Abstract
The present study was carried out to determine the prevalence of Theileria annulata in large ruminants in Southern Punjab (Pakistan). Blood samples were collected from 144 large ruminants, consisting of 105 cattle and 39 buffaloes, from six districts of Southern Punjab including Multan, Layyah, Muzaffar Garh, Bhakar, Bahawalnagar, and Vehari. Data on the characteristics of the animals and herds were collected through questionnaires. The age of animals (P = 0.02), presence of ticks on animals (P = 0.02), and presence of ticks on dogs associated with herds (P = 0.05) were among the major risk factors involved in the spread of tropical theileriosis in the study area. Two different parasite detection techniques, PCR amplification and screening of Giemsa-stained slides, were compared, and it was found that PCR amplification is a more sensitive tool (19% parasite detection) as compared to smear scanning (3% parasite detection) for the detection of T. annulata. Twenty eight out of 144 animals produced the 721-bp fragment specific for T. annulata from five out of six sampling districts. Different blood (hemoglobin, glucose) and serum (ALT, AST, LDH, cholesterol) parameters of calves and cattle were measured and compared between parasite-positive and parasite-negative samples to assess the effect of T. annulata on the blood and serological profile of infected animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cattle are raised as livestock for meat, as dairy animals for milk and other dairy products, and as draft animals. Other products include leather and dung for manure or fuel (Durrani et al. 2006). The role of livestock in Pakistan’s rural economy may be realized from the fact that 30–35 million of the rural population is engaged in livestock raising which helps them to derive 30–40% of their income from it (Livestock Census 1996).
Tick infestation in cattle is one of the major constraints to the livestock industry in developing countries which adversely affects economic performance, mainly by transmission of serious pathogens of animals (Das et al. 2005). Tropical theileriosis is caused by one of the most devastating apicomplexan protozoan parasite, Theileria annulata (belonging to the family Theileriidae), found in lymphocytes, histiocytes, and erythrocytes of ruminants and mainly transmitted by ixodid ticks (Soulsby 1982; Bazarusanga 2008). It affects cattle, yak, and buffalo, with milder infections usually seen in buffalo (The Center for Food Security and Public Health 2005). A marked rise in body temperature, reaching 40–41.5°C, is followed by depression, lacrimation, nasal discharge, and swelling of the superficial lymph nodes; anemia is among the characteristic features of tropical theileriosis. As a result, weight loss is rapid, and hemoglobinuria may occur (Oliveira et al. 1995; Gubbels et al. 2000).
Advances in molecular biological techniques have resulted in the improved detection, identification, and genetic characterization of many hemoparasites. Species-specific polymerase chain reaction (PCR) has been developed for the detection and identification of various Theileria species and has been shown to have higher sensitivity and specificity compared with serological assays and examination of Giemsa-stained blood smears (Bhoora et al. 2009).
The aim of the present study was to establish and optimize a specific, reliable, and sensitive molecular tool, the PCR, for the detection of T. annulata in cattle and buffaloes of Southern Punjab. Furthermore, the present study provided baseline data regarding T. annulata prevalence and risk factors involved in the spread of tropical theileriosis in large ruminants, and we also compared various hematological and serum biochemical parameters between parasite-positive and parasite-negative cattle/buffaloes.
Materials and methods
Sample and data collection
Blood samples were collected from 144 clinically healthy large ruminants (105 cattle and 39 buffaloes) from randomly selected herds located in the important livestock production regions of Southern Punjab. Samples were collected from six districts including Multan, Layyah, Muzaffar Garh, Bhakar, Bahawalnagar, and Vehari. Blood was collected from the jugular vein of the animals and immediately preserved in Eppendorf tubes by adding a few drops of 0.5 M EDTA. Data regarding the characteristics of animals (species, gender, age, and presence of ticks) and herds (location, size, species of animals, dogs associated with the herds, presence of ticks on dogs associated with the herds) were collected through questionnaires completed by investigators on sampling sites in order to calculate the risk factors involved in the spread of tropical theileriosis.
Blood smear formation
Blood smears were prepared, fixed with methanol, stained with Giemsa, and microscopically observed for the detection of Theileria sp. in blood.
DNA extraction
An inorganic method of DNA extraction was used following Shaikh et al. (2005). The quality of the DNA extract in regard to purity and integrity was assessed with optical density counts at 260/280 nm and submerged gel electrophoresis.
PCR amplification
A set of oligonucleotide primers was used to amplify the 30-kDa gene sequences of T. annulata as previously described by Oliveira et al. (1995). The nucleotide sequence of the primer pair was: (N516) 5′ GTAACCTTTAAAAACGT 3′ and (N517) 5′ GTTACGAACATGGGTTT 3′. PCR was performed in a final reaction volume of 25 μl. Each reaction contained 50 mM KCl, 10 mM Tris–HCl (pH 8.3), 1.5 mM MgCl2, 0.1% Triton X-100, 200 μM (each) deoxynucleotide triphosphate, 2.5 U of Taq DNA polymerase (Merck, USA), 20 pmol of primers, and 5 μl of extracted DNA sample. T. annulata DNA (previously isolated from the blood of naturally infected cattle) and distilled water (without DNA) were run during every PCR amplification as positive and negative controls, respectively. DNA amplification was carried out in a thermal cycler (Gene Amp® PCR system 2700, Applied Biosystems Inc., UK). The thermo-profile used by Oliveira et al. (1995) was modified for the present study. An initial denaturing step of 5 min at 94°C was followed by 5 cycles: a denaturing step of 1 min at 94°C, an annealing step of 1 min at 56°C, and an extension step of 1 min at 72°C. These 5 cycles were followed by 30 cycles. Each cycle consisted of a denaturing step of 1 min at 94°C, an annealing step of 1 min at 54°C, and an extension step of 1 min at 72°C. The PCR program ended with a final extension step of 7 min at 72°C. Amplified products were separated by electrophoresis on a 1% agarose gel and visualized under a UV Transilluminator (Biostep, Germany).
Hematological and serological analyses
Blood glucose concentration was measured by using ACCU-CHEK® Active blood glucose meter (Roche, Germany) while hemoglobin was determined by using Metertek SP-8SO spectrophotometer (Korea) and Randox Laboratories Ltd. kits (UK). For the determination of serum biochemical activity, the blood samples were centrifuged at 13,000 rpm for 10 min to separate the serum and stored at −20°C until use. Serological biochemical parameters including cholesterol, aspartate transaminase (AST), alanine transaminase (ALT), and lactate dehydrogenase (LDH) were determined by using APEL PD-303S spectrophotometer (Japan) and diagnostic kits manufactured by Spinreact, Spain, following their user’s manuals.
Statistical analysis
Animals were grouped into two age categories: less than 1 year to 1 year (calf) and more than 1 year old (adult). Herds were divided into two size categories: herds having 1–15 animals and herds with more than 15 animals. Also, herds were divided according to their composition into three categories: herds with cattle only, herds with buffalo only, and herds with cattle and buffalo together. The absence or presence of ticks on cattle, buffalo, calves, and dogs associated with the herds was also recorded. The association between the presence (positive and negative blood samples) of T. annulata and the various parameters, i.e., herd location, herd size, species, gender and age of the animals, and absence or presence of ticks on cattle, buffalo, calves, and dogs in the herd were assessed by contingency table analysis using Fisher’s exact test (for 2 × 2 tables). The association of parasite prevalence with herd composition and herd location was determined by one-way analysis of variance (ANOVA). Similarly, one-way ANOVA was also calculated for parasite prevalence and various blood and serological parameters (glucose, hemoglobin, cholesterol, ALT, AST, and LDH) in calves, cattle, and buffaloes. All the values are expressed as means ± standard deviations. Mini Tab (version 16) was used for statistical analysis.
Results
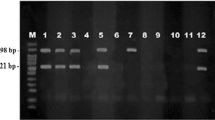
PCR results showed that 28 (19%) out of 144 examined ruminant blood samples, collected from six districts of Southern Punjab, produced the 721-bp fragment specific for T. annulata. The 28 parasite-positive blood samples included 17 cattle and 11 buffalo samples. On the other hand, only 4 (3%) of 144 blood samples were found parasite positive during microscopic examination of Giemsa-stained blood smears. Out of the six districts, five (83%) had ruminant samples positive for T. annulata, and the parasitic prevalence varied between 10% and 41% (Table 1).
Statistical analysis of the characteristics of the animals showed that age (P = 0.02) and presence of ticks on animals (P = 0.02) may play an important role in the spread of theileriosis as animals less than 1 year old and those having ticks present on them were more infected by the parasite. Regarding the characteristics of herds, results indicated that the presence of ticks on dogs associated with herds had a significant correlation with increasing parasitic prevalence (P = 0.05) (data not shown).
Blood and serum biochemical parameters, such as hemoglobin, cholesterol, ALT, AST, and LDH, significantly varied between parasite-positive and parasite-negative blood samples, except for blood glucose levels, indicating that the presence of T. annulata, being a blood parasite, affects the blood biochemistry of the host (Tables 2 and 3).
Discussion
The prevalence, morbidity, and mortality of tropical theileriosis are considerably high. It is estimated that 250 million cattle in many countries including Iran, Turkey, India, and China are at a risk of the disease, which causes serious economic losses through bovine mortality and productivity losses (Hasanpour et al. 2008). It is the demand of time to develop sensitive tools for the effective detection and drugs for the treatment of Theileria sp. in order to decrease the economic losses by the parasite. In the present study, the major merozoite surface antigen gene of the parasite (Tams 1) was amplified by PCR. The Tams 1 gene is the most abundant and immuno-dominant antigen on the surface of merozoites (asexual blood stage) of T. annulata, having a molecular mass of approximately 30 kDa (Rady et al. 2010). This gene has been targeted by several previous studies for the molecular detection of T. annulata in cattle and buffaloes (Oliveira et al. 1995; Kirvar et al. 1999; Dumanli et al. 2005; Durrani et al. 2010). The parasite was present in five out of six districts included in the study. Vehari was the only district where the parasite was absent. The highest prevalence of the parasite was recorded in Bhakar (41%) while the lowest prevalence was found in Layyah (10%) (Table 1).
Several conventional and modern methods are used for the detection of Theileria sp. in host animals. The traditional one is by microscopic examination of blood smears stained with Giemsa. This technique is usually adequate for detection of acute infections, but not for detection of carrier animals, where parasitemia may be low (Altay et al. 2008). Several studies documented that PCR is more specific and sensitive than conventional techniques for the detection of carrier ruminants having Theileria sp. present in the blood without any apparent signs of theileriosis (Oliveira et al. 1995; Martin-Sanchez et al. 1999; Kirvar et al. 1999). We had similar experience as the prevalence of T. annulata detected through PCR was 19% to 3% (N = 4) parasitic detection by microscopic examination of Giemsa-stained blood smears. Furthermore, the four blood samples were also found to be parasite positive by PCR. Only the microscopic examination of PCR-positive samples would have declared them parasite free. A similar comparison was made by Durrani et al. (2010) in Sahiwal (Pakistan) and found 23% prevalence for T. annulata in cattle by PCR as compared to 5% prevalence detected by blood smear examination.
Analysis of data revealed that calves (33%) were more infected by T. annulata as compared to adult animals (14%) (P = 0.02). Further analysis showed that the calves of buffaloes (50%) were more prone to theileriosis than their adults (10%) (P = 0.01). This result was in accordance with the findings of Qayyum et al. (2010) who found a high incidence of T. annulata in calves as compared to adult cattle in various livestock farms of Sahiwal, Pakistan. The presence of ticks on animals proved to be an important risk factor for the spread of theileriosis during the present study. Ticks were found on 34% of the infected animals (P = 0.02). The incidence of theileriosis due to tick infestation was more important in buffaloes than in cattle during the present study as in cattle, ticks were found on only 12% of the infected animals while 60% of the T. annulata-positive buffaloes had ticks detected on their bodies (P = 0.0009). A similar trend of tick infestation and theileriosis was previously reported by Kosar (1965), Hussain (1980), Rehman et al. (2004), and Ghosh et al. (2007). Results from the present study confirmed that ticks act as a vector for the transmission of Theileria sp. in large ruminants (data not shown here).
The prevalence of T. annulata was higher in large herds (22%) than in small herds (15%) (P = 0.3). A similar association between parasite prevalence and herd size was observed by Theodoropoulos et al. (2006), but their study involved small ruminants. In the present study, the high prevalence of T. annulata in larger herds might be an indication that this parasite spreads more rapidly in places where animals are kept in over-crowded conditions. During the study, prevalence of the parasite was higher in herds having tick-infested dogs (27%) than in herds having dogs without tick infestation (14%) (P = 0.05) which indicates that dogs may act as tick carriers and are indirectly involved in the spread of theileriosis. Criado et al. (2006) detected T. annulata in various animals and showed that the parasite was capable of infecting dogs associated with herds (data not shown).
As T. annulata is a blood parasite, various hematological and serum biochemical parameters were measured in all large ruminant samples. Blood hemoglobin and glucose values were reduced in infected calves and cattle as compared to the parasite-free ones (Tables 2 and 3). These observations were similar to those of Yadav and Sharma (1986), Geerts et al. (2001), Madder and Taeymans (2001), Col and Uslu (2007), Hasanpour et al. (2008), and Ananda et al. (2009). The decrease in blood glucose concentration could be due to the utilization of glucose by parasites and damage to the liver in large ruminants infected with T. annulata.
Serum AST and ALT concentrations are the indicators of hepatic function (Forsyth et al. 1999). The serum ALT concentration of infected calf samples was lower than that of parasite-negative calves while the serum ALT concentration of infected cattle was higher as compared to that of non-infected cattle. The level of serum AST in infected calves and cattle was higher as compared to that of the healthy ones (Tables 2 and 3). Similar findings were observed by Col and Uslu (2007) who reported an increase in ALT concentration in Theileria-infected cattle and by Hasanpour et al. (2008) who detected elevated AST and ALT levels in Theileria sp.-positive buffaloes. In the present study, the increase in ALT and AST levels in infected animals compared with healthy animals might indicate hepatic dysfunction in parasite-positive animals.
In infected calves, the serum cholesterol concentration was lower as compared to that of parasite-negative blood samples (Table 2). A similar result was reported by Singh et al. (2001). This significant decrease in cholesterol levels can be ascribed to anorexia and diarrhea (Col and Uslu 2007). Contrary to the above-mentioned finding, the serum cholesterol concentration of infected cattle was higher as compared to that of non-infected cattle (Table 3). Yadav and Sharma (1986) reported a similar increase of serum cholesterol and argued that this is due to liver damage that results in a concurrent increase in the level of fats with the reduction of sugar and protein. The serum LDH concentrations of infected calves and cattle were found to be higher than parasite-negative calves and cattle (Tables 2 and 3). Similar results were reported by Nazifi et al. (2008) who had observed an increase in LDH concentration in cattle suffering from theileriosis. They suggested that the increased activity of LDH is probably due to vascular thrombosis, hemorrhage, and tissue breakdown especially in the liver and kidney of infected animals.
References
Altay K, Aydin MF, Dumanli N, Aktas M (2008) Molecular detection of Theileria and Babesia infections in cattle. Vet Parasitol 158:295–301
Ananda KJ, D’Souza PE, Puttalakshmamma GC (2009) Prevalence of haemoprotozoan diseases in crossbred cattle in Bangalore north. Vet World 2:15–16
Bazarusanga T (2008) The epidemiology of theileriosis in Rwanda and implications for the control. PhD thesis, Dept. Vet Med, Ghent University, Rwanda
Bhoora R, Franssen L, Oosthuizen MC, Guthrie AJ, Zweygarth E, Penzhorn BL, Jongejan F, Collins NE (2009) Sequence heterogeneity in the 18S rRNA gene within Theileria equi and Babesia caballi from horses in South Africa. Vet Parasitol 159:112–120
Col R, Uslu U (2007) Changes in selected serum components in cattle naturally infected with Theileria annulata. Bull Vet Inst Pulawy 51:15–18
Criado A, Martinez AJ, Buling A, Barba JC, Merino S, Jefferies R, Irwin PJ (2006) New data on epizootiology and genetics of piroplasms based on sequences of small ribosomal subunit and cytochrome b genes. Vet Parasitol 142:238–247
Das G, Ghosh S, Ray DD (2005) Reduction of Theileria annulata infection in ticks fed on calves immunized with purified larval antigens of Hyalomma anatolicum anatolicum. Trop Anim Health Prod 37:345–361
Dumanli N, Aktas M, Cetinkaya B, Cakmak A, Koroglu E, Saki CE, Erdogmus Z, Nalbantoglu S, Ongor H, Simsek S, Karahan M, Altay K (2005) Prevalence and distribution of tropical theileriosis in eastern Turkey. Vet Parasitol 127:9–15
Durrani AZ, Kamal N, Khan MS (2006) Incidence of theileriosis and estimation of packed cell volume, total erythrocyte count and hemoglobin in buffaloes. J Anim Pl Sci 16:85–88
Durrani AZ, Mehmood N, Shakoori AR (2010) Comparison of three diagnostic methods for Theileria annulata in Sahiwal and Friesian cattle in Pakistan. Pak J Zool 42:467–472
Forsyth LMG, Minns FC, Kirvar E, Adamson RE, Hall FR, McOrist S, Brown CGD, Preston PM (1999) Tissue damage in cattle infected with Theileria annulata accompanied by metastasis of cytokine producing, schizont-infected mononuclear phagocytes. J Comp Pathol 120:39–57
Geerts S, Holmes PH, Diall O, Eisler MC (2001) African bovine theileriosis: the problem of drug resistance. Trends Parasitol 17:25–28
Ghosh S, Bansal GC, Gupta SC, Ray D, Khan MQ, Irshad H, Shahiduzzaman M, Seitzer U, Ahmed JS (2007) Status of tick distribution in Bangladesh, India and Pakistan. Parasitol Res 101:207–216
Gubbels MJ, d’Oliveira C, Jongejan J (2000) Development of an indirect Tamsl enzyme-linked immunosorbent assay for diagnosis of Theileria annulata infection in cattle. Clin Diagn Lab Immunol 7:404–411
Hasanpour A, Moghaddam GA, Nematollahi A (2008) Biochemical, hematological, and electrocardiographic changes in buffaloes naturally infected with Theileria annulata. KJ Parasitol 46:223–227
Hussain SI (1980) Studies of ectoparasites of livestock of Sindh. Final Tech Report. Univ Sindh, Jamshoro, Sindh
Kirvar E, Ilhan T, Katzer F, Hooshmand-Rad P, Zweygarth E, Gerstenberg C, Phipps P, Brown CGD (1999) Detection of Theileria annulata in cattle and vector ticks by PCR using the Tams1 gene sequences. Parasitology 120:245–254
Kosar MH (1965) Taxonomy and bionomics of the species of genus Rhipicephalus. MSc Thesis, Dept Vet Parasitol, Univ Agri, Faisalabad, Pakistan
Livestock Census (1996) Data on Punjab Livestock, Livestock Census Organization, Govt. of Punjab
Madder M, Taeymans J (2001). Merogony in vitro cultures of Theileria parva. In: The 18th International Conference of the World Association for the Advancement of Vet. Parasitology in Italy. pp. 26–30
Martin-Sanchez J, Viseras J, Adroher FJ, Garcia-Fernandez P (1999) Nested polymerase chain reaction for detection of Theileria annulata and comparison with conventional diagnostic techniques: its use in epidemiology studies. Parasitol Res 85:243–245
Nazifi S, Razavi SM, Mansourian M, Nikahval B, Moghaddam M (2008) Studies on correlations among parasitaemia and some hemolytic indices in two tropical diseases (theileriosis and anaplasmosis) in Fars province of Iran. Trop Anim Health Prod 40:47–53
Oliveira DC, Van Der Weide M, Habela MA, Jacquiet P, Jongejan F (1995) Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol 33:2665–2669
Qayyum M, Farooq U, Samad HA, Chauhdry HR (2010) Prevalence, clinicotherapeutic and prophylactic studies on theileriosis in district Sahiwal (Pakistan). J Anim Plant Sci 20:266–270
Rady AA, Ahmed LS, Mohamed A, Hosary AA (2010) Using polymerase chain reaction (PCR) for diagnosis of bovine theileriosis in upper Egypt. IJAVMS 4:67–74
Rehman WU, Khan IA, Qureshi AH, Hussain S (2004) Prevalence of different species of ixodidae (hard ticks) in Rawalpindi and Islamabad. Pakistan J Med Res 43
Shaikh R, Ramzan K, Nazil S, Sattar S, Khan SN, Raizuddin S, Ahmed ZM, Friedman TB (2005) A new locus for nonsyndromic deafness DFNB51 maps to chromosomes 11p 13-p12. Am J Med Genet A 138:295–392
Singh A, Singh J, Grewal AS, Brar RS (2001) Studies on some blood parameters of crossbred calves with experimental Theileria annulata infection. Vet Res Commun 25:289–300
Soulsby EJL (1982) Helminths, arthropods and protozoa of domesticated animals. Bailliere, London
The Center for Food Security and Public Health (2005) Animal disease fact sheet. The Center for Food Security and Public Health, Iowa State University
Theodoropoulos G, Gazouli M, Ikonomopoulos JA, Kantzoura V, Kominakis A (2006) Determination of prevalence and risk factors of infection with Babesia in small ruminants from Greece by polymerase chain reaction amplification. Vet Parasitol 135:99–104
Yadav CK, Sharma NN (1986) Changes in blood chemical components during experimentally induced Theileria annulata infections in cattle. Vet Parasitol 21:91–98
Acknowledgments
This project was financed by the Directorate of Research and External Linkages, Bahauddin Zakariya University, Multan (Pakistan). The authors would like to thank all the veterinarians for their kind help during sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahnawaz, S., Ali, M., Aslam, M.A. et al. A study on the prevalence of a tick-transmitted pathogen, Theileria annulata, and hematological profile of cattle from Southern Punjab (Pakistan). Parasitol Res 109, 1155–1160 (2011). https://doi.org/10.1007/s00436-011-2360-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2360-1