapopulations. In contrast to RAPD markers, a 1,125-bp fragment of cox1 mt gene failed to provide any significant within-species structure. The lack of geographic structuring was detected using unique haplotypes which were determined in the current work for Moscow and Western Siberian local populations as well as obtained previously for European isolates (Czech Republic and Germany). All T. szidati/Trichobilharzia ocellata haplotypes were found to be mixed across their geographical origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichobilharzia szidati Neuhaus, 1952, visceral avian schistosome, is a member of the largest genus in the family Schistosomatidae (Trematoda) covering over 40 species. The members of Trichobilharzia are the most frequent causative agents of cercarial dermatitis in humans. Cercarial dermatitis is caused by cercariae when they mistake humans for waterfowl and penetrate human skin. These parasites have a two-host life cycle, with water snails and birds as the intermediate and final hosts, respectively. Usually, an egg of visceral Trichobilharzia is deposited in the circulation (capillaries) of the final host and needs to pass through different tissues in order to reach the lumen of the intestine and, subsequently, leave the host with feces. The eggs mature within the host and the released eggs contain fully developed miracidia that are able to hatch. Once in the water, the miracidium leaves the egg and searches for the intermediate host. Upon finding a proper molluskan host, the miracidium penetrates and develops into a mother sporocyst. The latter stage produces daughter sporocysts which migrate to the hepatopancreas where further development with subsequent release of cercariae continues. Once in the water, the cercariae find a proper final host, penetrate the skin, transform to schistosomula, and start to migrate to the preferred location for egg laying. Depending on the parasite species, two different routes are employed to reach these places: migration via the blood circulation or passage through the nervous tissues (peripheral nerves and the central nervous system). The first route is typical for visceral species; the second one is used by the nasal schistosome Trichobilharzia regenti (Horák et al. 2002; Horák and Kolářová 2011).
The European “Trichobilharzia ocellata” isolates from Lymnaea stagnalis are regarded as a synonymous of T. szidati based on molecular analysis (Rudolfová et al. 2005). The intermediate host spectrum of T. szidati/T. ocellata is limited to L. stagnalis and Stagnicola palustris (Kock 2001). Usually L. stagnalis snails are infected by T. szidati only. However, Rudolfová et al. (2005) found a rare case of Trichobilharzia franki infecting L. stagnalis in Czech Republic. Prevalence of T. szidati in snails differs across localities and depends on the season ranging in Europe (Finland, Germany, France) from 0.17% to 0.82% (reviewed in Horák and Kolářová 2011). The first finding of T. szidati in natural definitive host—mallard (Anas platyrhynchos)—was reported recently by Rudolfová et al. (2007).
There are many studies focusing on taxonomical identification of Trichobilharzia spp. and phylogenetic relationships assessment between different representatives of the genus. These studies involved nuclear (28S and ITS rDNA) and mt (cox1) markers, and many different haplotypes were discovered using them within Trichobilharzia spp. across geographical scale (reviewed in Jouet et al. 2010). However, population genetic structure of Trichobilharzia spp. has not been studied yet. Examination of parasitic genetic structure across geographic areas as well as within and among hosts on a local scale may provide information about transmission. On the other hand, this knowledge is necessary for understanding the coevolutionary dynamics and revealing host–parasite interactions involving mechanisms of local adaptations of hosts to parasites.
Molecular markers have been widely used in population genetics. Among nuclear markers, randomly amplified polymorphic DNA (RAPD) are the most popular and have been widely used for parasite identification and differentiation (e.g., Williamson et al. 1997). RAPD were applied to estimate genetic variation of mammalian schistosomes within and among hosts or within and among populations (e.g., Dabo et al. 1997; Davies et al. 1999; Sire et al. 2001). The population genetic studies involving different mt markers were also reported for Schistosoma parasites (Morgan et al. 2005; Attwood et al. 2008; Stothard et al. 2009).
Previously, using two random primers, we observed cercariae RAPD variability between two Trichobilharzia species—T. szidati and T. franki (Semenova et al. 2005). In addition, we demonstrated the utility of RAPD to study population genetic structure of Bucephalus polymorphus (Trematoda: Bucephalidae) using cercariae from daughter sporocysts (Korsunenko et al. 2009). A comparative analysis based on the cox1 mt gene was suitable to reveal among- and within-species variation in cercariae of T. szidati, T. franki, T. regenti, T. sp. var. narochanica originating from Russia and Belarus (Chrisanfova et al. 2009; Lopatkin et al. 2010). In another study, three groups of haplotypes were determined for cercariae of avian schistosome Bilharziella polonica from Belarus based on cox1 polymorphism (Chrisanfova et al. 2011). Here we combined nuclear (RAPDs) and mt (cox1) markers to examine genetic diversity in seven cercariae infrapopulations of T. szidati from three geographically isolated local groups (populations) inhabiting Russia.
Material and methods
Collection sites
T. szidati cercariae were sampled after shedding from seven naturally infected snails of L. stagnalis posing seven infrapopulations (all the individuals of a parasite species inhabiting a host individual at one time, Bush et al. 1997). The snails were collected in 2002 and 2005 from three localities in Russia. Among them are those collected from two Moscow freshwater ponds—Altufyevo (Lsm1, Lsm7, Lsm20) and Olympiyskaya derevnya (Lsm2, Lsm3, Lsm4) as well as Kargat River (Lsk2), basin of Chany Lake, at Novosibirsk region in Western Siberia (Table 1). We estimated T. szidati prevalence in snails for the season of 2005. In total snail batch, two (2.5%) out of 80 snails from Altufyevo were infected by T. szidati cercariae, whereas three (3.4%) out of 88 snails from Olympiyskaya derevnya were found positive. Only one (0.38%) out of 263 snails from Kargat emitted ocellate furcocercariae.
DNA extraction, amplification, and sequencing
DNA was extracted from cercariae that have been shed by each host snail using a Chelex-100 suspension containing proteinase K (0.1 mg/ml) at 50°C for 30 min (a slight modification of Walsh et al. 1991). For RAPD analysis, DNA was extracted from individual cercariae; in total, 39 cercariae were used (Table 1). For ITS2-based species identification and cox1 amplification, DNA was extracted using ten cercariae from each infrapopulation.
Species identification was verified based on ITS2 rDNA; the amplification reactions were performed using the primers its3Trem and its4Trem by methods according to Dvořák et al. (2002).
Since DNA amount extracted from individual cercaria is limited; the most intensely stained and unambiguous RAPD profiles were obtained only when the DNA sample was used for no more than three primers. Among tested random primers, those which detected the most of polymorphic markers were selected for this study. RAPD-PCR was performed with each of the selected 10-mer primers, OPA09 (5′-ACCGGACACT-3′), OPA10 (5′-AACGGGCACC-3′), and P29 (5′-CCGGCCTTAC-3′), in 25 μl volume containing 10 ng of total DNA, 75 mM Tris–HCl (pH 8.8), 20 mM (NH4)2SO4, 0.01% Tween 20, 5 mM MgCl2, 0.25 mM of each dNTPs, 1 μM of primer, and 0.6–0.7 U of Taq DNA polymerase (Fermentas, Vilnius, Lithuania). The following amplification profile was used: 2 min of denaturation at 95°C, 35 cycles of 1 min at 94°C, 1 min at 38°C, 15 s at 45°C and 2 min at 72°C, followed by the final 10 min extension at 72°C. Reaction mixture containing no template DNA was used as negative control for PCR assays.
Amplification of 1,125 bp cox1 fragment was performed using the primer pair (Cox1_schist_5′ and Cox1_schist_3′) and conditions developed by Lockyer et al. (2003). The PCR products were processed using the automatic sequencing system ABI PRISM 3100-Avant (Applied Biosystems Inc, Foster City, CA, USA). The ITS2 sequences were deposited in GenBank under the accession numbers HM016851–HM016857 and GenBank accession numbers of cox1 sequences generated from this study: JF838197–JF838203 (Table 1).
Data analysis
Reproducible, clear, scorable RAPD fragments obtained from T. szidati cercariae using the three primers were used for statistical analysis. For each T. szidati cercariae, a 1/0 (the presence/absence of the amplified bands) binary data matrix was analyzed. Intra- and interpopulation genetic diversity was estimated based on percentage of polymorphic loci (P) and mean values of genotypic diversity (Shannon’s index, I). These parameters were calculated using population genetic analysis software POPGENE ver. 1.32 (Yeh et al. 1999). Furthermore, the unweighted pair-group means of analysis (UPGMA) dendrogram based on Nei and Li’s genetic distances (GD xy ) (Nei and Li 1979) was constructed using TREECON for Windows (Van de Peer and De Wachter 1994). Nodal support was assessed by bootstrap resampling (1,000 replicates). Analysis of molecular variance (AMOVA) was employed to quantify the amount of variation between cercariae within hosts as well as among populations and hosts using ARLEQUIN ver 3.11 (Excoffier et al. 2005).
Nucleotide sequences of 1,125 bp cox1 mtDNA fragment were aligned and used for phylogenetic tree construction. Maximum likelihood (ML) analysis was performed in MEGA version 4.0 software (Tamura et al. 2007) using haplotypes obtained in this study and those deposited in GenBank by Lockyer et al. (2003): T. ocellata (AY157189, Germany), T. szidati (AY157191, Czech Republic), and T. regenti (AY157190, Czech Republic); the latter was used as the outgroup. The Hasegawa, Kishino, and Yano (HKY) model and the parameters were chosen using the hierarchical likelihood ratio test implemented in Modeltest 3.7 (Posada and Crandall 2001). Internal node support was assessed by bootstrapping (500 replicates). Analysis of nucleotide (π) and haplotype (h) diversities were performed using MEGA version 4.0 software.
Results
Initially, species identification of cercariae was verified based on the amplification and sequence analysis of ITS2 rDNA (318 bp) through the comparison with nucleotide sequences available in GenBank. As a next step using RAPD-PCR, we analyzed a total of seven cercariae infrapopulations of T. szidati from three geographically isolated locations: Kargat River (Novosibirsk region) and two freshwater ponds in Altufyevo and Olympiyskaya derevnya (Moscow). A set of three random primers (OPA09, OPA10, P29) was suitable for observing genetic diversity within populations (within infrapopulations and between them) and among them. Banding patterns obtained with the P29 primer for three cercariae from each studied infrapopulation are shown as examples in Fig. 1. RAPD profiles differed among three cercariae populations: Kargat (lanes 1–3), Altufyevo (lanes 4–12), and Olympiyskaya derevnya (lanes 13–21). Moreover, each cercariae infrapopulation within species had a unique set of RAPD profiles: Lsk2 (lanes 1–3), Lsm7 (lanes 4–6), Lsm20 (lanes 7–9), Lsm1 (lanes 10–12), Lsm2 (lanes 13–15), Lsm3 (lanes 16–18), and Lsm4 (lanes 19–21).
RAPD profiles of T. szidati cercariae from three local populations (Kargat, Altufyevo, and Olympiyskaya derevnya) obtained with the P29 primer. Lane M molecular size marker (100-bp ladder), lanes 1–3 cercariae from Lsk2 infrapopulation, lanes 4–6 cercariae from Lsm7 infrapopulation, lanes 7–9 cercariae from Lsm20 infrapopulation, lanes 10–12 cercariae from Lsm1 infrapopulation, lanes 13–15 cercariae from Lsm2 infrapopulation, lanes 16–18 cercariae from Lsm3 infrapopulation, lanes 19–21 cercariae from Lsm4 infrapopulation
The three primers selected yielded a total of 57 RAPD fragments in the range of 250 to 2,200 bp. The number of reproducibly amplified fragments in each cercariae pattern varied from 13 to 20, depending on the primer used. The high level of within-snail diversity was observed. The percentage of polymorphic loci (P) within infrapopulations ranged from 17.9% (Lsm2) to 45.3% (Lsm1) (Table 2). The obtained data also demonstrate high within-population genetic diversity with population estimates being 58.4% and 70.0% for Altufyevo and Olympiyskaya derevnya respectively. The percentage of polymorphic bands within these two populations was 86.3%. Total genetic diversity within three studied populations reached 98.4%. The same tendency was found with Shannon’s index (I) estimates. The mean I values within infrapopulations were 0.19, 0.12, and 0.18 for populations from Altufyevo, Olympiyskaya derevnya, and Kargat, respectively. Estimated genetic diversity obtained with I within Altufyevo (0.31) and Olympiyskaya derevnya (0.30) populations were higher and of the same order of magnitude compared with both values obtained for within Moscow strain (0.33) or within three populations in total (0.37).
An UPGMA dendrogram was constructed based on RAPD bands obtained with primers used (Fig. 2). The dendrogram shows the division of all specimens into three distinct clusters. These clusters corresponded to populations examined in this study from geographically isolated water bodies—Altufyevo and Olympiyskaya derevnya ponds and Kargat River as well. The studied T. szidati populations were strongly bootstrap-supported (67, 83, 100). The topology of dendrogram also demonstrates the infrapopulation substructuring. Cercariae from Lsm1, Lsm7, and Lsm20 infrapopulations within Altufyevo population, Lsm2, Lsm3, Lsm4 within Olympiyskaya derevnya population, and Lsk2 infrapopulation formed distinct corresponding subclusters separated from each other. These subclusters had high bootstrap values (80–100). The differences between cercariae within Lsk2 infrapopulation varied in range 0.03–0.44 respectively, while the minimum and maximum values of genetic distance for the other infrapopulations were 0.21 and 0.47 (Lsm1), 0.22 and 0.50 (Lsm7), 0.18 and 0.27 (Lsm20), 0.14 and 0.23 (Lsm2), 0.21 and 0.34 (Lsm4), and 0.30 and 0.34 (Lsm3), respectively. The Lsk2 infrapopulation is the most distant relative to the others. The level of differences between populations from Altufyevo (Lsm1, Lsm7, Lsm20) and Olympiyskaya derevnya (Lsm2, Lsm3, Lsm4) was 0.74.
Genetic data were also examined using an AMOVA. Three sources of variation (differences within infrapopulations; among infrapopulations within Altufyevo and Olympiyskaya derevnya populations; between Altufyevo, Olympiyskaya derevnya, and Kargat populations) made a statistically significant contribution to the total variance observed (Table 3). The highest contribution corresponds to the differences among individual cercariae within infrapopulations (48.9%, p < 0.0001); 32.3% (p < 0.0001) of variability accounted by differences among infrapopulations within Altufyevo and Olympiyskaya derevnya populations. Of total genetic diversity, 18.8% (p < 0.01) were partitioned between populations.
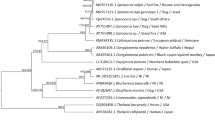
We also used the 1,125-bp fragment of cox1 mt gene to examine intraspecific genetic variance of T. szidati cercariae. The high haplotype diversity was observed (h = 100%), i.e., for each cercariae infrapopulation, a unique haplotype was detected, while the nucleotide diversity in the total sample was low (π = 0.9%). ML dendrogram was constructed based on the cox1 nucleotide sequences obtained for seven studied cercariae infrapopulations and two 1,125-bp cox1 sequences available in GenBank (GenBank accession numbers AY157189, AY157191) (Fig. 3). The topology of tree did not reveal subdivision into haplotype lineages. All T. szidati/T. ocellata haplotypes were found to be mixed across their geographical origin. The Moscow haplotypes from Altufyevo (Lsm1, Lsm7, Lsm20) and Olympiyskaya derevnya (Lsm2, Lsm3, Lsm4) were not segregated according to their geographical origin. The Western Siberian haplotype (Lsk2) was placed among the Moscow (Lsm20), Czech Republic (GenBank accession number AY157191), and Germany (GenBank accession number AY157189) haplotypes. The European haplotypes also did not form distinct lineage. Thus, compared with results coming from RAPD analysis, cox1 domain failed to differentiate neither the Russian populations nor the Russian and European sets of haplotypes between each other.
Phylogenetic tree based on the 1,125-bp cox1 of T. szidati/T. ocellata cercariae constructed using maximum likelihood method (HKY model of substitution). The scale shows the number of nucleotide substitutions per site between DNA sequences. Sequence of T. regenti (GenBank accession number AY157190) was used as outgroup
Discussion
In the present study, combined analysis based on RAPD markers and cox1 mt gene have been applied to investigate the intraspecific genetic structure of T. szidati cercariae sampled from three geographically isolated populations. Cox1 failed to provide any significant within-species structure. The lack of geographic structuring was detected using unique haplotypes which were determined in the current work for Moscow and Western Siberian local populations as well as obtained previously by Lockyer et al. (2003) for European isolates of T. szidati/T. ocellata (Czech Republic and Germany). Likewise, the high haplotype diversity (h = 85.6%) and no geographical substructuring was determined based on the 1,125-bp-long cox1 fragment for another avian schistosome (B. polonica) originating from Belarusian lakes (Chrisanfova et al. 2011). In total, 11 haplotypes forming three lineages were detected with one of the lineages being intermediate between two others. However, these haplotype lineages did not demonstrate any geographical specificity. The high values of cox1 haplotype diversity (h = 80–100%) were previously determined for T. szidati, T. franki, and T. sp. var. narochanica (proposed new species) cercariae originating from Belarus (Chrisanfova et al. 2009) as well as for T. szidati, T. franki, and T. regenti cercariae infrapopulations from Russia (Moscow and Moscow region) (Lopatkin et al. 2010). The unique cox1 haplotypes obtained through these studies appeared to indicate the high T. szidati haplotype diversity across the species geography in general. Additionally, for cox1 haplotypes obtained herein, genealogical network was constructed (data not shown) which demonstrated a “star-tree” structure. A starburst phylogeographic pattern is an expected signature for an abundant species that has expanded its range rather recently from small numbers of founders (Avise 2000). In contrast to the avian schistosomes, geography-based differentiation was reported for mammalian schistosomes. Attwood et al. (2008) found that three of four studied Schistosoma mekongi populations from Cambodia and Southern Laos were distinguishable at cox1 and 12S mt loci. Furthermore, the unique haplotype patterns representing two evolutionary lineages with strong spatial specificity were detected for Ugandan Schistosoma mansoni from Lake Albert and Lake Victoria using DNA barcoding, sequence analysis of two partially overlapping cox1 regions—ASMIT (396 bp) and MORGAN (617 bp) (Stothard et al. 2009). Morgan et al. (2005) used partial fragments of cox1, rrnL-rrnS, cytb-nad4L-nad4, and nad1 covering more than 2,500 bp of mtDNA to describe within-species diversity of S. mansoni originating from Africa and the New World. Considerable geographical structure, at global and regional scales, was found based on 85 unique mt haplotypes separated into five distinct lineages.
Using RAPD-PCR, we demonstrate cercariae genetic variation among populations, thus posing genetic structure across geographic sites. Cercariae from Altufyevo were found to be more similar to those from Olympiyskaya derevnya than compared with Kargat samples. The geographical distance between two Moscow populations is about 25 km. Kargat population located in Western Siberia is much more geographically isolated from two others (about 2,600 km). Moreover, using random primers, it was shown that T. szidati cercariae have been genetically structured not only between local populations but among hosts within geographic sites as well. Clustering of cercariae into seven groups corresponding to their snail hosts as visualized on UPGMA dendrogram is seemed to indicate the occurrence of parasite–host interactions. In our previous work, substructuring at the both infrapopulation and population levels was detected based on RAPD markers for B. polymorphus cercariae from daughter sporocysts (Korsunenko et al. 2009). In general, the parasite substructuring among hosts as well as their high heterogeneity within hosts both detected by RAPDs may reflect some molecular adaptations occurring between intermediate host and parasite. Lively (1989) showed strong local adaptation by parasite in reciprocal cross-infection experiment using Potamopyrgus antipodarum as the host and Microphallus sp. (Trematoda: Microphallidae) as the parasite. Snails from several New Zealand lake populations were experimentally exposed to parasites from the same lakes. The results showed that snails are more susceptible to parasites from the same locality, i.e., the parasites are adapted to infecting snails from their local host population.
AMOVA analysis confirmed that the percentage of RAPD variance attributable to differences between cercariae within infrapopulations (snails) is significant and higher than the percentage of variation due to differences among infrapopulations within Altufyevo and Olympiyskaya derevnya populations as well as differences between the three populations. Unfortunately, it was not possible to estimate the contribution of differences among infrapopulations within population from Kargat River since only one infrapopulation was available for the study. Previously, the prevalence of within-hosts/within-populations component rather than among-hosts/among-populations component in total parasitic diversity and multiple snail infections were determined based on RAPD markers in mammalian schistosome Schistosoma haematobium (Dabo et al. 1997; Davies et al. 1999).
In our study, the genetic heterogeneity of T. szidati cercariae within infrapopulations was demonstrated based on UPGMA analysis and AMOVA results. Snail infections with multiple parasite genotypes could be responsible for parasite genetic diversity within hosts. In natural populations, over 50% of snails which are infected by S. mansoni and S. haematobium contained multiple genotypes, with the maximum genotypically unique miracidia per snail being nine (reviewed in Yin et al. 2008). On the other hands, cercariae heterogeneity could be generated during the clonal reproduction in intermediate host. The variable occurrence of W1 and W2 elements originating from genomic instability of these repetitive elements was detected within and among daughter sporocyst generations of S. mansoni cultured in vitro (Grevelding 1999; Bayne and Grevelding 2003). Because an unexpected heterogeneity was found even among clonal cercariae originating from monomiracidial snail infections, the authors suggested that mitotic recombination events could occur during the asexual life stage of schistosomes. Previously, based on multi-locus microsatellite analysis, near-identical multi-locus genotypes (niMLGs), i.e., genotypes that differed at one or a very few loci from a frequently observed MLGs (Yin et al. 2008), were identified for S. mansoni (Gower et al. 2007) and Schistosoma japonicum (Yin et al. 2008). Yin et al. (2008) revealed the presence of at least nine niMLG among worms arising from monomiracidial snail infection and regarded somatic mutations as the most likely explanation of this phenomenon. Recently, based on RAPD markers, we revealed significant genetic heterogeneity between cercariae within parthenites (sporocysts) for six trematode species from Schistosomatidae, Strigeidae, Gorgoderidae, Bucephalidae, Diplostomatidae, and Plagiorchiidae families (Semyenova et al. 2007). RAPD variability within daughter sporocysts was also determined in population genetic studies of some trematodes: Microphallus pygmaeus and Microphallus pseudopygmaeus (Khalturin et al. 2000) and B. polymorphus (Korsunenko et al. 2009). Using cox1 fragment, we did not reveal more than one haplotype in each of the cercariae infrapopulations that appeared to indicate the monomiracidial infection of the studied snails. However, such finding may be the consequence of snail infection caused by several closely related miracidia with cox1 identical haplotypes. The second explanation for T. szidati cercariae heterogeneity was detected in this study—somatic mutation events during parthenogenesis rounds within snails. Furthermore, we also suggest that both explanations may be the case.
Migration of definitive host is known to be the major factor which forms the population structure of parasite (Jarne and Theron 2001). In the case of our study migration of T. szidati, definitive host (anatid birds) in large distances and in microspatial scale (between localities within Moscow region) appeared to promote gene flow between parasitic populations influencing the distribution of parasite genetic variability. Such factors as genetic drift and mutation, founder effect, etc. may also be responsible for the distribution of genetic diversity within and among T. szidati populations. Population genetic structure of T. szidati intermediate and definitive hosts should be studied to analyze the forces acting upon the parasite population structure. The overall lack of genetic structure in mallards (the main definitive host of trichobilharzians) from Western Russia and North Asia was demonstrated using 5′-end of the mtDNA control region (Kulikova et al. 2005). It was suggested that mallards exhibit considerable population connectedness and relatively high gene flow. To assess the T. szidati population genetic structure in details, additional studies that involve the use of different nuclear and mt markers and more cercariae isolates covered the larger geographical area should be carried out. The genetic structure inferring of other Trichobilharzia species is of the most interest as well.
References
Attwood SW, Fatih FA, Upatham ES (2008) DNA-sequence variation among Schistosoma mekongi populations and related taxa; phylogeography and the current distribution of Asian schistosomiasis. PLoS Negl Trop Dis 2(3):e200. doi:10.1371/journal.pntd.0000200
Avise J (2000) Phylogeography: the history and formation of species. Harvard University Press, Cambridge
Bayne CJ, Grevelding CG (2003) Cloning of Schistosoma mansoni sporocysts in vitro and detection of genetic heterogeneity among individuals within clones. J Parasitol 89(5):1056–1060. doi:10.1645/GE-3186RN
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83(4):575–583
Chrisanfova GG, Lopatkin AA, Mishchenkov VA, Kheidorova EE, Dorozhenkova TE, Zhukova TV, Ryskov AP, Semyenova SK (2009) Genetic variability of bird schistosomes (class Trematoda, family Schistosomatidae) of Naroch Lake: identification of a new species in the Trichobilharzia ocellata group. Dokl Biochem Biophys 428:268–272
Chrisanfova G, Lopatkin A, Shestak A, Mishchenkov V, Zhukova T, Akimova L, Semyenova S (2011) Polymorphism of the cox1 mtDNA gene from cercarial isolates of the avian schistosome Bilharziella polonica (Trematoda: Schistosomatidae) from Belarussian lakes. Russ J Genet 47(5):603–609. doi:10.1134/s1022795411050097
Dabo A, Durand P, Morand S, Diakite M, Langand J, Imbert-Establet D, Doumbo O, Jourdane J (1997) Distribution and genetic diversity of Schistosoma haematobium within its bulinid intermediate hosts in Mali. Acta Trop 66(1):15–26. doi:S0001-706X(97)00670-0
Davies CM, Webster JP, Kruger O, Munatsi A, Ndamba J, Woolhouse ME (1999) Host-parasite population genetics: a cross-sectional comparison of Bulinus globosus and Schistosoma haematobium. Parasitology 119(Pt 3):295–302
Dvořák J, Vanáčová Š, Hampl V, Flegr J, Horák P (2002) Comparison of European Trichobilharzia species based on ITS1 and ITS2 sequences. Parasitology 124(Pt 3):307–313
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Gower CM, Shrivastava J, Lamberton PH, Rollinson D, Webster BL, Emery A, Kabatereine NB, Webster JP (2007) Development and application of an ethically and epidemiologically advantageous assay for the multi-locus microsatellite analysis of Schistosoma mansoni. Parasitology 134(Pt 4):523–536. doi:10.1017/S0031182006001685
Grevelding CG (1999) Genomic instability in Schistosoma mansoni. Mol Biochem Parasitol 101(1–2):207–216. doi:S0166-6851(99)00078-X
Horák P, Kolářová L (2011) Snails, waterfowl and cercarial dermatitis. Freshw Biol 56(4):779–790. doi:10.1111/j.1365-2427.2010.02545.x
Horák P, Kolářová L, Adema CM (2002) Biology of the schistosome genus Trichobilharzia. Adv Parasitol 52:155–233
Jarne P, Theron A (2001) Genetic structure in natural populations of flukes and snails: a practical approach and review. Parasitology 123(Suppl):S27–S40
Jouet D, Skirnisson K, Kolářová L, Ferte H (2010) Molecular diversity of Trichobilharzia franki in two intermediate hosts (Radix auricularia and Radix peregra): a complex of species. Infect Genet Evol 10(8):1218–1227. doi:10.1016/j.meegid.2010.08.001
Khalturin KV, Mikhailova NA, Granovich AI (2000) Genetic heterogeneity in natural populations of Microphallus piriformes and M. pygmaeus parthenites (Trematoda: Microphallidae). Parazitologiia 34(6):486–501
Kock S (2001) Investigations of intermediate host specificity help to elucidate the taxonomic status of Trichobilharzia ocellata (Digenea: Schistosomatidae). Parasitology 123(Pt 1):67–70
Korsunenko AV, Tiutin AV, Semenova SK (2009) Clonal and population RAPD variation of cercariae obtained from Bucephalus polymorphus sporocysts (Trematoda: Bucephalidae). Genetika 45(1):73–80
Kulikova I, Drovetski S, Gibson D, Harrigan R, Rohwer S, Sorenson M, Winker K, Zhuravlev Y, McCracken K (2005) Phylogeography of the Mallard (Anas platyrhynchos): hybridization, dispersal and lineage sorting contribute to complex geographic structure. Anglais 122(3):949–965
Lively CM (1989) Adaptation by a parasitic trematode to local populations of its snail. Evolution 43:1663–1671
Lockyer AE, Olson PD, Ostergaard P, Rollinson D, Johnston DA, Attwood SW, Southgate VR, Horák P, Snyder SD, Le TH, Agatsuma T, McManus DP, Carmichael AC, Naem S, Littlewood DT (2003) The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitology 126(Pt 3):203–224
Lopatkin AA, Khrisanfova GG, Voronin MV, Zazornova OP, Beer SA, Semenova SK (2010) Polymorphism of the cox1 gene in bird schistosome cercaria isolates (Trematoda, Schistosomatidae) from ponds of Moscow and Moscow oblast. Genetika 46(7):981–989
Morgan JA, Dejong RJ, Adeoye GO, Ansa ED, Barbosa CS, Bremond P, Cesari IM, Charbonnel N, Correa LR, Coulibaly G, D'Andrea PS, De Souza CP, Doenhoff MJ, File S, Idris MA, Incani RN, Jarne P, Karanja DM, Kazibwe F, Kpikpi J, Lwambo NJ, Mabaye A, Magalhaes LA, Makundi A, Mone H, Mouahid G, Muchemi GM, Mungai BN, Sene M, Southgate V, Tchuente LA, Theron A, Yousif F, Zanotti-Magalhaes EM, Mkoji GM, Loker ES (2005) Origin and diversification of the human parasite Schistosoma mansoni. Mol Ecol 14(12):3889–3902. doi:10.1111/j.1365-294X.2005.02709.x
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A 76(10):5269–5273
Posada D, Crandall KA (2001) Selecting the best-fit model of nucleotide substitution. Syst Biol 50(4):580–601
Rudolfová J, Hampl V, Bayssade-Dufour C, Lockyer AE, Littlewood DT, Horák P (2005) Validity reassessment of Trichobilharzia species using Lymnaea stagnalis as the intermediate host. Parasitol Res 95(2):79–89. doi:10.1007/s00436-004-1262-x
Rudolfová J, Littlewood DT, Sitko J, Horák P (2007) Bird schistosomes of wildfowl in the Czech Republic and Poland. Folia Parasitol (Praha) 54(2):88–93
Semenova SK, Khrisanfova GG, Filippova EK, Beer SA, Voronin MV, Ryskov AP (2005) Individual and population variation in cercariae of bird schistosomes of the Trichobilharzia ocellata species group as revealed with the polymerase chain reaction. Genetika 41(1):17–22
Semyenova SK, Khrisanfova GG, Korsunenko AV, Voronin MV, Beer SV, Vodyanitskaya SV, Serbina EA, Yurlova NI, Ryskov AP (2007) Multilocus variation in cercariae, parthenogenetic progeny of different species of the class Trematoda. Dokl Biol Sci 414:235–238
Sire C, Langand J, Barral V, Theron A (2001) Parasite (Schistosoma mansoni) and host (Biomphalaria glabrata) genetic diversity: population structure in a fragmented landscape. Parasitology 122(Pt 5):545–554
Stothard JR, Webster BL, Weber T, Nyakaana S, Webster JP, Kazibwe F, Kabatereine NB, Rollinson D (2009) Molecular epidemiology of Schistosoma mansoni in Uganda: DNA barcoding reveals substantial genetic diversity within Lake Albert and Lake Victoria populations. Parasitology 136(13):1813–1824. doi:10.1017/S003118200999031X
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24(8):1596–1599. doi:10.1093/molbev/msm092
Van de Peer Y, De Wachter R (1994) TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci 10(5):569–570
Walsh PS, Metzger DA, Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10(4):506–513
Williamson VM, Caswell-Chen EP, Westerdahl BB, Wu FF, Caryl G (1997) A PCR assay to identify and distinguish single juveniles of Meloidogyne hapla and M. chitwoodi. J Nematol 29(1):9–15
Yeh FC, Yang R, Boyle T (1999) POPGENE. Version 1.31 edn. University of Alberta, Edmonton
Yin M, Hu W, Mo X, Wang S, Brindley PJ, McManus DP, Davis GM, Feng Z, Blair D (2008) Multiple near-identical genotypes of Schistosoma japonicum can occur in snails and have implications for population-genetic analyses. Int J Parasitol 38(14):1681–1691. doi:10.1016/j.ijpara.2008.05.015
Acknowledgments
We would like to thank Dr. Natalya Yurlova and Dr. Elena Serbina for their help in collecting of samples. This work received financial support from the Russian Foundation for Basic Research (09-04-01611, 08-04-12204), RFC (02.740.11.0088), and President RF Program of Leading Scientific Schools (S. S. -2107.2008.4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korsunenko, A., Chrisanfova, G., Lopatkin, A. et al. Genetic differentiation of cercariae infrapopulations of the avian schistosome Trichobilharzia szidati based on RAPD markers and mitochondrial cox1 gene. Parasitol Res 110, 833–841 (2012). https://doi.org/10.1007/s00436-011-2562-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2562-6