Abstract
The aim of this study was to determine the prevalence of sarcosporidiosis in semi-intensively bred cattle in northwestern Italy. A diagnostic protocol was setup in which infected animals were identified by rapid histological examination of the esophagus, diaphragm, and heart and the detected Sarcocystis spp. were subsequently typed using conventional electron microscopy in combination with molecular techniques. Sarcosporidia cysts were detected in 78.1% of the animals and were seen most often in the esophagus. The cattle is intermediate host for Sarcocystis hominis (final host, humans and some primates), Sarcocystis cruzi (final host, domestic and wild canids), and Sarcocystis hirsuta (final host, wild and domestic cats).All these three species of Sarcocystis were identified, variously associated, with the following prevalence: S. cruzi (74.2%), S. hirsuta (1.8%), and S. hominis (42.7%). Furthermore, a new S. hominis-like (prevalence 18.5%), characterized by hook-like structures of villar protrusion and a different sequence of the 18S rRNA gene, was identified. The cattle sheds testing positive for zoonotic Sarcocystis were assessed for risk factors contributing to the maintenance of the parasite’s life cycle. Significant associations emerged between consumption of raw meat by the farm owner, mountain pasturing, and absence of a sewerage system on the farm and cattle breed. Our study demonstrates that sarcosporidiosis may constitute a public health problem in Italy and indicates several issues to be addressed when planning surveillance and prevention actions. The applied diagnostic approach revealed that cattle can harbor a further type of Sarcocystis, of which life cycle and zoonotic potential should be investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcosporidiosis is a parasitic disease caused by intracellular protozoa of the genus Sarcocystis. The biological cycle of these parasites is based on a prey–predator relationship between a definitive host (usually carnivores) and an intermediate host (usually herbivores, omnivores, rodents, and birds). All species of Sarcocystis complete the life cycle in specific intermediate and definitive hosts or within closely related host species (Fayer 2004). Domestic cattle, and other species of the genera Bos, Bison, and Bubalus, can act as an intermediate host for Sarcocystis hominis, Sarcocystis cruzi, and Sarcocystis hirsuta. Definitive hosts for S. hominis, the only zoonotic species besides Sarcocystis suihominis (intermediate host, domestic and wild Suidae), are humans and, only at an experimental level, some primates (Chimpanzee trogloditis, Macaca mulatta, Papyo cinocephalus). The definitive hosts for S. cruzi are domestic and wild canids (Canis latrans, Canis lupus, and Vulpes vulpes) and probably some procions (Procyon lator), while for S. hirsuta, the definitive hosts are wild and domestic cats (Felis catus) (Odening 1998).
Following the consumption of meat containing protozoan cysts by a definitive host, the sexual phase of the parasite’s life cycle takes place in the host’s intestinal wall and ends with the release of oocysts or sporocysts with the feces into the environment. In the intermediate host, after oral intake of oocysts, the parasites enter the asexual reproductive phase, which through four generations of merozoites leads to the formation of infectious cysts (sarcocysts) inside the host’s muscle cells (striated, smooth, or cardiac). Protozoan cysts can develop virtually in all muscle tissues, but the heart, esophagus, diaphragm, and tongue are the organs most often affected.
Usually, the cysts can only be observed by light microscopy, but in severe cases of generalized sarcosporidiosis, they can grow enough to be appreciated with the naked eye on the muscle surfaces of the carcass. The various steps leading to the formation of intramuscular cysts in cattle can cause perivascular monocytic infiltration, petechial hemorrhages, weakness, fever, abortion in pregnant females, encephalomyelitis, and also death in cases of massive infestation (Fayer 2004; Gunning et al. 2000; Johnson et al. 1974; Johnson et al. 1975). In humans, zoonotic Sarcocystis may cause gastrointestinal symptoms as reported by many authors (Rommel and Heydorn 1972; Heydorn 1977).
Given that some sarcosporidia have zoonotic importance, Directive 2003/99/EC requires the reporting of information on Sarcocystis spp. based on the epidemiological situation in member states. References relating to the spread of parasites in European cattle are scarce and often rather vague about Sarcocystis typing, which is sometimes conducted only by assessment of cyst wall thickness. Since the cysts of S. cruzi are thin-walled, they are easily identifiable by light microscopy; however, the same cannot be said for S. hominis and S. hirsuta, both of which have thick-walled cysts that make them indistinguishable from each other (Mehlhorn and Heydorn 1978; Vercruysse et al. 1989).
The sarcosporidiosis prevalence reported by some European countries in slaughtered cattle is 84% in Czechoslovakia (Cerná and Merhautová 1981), 98.7% in Southern Germany (Boch and Erber 1981), 77% in Croatia (Wikerhauser et al. 1981), 100% in Holland (Van Knapen et al. 1987), and 97% in Belgium (Vercruysse et al. 1989). For Italy, the reports chiefly concern isolated cases of generalized sarcosporidiosis in swine and cattle (Domenis et al. 2002; Ferrantelli et al. 2004) or monitoring of domestic and wild Suidae (Brindani et al. 1982; Brindani et al. 1983; Leoni et al. 1995); recently, a prevalence and distribution study has been carried out in cattle slaughtered in Sicily (Bucca et al. 2010).
“The Community Summary Report of EFSA (European Agency for Food Security) on Trends and Sources of Zoonoses, Zoonotic Agents, Antimicrobial Resistance and Foodborne Outbreaks in the European Union” issued in 2005 and 2006 suffers from a lack of information about the spread of sarcosporidiosis, mentioning only that the prevalence of Sarcocystis in slaughtered cattle was 0.002% and 0.01% in Belgium and Luxembourg, respectively; since 2007, the report has provided no data about this parasitosis.
A similar gap in information can be noted in the “Development of harmonized schemes for the monitoring and reporting of Sarcocystis in animals and foodstuffs in the European Union” Taylor et al. 2009, a scientific report prepared by a group of experts in response to a specific request of the EFSA (call for proposals CFP/EFSA/Zoonoses/2007/01). In brief, the document reiterates that the data in almost all member states are insufficient, defining as “unclear” the situation in animal populations, as well as the impact of the disease on human health. The report also underlines the limitations of Sarcocystis surveillance, considering that mere visual inspection of the carcass at the slaughterhouse does not allow the detection of microscopic sarcocysts or the differentiation between zoonotic and non-zoonotic species when the sarcosporidiosis produces gross lesions, which is only possible with inspection of the cyst wall by electron microscopy.
The aim of our study was to determine the prevalence of sarcosporidiosis in semi-intensively bred cattle. A diagnostic protocol was setup to identify infected animals by rapid histological exam and typing of involved Sarcocystis, thus combining conventional electron microscopy with molecular techniques. Given the high prevalence of zoonotic infestations, a case-control study was carried out to identify the major risk factors for the maintenance of the parasite cycle in the cattle sheds found infected.
A novel variant of Sarcocystis, genetically related to S. hominis but ultrastructurally different from any other Sarcocystis spp. so far described, was detected in the context of our epidemiological survey and is presented here.
Materials and methods
All the cattle included in the study were born and reared in the Province of Biella located in northwestern Italy and characterized by a predominantly foothill landscape and a continental climate. Given the estimated cattle population size in the Province of Biella of about 14,700 heads (as reported by the Regional Veterinary Office in 2008), a sample of 296 animals was needed to be able to reveal a prevalence of 1% [95% confidence interval (CI)]. We therefore sampled 384 cattle, which included all animals slaughtered for self-consumption within the province. “Self-consumption slaughtering” means slaughtering done by the breeder at an authorized slaughterhouse for private consumption. We chose this type of animals (raised identically to animals for commercial sale) because we considered self-consumption as an important factor for perpetuating the predator–prey cycle of zoonotic Sarcocystis in the cattle shed. The target organs for Sarcocystis spp. (heart, diaphragm, and esophagus) of each animal were analyzed. The diagnostic protocol included a screening phase (using rapid histological exam by cryostat) followed by molecular typing of detected sarcosporidia and electron microscopy.
Diagnostic methods
-
1.
Rapid histological exam by cryostat
The tissue sections were prepared as follows: a tissue block about 1 cm wide was constructed, then rapidly frozen in cryostat-embedding medium (at −20°C); sections were cut to about 5 μm thickness from at least two cutting planes 4–5 mm apart; the sections were fixed in 95% alcohol, stained with hematoxylin and eosin, dehydrated through the increasing alcohol scale, cleared in Bioclear, and mounted on glass slides and coverslipped with Eukitt balsam. The preparations were observed under an Olympus BX60 microscope (×40 magnification) and the digitized images of the more interesting features were acquired with an Olympus Camedia C-40-40 Zoom Digital Camera, using Olympus DP-Soft version 3.2 imaging software.
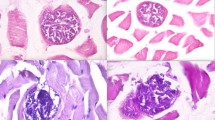
The sarcocysts in the muscle cells appear as basophilic bodies, round or elongated in shape depending on the cutting plane, bordered by a radial fairly thick wall (Fig. 1). The degree of infestation was semiquantitatively graded (1+, 2+, and 3+) according to the number of cysts observed (one cyst per section, one cyst per microscopic field, or more than one cyst per microscopic field, respectively) at a final magnification of ×40.
-
2.
Molecular typing
Total DNA was isolated from 25-mg tissue samples using a GenElute Mammalian Genomic DNA kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol. Molecular typing was initially performed by sequencing and analysis of the complete 18S ribosomal DNA as described by Fischer and Odening (1998). A high throughput method based on the differential PCR described by Vangeel et al. (2007) was then applied using a modified reverse primer labeled with Hex fluorescent dye at the 5′. This PCR amplifies a region of the 18S rRNA gene generating amplicons of different lengths depending on the Sarcocystis species: 164 bp for S. hominis, 172 bp for S. cruzi, and 186 bp for S. hirsuta. The PCR products were submitted to capillary electrophoresis on an ABI 3130 Genetic Analyser (Applied Biosystems, Foster City, CA, USA), and the size was determined by GeneMapper software analysis. From selected samples showing unknown 168 bp peaks, PCR products were cloned into a TOPO TA cloning vector (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. At least ten recombinant cloned plasmids were analysed by multiple sense sequencing, using the oligos provided with the kit [M13 forward (+) 5′-CGCCAGGGTTTTCCCAGTCACGA-3′ and M13 reverse (−) 5′-AGCGGATAACAATTTCACACAGGA-3′]. The obtained sequences were aligned using SeqMan (DNASTAR, Madison, WI, USA) and submitted to BLAST analysis.
-
3.
18S rRNA gene sequencing and phylogenetic analysis of S. hominis-like
Two selected samples were submitted to PCR amplification using primers specifically designed for the novel S. hominis-like. The sequences of these primers, which were obtained from Invitrogen (Carlsbad, CA, USA), were SHV_F (+) 5′-GTTTATTAGATACAGAACCAATAA-3′ and SHV_R (−) 5′-CCCCAAAAAGGAGCGTGTTA-3′ hybridizing at their 3′-end on a S. hominis-like specific nucleotide triplet. Primers SHV_F and SHV_R were coupled with primers S4 and S1 (Fischer and Odening 1998), respectively. PCR reactions were performed as follows: 5 μl of genomic DNA, 50 pmol of each primer, 100 μM of dNTPs (Fermentas UAB, Vilnius, Lithuania), 1.5 U of Taq polymerase (Platinum Taq, Invitrogen, Carlsbad, CA, USA) in a final volume of 50 μl consisting of 1× PCR buffer and 1.5 mM MgCl2. Thermocycling parameters consisted of an initial denaturation step (95°C, 2.5 min) followed by 45 cycles of denaturation (94°C, 1 min), annealing (60°C, 1 min), and extension (72°C, 2 min) on temperature gradient cycler (GeneAmp 9700, Applied Biosystems, Foster City, CA, USA). The purified PCR products were cloned into TOPO TA cloning vector (Invitrogen, Carlsbad, CA, USA) and ten recombinant clones for each sample were double strand sequenced by Big Dye terminator cycle sequencing using the amplification primer pairs and internal primers S2 and S3 (Fischer and Odening 1998), and run on an ABI Prism 3130 Genetic Analyser (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. The 18S rRNA gene sequences of S. hominis-like were analysed using the Lasergene package (DNASTAR Inc., Madison, WI, USA). The obtained consensus sequence was submitted to BLAST search and aligned with the sequences of other known Sarcocystis species available in the GenBank database. Phylogenetic analysis was based on a representative sample of the Sarcocystidae, including 16 Sarcocystis species with different intermediate and definitive hosts. Genetic distances were computed using MEGA 4 (Tamura et al. 2007). Distance matrices were determined under the assumptions of Kimura’s two parameter model and were used to infer dendrograms by the neighbor-joining method (Saitou and Nei 1987). Confidence values for individual branches of the resulting tree were determined by bootstrap analysis with 1,000 replicates (Felsenstein 1981). The newly determined Sarcocystis sequence has been deposited in the GenBank database under accession number JF327857.
-
4.
Electron microscopy
Under an inverted microscope (Olympus CK2), the sarcocysts were isolated from the muscle tissue using surgical needles; the samples were fixed in 0.1 cacodylate buffer (pH 7.2) containing glutaraldehyde for 3 h at 4°C. The samples were then washed and fixed in 1% OsO4 in cacodylate buffer for 1.5 h at 4°C. All samples were then dehydrated in ethanol and embedded in Epon 812. Semithin sections were stained with 0.5% toluidine blue in 1% sodium tetraborate and examined by optical microscopy. Thin sections (80 nm) were stained with uranyl acetate band lead citrate and examined under a Zeiss EM 900 transmission electron microscope (TEM) at 80 kV.
a Example of high infestation by Sarcocystis spp. (corresponding to the level 3+) detected by histological screening in the esophageal muscle layer (HE staining). The sarcocysts are housed inside the muscle fibers and appear as elongated (if cut lengthwise, see arrow) or rounded (if cut crosswise, see arrowhead) elements, intensely basophilic. b Sarcocyst cut lengthwise (higher magnification of the particular pointed by the arrow in a). c Sarcocysts cut crosswise (higher magnification of the particular pointed by the arrowhead in b)
Epidemiologic survey
Variables such as age, size, and breed livestock were categorized. Animal age was grouped into quintiles and the farm size into tertiles. The frequencies of possible associations between the presence of the parasite and individual or farming variables were described.
Due to the high prevalence of Sarcocystis recorded in the sample unit, we evaluated the risk factors for transmission of the parasite by setting up a case-control study. A questionnaire was drawn up to collect information on the potential risk factors. The questionnaire was administered to the 45 farms out of those found positive for S. hominis and to 49 farms were found negative. A cattle shed was considered negative when at least four animals tested negative; this level of resolution was adopted because during the survey to verify the frequency of Sarcocystis, at least one individual was always found positive on farms with a minimum of four animals tested. Exposure variables investigated in the questionnaire were those considered as major risk factors, namely, use of pastures, absence of a sewerage system for wastewater from animal manure, practice of pasturing, raw meat consumption by the farmer, and concurrent presence of other animal species.
In this way, we followed the classic pathway of analytic epidemiology: investigation of possible associations, calculation of disease risk for the variables significantly associated with disease [odds ratio (OR)], and then detection of confounding parameters that could alter the real associations (Maentel-Haenszel correction). Multivariate analysis (logistic regression) was conducted to confirm the significance revealed by the univariate analysis.
An ad hoc database was set up to collect data from the survey and information from the questionnaire. Data were analyzed using Stata SE version 10.1 (StataCorp, College Station, TX, USA).
Results
Rapid histological exam by cryostat
On histological screening, 78.1% (300/384) of the animals tested positive for the presence of sarcocysts in at least one organ. The anatomic structure most often affected was the esophagus (n = 279), followed by the diaphragm (n = 228) and the heart (n = 225); the anatomic district with the highest density of sarcocysts was the esophagus (22.9% of samples graded 3+), followed by the heart (12.4% of samples graded 3+) and the diaphragm (4.8% of samples graded 3+). Table 1 reports the distribution of sarcocysts in the investigated organs.
Molecular typing
All three species of Sarcocystis affecting cattle (S. cruzi, S. hominis, and S. hirsuta) were identified by molecular typing. The combination of differential PCR and capillary electrophoresis analysis greatly improved the method’s resolution, ensuring unambiguous interpretation of results. Beside peaks of the expected size, some samples showed a peak of 168 bp nucleotides by GeneMapper analysis. Cloning of PCR products from a selection of these samples and BLAST analysis of recombinant clone sequences revealed an unreported sequence showing 94% similarity with the 18S rRNA gene of S. hominis. This finding was interpreted as a genetic variant of S. hominis, characterized by a different number of bases (168 bp) and defined as S. hominis-like (Fig. 2). The prevalence of the various species of Sarcocystis in the sample population was: S. cruzi 74.2% (n = 285), S. hominis 42.7% (n = 164), S. hominis-like 18.5% (n = 71), and S. hirsuta 1.8% (n = 7).
The distribution of each Sarcocystis spp. in the anatomic districts is reported in Table 2. Types of observed infestations (single infections and multiple infections) with relative prevalence are given in Table 3. The esophagus had the highest number of associations (n = 9) among the different species, followed by the diaphragm (n = 6) and the heart (n = 4).
18S rRNA gene sequencing and phylogenetic analysis of S. hominis-like
To obtain specific amplification of the complete 18S rRNA gene sequence, two samples co-infected with S. hominis and S. hominis-like were selected and submitted to PCR amplification using primers targeting a nucleotide triplet exclusive to S. hominis-like. This strategy was based on the genetic information retrieved by molecular typing of the 168 bp peak as described above. Agarose gel electrophoresis showed PCR products of the expected molecular sizes (199 and 1,408 bp for S1/SHV_R and SHV_F/S4 primer pairs, respectively). The consensus sequence of the 18S rRNA gene was determined by cloning and sequencing of these amplicons. Sequence distances calculated using the MegAlign applet of the Lasergene package showed the highest similarity (97.6%) of the newly determined 18S rRNA gene with the homologous gene of S. hominis and 88.4–95.5% with the members of the other Sarcocystis species selected for phylogenetic analysis. Sequence divergence within published S. hominis sequences included in the analysis was 0.1–0.9%, while the divergence between S. hominis-like and the S. hominis taxon was 0.7–1.4%. Multiple alignments of the 18S rRNA gene sequences showed that S. hominis-like differed from its most closely related homologous gene (the 18S rRNA of S. hominis) by insertions/deletions and single nucleotide polymorphisms (data not shown). Figure 3 shows the phylogenetic tree resulting from the analysis of the 18S rRNA gene of S. hominis-like together with other reported protozoan sequences.
Phylogenetic tree of similarity among the 18S rRNA gene sequences of the novel S. hominis-like and other protozoan sequences. The GenBank accession numbers of the analysed 18S rRNA sequences are indicated. Bootstraps values >50 (1,000 replicates) are shown at the internal nodes. The length of each pair of branches represents the distance between sequence pairs. The scale bar represents the percentage of nucleotide differences
Electron microscopy
Identification of S. cruzi and S. hominis by molecular typing was confirmed by transmission electron microscopy of cyst wall morphology in the tissue samples from eight and six animals, respectively. Two different cyst wall structures were identified corresponding to those described by Dubey et al. (1989) for S. cruzi and S. hominis. Due to the small number of affected animals and the low level of infection in the positive organs and anatomic structures, we were unable to characterize the wall of S. hirsuta.
S. cruzi: In the longitudinal sections, the cyst wall of was thin (0.4 μm) with flattened microvillar protrusions (up to 3.5 μm long) that contained no tubules or microtubules in the villar core (Fig. 4). The transverse section of the base of the cyst wall showed an electron-dense vesicle-like invaginations possibly involved in the absorption of nutrients (Fig. 5).
S. hominis: The structure of the fingerlike villar protrusions was uniform along its entire length. The protrusions were oriented perpendicularly to the sarcocyst surface and measured approximately 6.8 μm in length and 1 μm in width. Granular layer measure was 1.5 μm. The villar core contained numerous microfilaments and rows of electron-dense granules oriented parallel to the longitudinal axis (Fig. 6).
S. hominis-like: The ultrastructure of some cysts obtained from two different samples positive for S. hominis-like at molecular typing, the same was used for the phylogenetic analysis, displayed anomalous features never described previously. Specifically, hook-like structures were observed both at the base and at the apex of the villar protrusions (Fig. 7). Microfilaments, coarse granules, and vesicle-like invaginations of the villar surface were observed only at the base of the villar protrusions. The protrusions were oriented oblique to the sarcocyst surface and measured approximately 5.6 μm in length and 0.6 μm in width. Granular layer measure was 0.3 μm.
TEM micrograph of the morphological variant observed in S. hominis-like positive cattle. Hook-like structures are observable both at the base and the apex of the villar protrusions. Microfilaments (asterisks), coarse granules, and vesiclelike invaginations of the villar surface are present only at the base of the villar protrusions (g, granular layer, m, muscle)
Epidemiologic survey
As expected, a trend for increasing the risk of infection with aging was noted, an effect likely due to the accumulation of cysts over time (Table 4). Among the variables investigated in the questionnaire for the case-control study, the following were found to be significantly associated with the Sarcocystis infection:
-
1.
Consumption of raw meat by the farmer (OR 5.7; 95% CI, 1.1–55.3)
-
2.
Grazing of the animals (OR 5.3; 95% CI, 1.8–16.8)
-
3.
Breed: Pezzata Rossa d’Oropa, either pure breed or crossbred, appeared to be at higher risk than the other breeds included in the study (Holstein and Piedmontese) (OR 6.5; 95% CI, 2–24.6)
-
4.
Mountain pasturing (OR 3.6; 95% CI, 1.1–14)
-
5.
Absence of sewerage system on the farm (χ 2 = 5.2; p = 0.02); we were unable to calculate the OR because there were only cases and no controls in the cell of exposure
The association between breed and disease was confirmed on multivariate analysis. Moreover, the effect of meat consumption and of mountain pasturing, though high, barely missed reaching significance (p = 0.07 and p = 0.08, respectively) (Table 5).
Discussion
The data obtained in this study on the occurrence of sarcosporidiosis are relevant for animal and human health, since, to our knowledge, the prevalence of Sarcocystis spp. in Italian cattle is largely unknown. The prevalence (78.1%) detected by histological screening was high, though slightly lower than the rates reported for other European countries (generally 80–100%).
As regards the distribution of Sarcocystis spp., 62% of infected animals had parasites in all three anatomic structures (esophagus, heart, diaphragm) investigated underlining the advisability of sampling these sites. The esophagus, besides presenting the highest degree of infestation (sarcocyst density), was also the anatomic structure most commonly affected (in only 7.3% of positive animals was infestation limited to the heart and/or the diaphragm).
S. cruzi appeared to localize preferentially in the esophagus rather than the heart, in contrast with findings from previous studies (Vercruysse et al. 1989), and this species was clearly more common, either alone or in combination with other Sarcocystis, than the thick-walled species. Of these latter species, S. hirsuta was relatively rare and confined to the esophagus. S. hominis showed a tropism for the esophagus and the diaphragm, while it was rather rare in the heart.
These issues point to two conclusions that could be useful for offering a partial answer to certain questions put forward by the EFSA: given these tropisms, sampling of the esophagus and the diaphragm would be preferable in surveillance for zoonotic Sarcocystis at the slaughterhouse; in geographical areas similar to the study area, where monitoring showed that S. hirsuta is nearly absent, simple histological examination (evaluation of sarcocyst wall thickness) could be a useful tool to detect the presence of zoonotic sarcosporidia in cattle without the need for more complex, expensive techniques such as electron microscopy and molecular biology.
The relatively high presence of S. hominis is of concern, especially for the studied region that has a traditional cuisine characterised by the consumption of food prepared from raw or undercooked meat. The presence of sarcocysts has been reported (Bucca et al. 2010; Moré et al. 2010; Ono and Takayuki 1999) in all skeletal muscles, although these are generally less affected than target organs (Saito et al. 1998), and in food containing raw or undercooked meat such as hamburgers (Prayson et al. 2008) and kibbeh (Pena et al. 2001).
The infection can have a more or less benign course in humans. In a study involving Chinese volunteers who assumed from 1,467 to 14,740 cysts of S. hominis, the majority experienced pain and abdominal distension, watery diarrhea, and eosinophilia for 1–4 weeks followed by spontaneous resolution of symptoms (Chen et al. 1999). Conversely, six Thai persons who had consumed raw zebu meat infested by sarcocysts developed severe segmental necrotizing enteritis that required surgical treatment (Bunyaratvej et al. 1982). In any event, it would be necessary to evaluate the danger of infestation in relation to particular consumer categories such as people with immunosuppression of any origin. Velásquez et al. (2008) reported systemic sarcocystosis in AIDS patients, which being both an intermediate and a definitive host, developed the classic gastrointestinal symptoms and signs (myalgia and bronchial asthma) related to the presence of sarcocysts in muscle tissue.
The survey carried out on the farms that tested positive for zoonotic Sarcocystis showed significant correlations between the disease and consumption of raw meat by the farmers, lack of a sewerage system, mountain pasturing, and cattle breed. The risk factors identified through the survey suggest that the biological cycle of S. hominis can be maintained both on the farm, due to the farmer’s habits, and externally, outside the farm, through the use of pastures contaminated with human sewage. What is likely, therefore, is that the parasites spread through the farms because of different overlapping factors, not only related to indigenous origin (i.e., possible sarcocystosis of the owner).
The association between breed and Sarcocystis is probably linked to the particular management of Pezzata Rossa d’Oropa, which involves more mountain pasturing than with other breeds.
The results of our study thus demonstrate that zoonotic sarcosporidiosis may still constitute a public health problem in Italy. In addition, they indicate several issues to be addressed when planning surveillance and prevention actions. Specifically, major efforts should be placed on educational programs to prevent circulation of the parasite, for example making farmers more aware and helping them to change their habits and to improve infrastructures. If implemented, surveillance systems should include diagnostic protocols for systematic species identification to allow to take preventive measures for human health only in presence of zoonotic species.
The diagnostic protocol we applied in the present study proved to be both useful and efficient. The use of frozen material did not compromise either sarcocyst integrity, which is essential for detecting parasites in histological samples or the fine morphology of the cyst wall, which is necessary for typing the species at the electron microscope. Molecular typing of sarcosporidia was consistent with typing conducted (when applied) with transmission electron microscopy of sarcocysts isolated from the same samples. The application of capillary electrophoresis sizing to the differential PCR described by Vangeel et al. (2007) significantly improved the resolution of the molecular method, which can sometimes lead to misinterpretation of results when PCR products are loaded on agarose gels. The small difference in the number of nucleotides among the Sarcocystis spp. may give ambiguous results if the run is not performed in optimal conditions.
Remarkably, this approach allowed the identification of a S. hominis-like with genetic and ultrastructural features never described before. The taxonomy of Sarcocystis spp. is based on genetics, morphology, and life cycle of the parasites (Chen et al. 2010; Jehle et al. 2009; Yang et al. 2001a; Yang et al. 2001b). The newly identified Sarcocystis sp. presented an 18S rRNA gene sequence with maximum similarity with S. hominis and this is the reason we identified it as a S. hominis-like. However, this homology was 97.6%, much less than the homology observed within the S. hominis taxon. Fischer and Odening (1998) actually reported homology higher than 99% between Sarcocystis of the same species and 95–96% or less of interspecies homology. The homology value we found for this new S. hominis-like was thus at a borderline. The phylogenetic analysis showed that the general phylogenetic pattern of Sarcocystidae did not change by including the sequence of the S. hominis-like. The basic structure of the inferred phylogenetic tree was in agreement with that described in other studies (Dahlgren et al. 2008; Morrison et al. 2004; Yang et al. 2001b). The novel sequence places on a separate branch supported by 86% bootstrap value parallel to the cluster formed by the known S. hominis sequences. A similar topology is reported by Yang et al. (2001b) for a S. cruzi-like that was characterized also by a specific ultrastructural morphology. Combining morphology and the position on the phylogenetic tree, they interpreted this Sarcocystis sp. as a separate species. In our case, in the same samples submitted to genetic analysis for the S. hominis-like, we were able to observe important differences in the shape, size, and microscopical details of the cyst wall. In particular, the fingerlike protrusions presented hook-like structures both at the base and at the apex, whereas the protrusions of S. hominis are uniform along its entire length. Microfilaments, coarse granules, and vesicle-like invaginations were present only at the base of S. hominis-like, whereas in S. hominis, these structures are uniformly scattered along its entire structure. Finally, the villar protrusions and the granular layer were thinner than those of S. hominis.
In conclusion, both genetical and ultrastructural characteristics showed that cattle appear to harbor a further type of Sarcocystis. It has to be clarified whether this is a different form of S. hominis, perhaps undergoing a speciation process, or whether it is a new species, of which life cycle and zoonotic potential should be investigated.
References
Boch J, Erber M (1981) Prevalence, economic and hygienic importance of Sarcocystis spp. of cattle, sheep and pigs. Fleishwirtschaft 61(3):427–431
Brindani F, Perini S, Cabassi E, Marastoni G (1982) Sarcosporidosis in slaughter pigs in the province of Reggio Emilia: breeding pigs. Obiettivi Documenti Veterinari 3(10):51–53
Brindani F, Perini S, Cabassi E, Marastoni G (1983) Prevalence of sarcosporidiosis in swine slaughtered in the province of Reggio Emilia. 2a. Fattening swine. In: Atti IX Convegno Soc It Pat Allev Suini, pp 57–60
Bucca M, Brianti E, Giuffrida A, Ziino G, Cicciari S, Panebianco A (2010) Prevalence and distribution of Sarcocystis spp. cysts in several muscles of cattle slaughtered in Sicily, Southern Italy. Food Control 22(1):105–108
Bunyaratvej S, Bunyawongwiroj P, Nitiyanant P (1982) Human intestinal sarcosporidiosis: report of six cases. Am J Trop Med Hyg 28:819–844
Cerná Z, Merhautová V (1981) Sarcocystosis in cattle and sheep at Prague abattoir. Folia Parasitol Praha 28(2):125–129
Chen X, Zuo Y, Zuo W (1999) Observation on the clinical symptoms and sporocysts excretion in human volunteers experimentally infected with Sarcocystis hominis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 17(1):25–27
Chen X, Zuo Y, Rosenthal BM, He Y, Cui L, Yang Z (2010) Sarcocystis sinensis is an ultrastructurally distinct parasite of water buffalo that can cause foodborne illness but cannot complete its life-cycle in human beings. Vet Parasitol. doi:10.1016/j.vetpar.2010.12.026
Dahlgren SS, Gouveia-Oliveira R, Gjerde B (2008) Phylogenetic relationships between Sarcocystis species from reindeer and other Sarcocystidae deduced from ssu rRNA gene sequences. Vet Parasitol 151:27–35
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC (OJ L325, 12.12.2003 pp: 31)
Domenis L, Banchi C, Paletti N, Peracino V, Orusa R (2002) Diffusion of muscular parasites in the wild boar (Sus scrofa) of Aosta Valley Region: a case of co-infection by Trichinella sp. and Sarcocystis sp. In: Proceedings of EAZWV and EWDA meeting held at Heidelberg, Germany, pp 423
Dubey JP, Speer CA, Fayer R (1989) Sarcocystosis of animals and man. CRC Press, Boca Raton, pp 1–215
Fayer R (2004) Sarcocystis spp. in human infection. Clin Microbiol Rev 17:894–902
Felsenstein J (1981) Evolutionary trees from DNA sequences: maximum likelihood approach. J Mol Evol 17:368–376
Ferrantelli V, Vicari D, Di Iorio D, Chetta M, Riili S, Monteverde P, Strola M (2004) La sarcosporidiosi bovina: una parassitosi occulta. Un caso rilevato in sede di macellazione. Large Anim Rev 4:15–17
Fischer S, Odening K (1998) Characterization of bovine Sarcocystis species by analysis of their 18S ribosomal DNA sequences. J Parasitol 84(1):50–54
Gunning RF, Jones JR, Jeffrey M, Higgins RJ, Williamson AG (2000) Sarcocystis encephalomyelitis in cattle. Vet Rec 146(11):328
Heydorn AO (1977) Sarkosporidieninfiziertes Fleisch als mögliche Krankheitsursache für den Menschen. Arch Lebensmittelhyg 28:27–31
Jehle C, Dinkel A, Sander A, Morent M, Romig T, Luc PV, De TV, Thai VV, Mackenstedt U (2009) Diagnosis of Sarcocystis spp. in cattle (Bos taurus) and water buffalo (Bubalus bubalis) in Northern Vietnam. Vet Parasitol 166:314–320
Johnson AJ, Fayer R, Hildebrandt PK (1974) The pathology of experimental sarcosporidiosis in the bovine. Lab Investig 30:377–378
Johnson AJ, Hildebrandt PK, Fayer R (1975) Experimentally induced Sarcocystis infection in calves: pathology. Am J Vet Res 3:995–999
Leoni A, Scala A, Grippa G, Pirino S, Sanna P (1995) La sarcosporidiosi del cinghiale (Sus scrofa pmeridionalis) in Sardegna: aspetti epidemiologici, morfo-ultrastrutturali e anatomo-istopatologici. In: Atti del Convegno Nazionale sulle problematiche emergenti nelle aree protette, Teramo, pp 101
Mehlhorn H, Heydorn AO (1978) The sarcosporidia (Protozoa, Sporozoa): life cycle and fine structure. Adv Parasitol 16:43–91
Moré G, Abrahamovic P, Jurado S, Bacigalupe D, Marin JC, Rambeaud M, Venturini L, Venturini MC (2010) Prevalence of Sarcocystis spp. in Argentinean cattle. Vet Parasitol. doi:10.1016/j.vetpar.2010.11.036
Morrison DA, Bornstein S, Thebo P, Wernery U, Kinne J, Mattsson JG (2004) The current status of the small subunit rRNA phylogeny of the coccidia (Sporozoa). Int J Parasitol 34:501–514
Odening K (1998) The present state of species-systematics in Sarcocystis Lankester, 1882 (Protista, Sporozoa, Coccidia). Syst Parasitol 41:209–233
Ono M, Takayuki O (1999) Prevalence of Sarcocystis spp. cysts in Japanese and imported beef (Loin: Musculus longissimus). Parasitol Int 48:91–94
Pena HF, Ogassawara S, Sinhorini IL (2001) Occurrence of cattle Sarcocystis species in raw kibbe from Arabian food establishments in the city of Sao Paulo, Brazil, and experimental transmission to humans. J Parasitol 87(5):1439–1465
Prayson B, McMahon JT, Prayson RA (2008) Fast food hamburgers: what are we eating ? Ann Diagn Pathol 12:406–409
Rommel M and Heydorn AO (1972) Beiträge zum Lebenszyklus der Sarkosporidien. III. Isospora hominis (Railliet und Lucet, 1891) Wenyon, 1923, eine Dauerform der Sarkosporidien des Rindes und des Schweins. Berliner und Münchener TierärztlicheWochenschrift 85:14
Saito M, Shibata Y, Azuma H, Itagaki H (1998) Distribution of Sarcocystis cruzi cysts in bovine striated muscles. J Jpn Vet Med Assoc 51:453–455
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Taylor MA, Boes J, Boireau P, Boué F, Claes M, Cook AJC, Dorny P, Enemark H, van der Giessen J, Hunt KR, Howell M, Kirjušina M, Nöckler K, Pozio E, Rossi P, Snow L, Theodoropoulos G, Vallée I, Vieira-Pinto MM, Zimmer IA (2009) Development of harmonised schemes for the monitoring and reporting of Sarcocystis in animals and foodstuffs in the European Union. Scientific Report submitted to EFSA, pp 1–28
The Community Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents, Antimicrobial Resistance and Foodborne Outbreaks in the European Union in 2005. The EFSA Journal 2006, 94, pp 199
The Community Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents, Antimicrobial Resistance and Foodborne Outbreaks in the European Union in 2006. The EFSA Journal 2007, 130, pp 238
Van Knapen F, Bouwman D, Greve E (1987) Study of the incidence of Sarcocystis spp. in Dutch cattle using various methods. Tijdschr Diergeneeskd 112(19):1095–1100
Vangeel L, Houf K, Chiers K, Vercruysse J, D’Herde K, Ducatelle R (2007) Molecular-based identification of Sarcocystis hominis in Belgian minced beef. J Food Prot 70(6):1523–1526
Velásquez JN, Di Risio C, Etchart CB, Chertcoff AV, Mendez N, Cabrera MG, Labbé JH, Carnevale S (2008) Systemic sarcocystosis in a patient with acquired immune deficiency syndrome. Hum Pathol 39(8):1263–1267
Vercruysse J, Franse J, van Goubergen M (1989) The prevalence and identity of Sarcocystisc cysts in cattle in Belgium. J Vet Med B 36:148–153
Wikerhauser T, Dzakula N, Rapic D, Majurdzic D (1981) A study of sarcocystosis in cattle and pigs. Veterinarski Arch 51(6):275–282
Yang ZQ, Zuo YX, Ding B, Chen XW, Luo J, Zhang YP (2001a) Identification of Sarcocystis hominis-like (Protozoa: Sarcocystidae) cyst in water buffalo (Bubalus bubalis) based on 18S rRNA gene sequences. J Parasitol 87(4):934–937
Yang ZQ, Zuo YX, Yao YG, Chen XW, Yang GC, Zhang YP (2001b) Analysis of the 18S rRNA genes of Sarcocystis species suggests that the morphologically similar organisms from cattle and water buffalo should be considered the same species. Mol Biochem Parasitol 115:283–288
Acknowledgments
This project (code B67) was funded by the Piedmont Region in the frame of the 2004 Applied Scientific Research Programme (Delibera Cipe 2004). We thank Dr. G. Ru for supporting the statistical analysis and Dr. F. Vottari, Dr. D. Cognata, Dr. S. Pellegrini, and Dr. L. Sala for collecting samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Domenis, L., Peletto, S., Sacchi, L. et al. Detection of a morphogenetically novel Sarcocystis hominis-like in the context of a prevalence study in semi-intensively bred cattle in Italy. Parasitol Res 109, 1677–1687 (2011). https://doi.org/10.1007/s00436-011-2441-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2441-1