Abstract
There are 48 members of the GP63 protease family in Trichomonas vaginalis according to our annotations; 37 of them are predicted to be transmembrane proteins. Because the GP63 protease family is the largest surface protease family and the second largest surface protein family, they are most likely to be involved in the interactions between T. vaginalis and the host cell surfaces, or otherwise participate in infection. We performed a preliminary study on the functions of GP63 in T. vaginalis (TvGP63). We demonstrated the cell surface localization of one highly expressed member of TvGP63 using indirect immunofluorescence assays in both isolate T016 and isolate 30236. The specific inhibitor of TvGP63 protease, 1,10-phenanthroline, was found to significantly inhibit the destruction of HeLa cells, whereas another chelator, EDTA, could not. Further tests showed that 1,10-phenanthroline did not inhibit the adherence of T. vaginalis cells to HeLa cells. The results presented in here suggest that GP63 protease plays a vital role in T. vaginalis infection process, but may not be related to the adherence of parasitic cells to their hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichomonas vaginalis is the major causative agent of non-viral vaginitis, which is a sexually transmitted disease in humans, and there are more than 170 million cases reported worldwide every year. Women who are infected with T. vaginalis will not only get vaginitis but may also have a higher risk of premature delivery, low birth rate, atypical pelvic inflammatory disease, infertility, predisposition to developing invasive cervical cancer, and increased susceptibility to HIV infection (Minkoff et al. 1984; Cotch et al. 1997; Zhang and Begg 1994; Kharsany et al. 1993; Grodstein et al. 1993; Yap et al. 1995; Sorvillo and Kerndt 1998; Sorvillo et al. 2001). T. vaginalis can infect the urogenital tracts of both sexes. In men, it can cause non-gonoccogal urethritis and chronic prostatitis (Soper 2004). The mechanisms of the disease caused by T. vaginalis have become a hot topic of study in recent years.
In 1985, Krieger et al. (1985) reported that the cytopathogenic mechanism that T. vaginalis uses is contact-dependent, where adherence to a cell is the first step leading to cytotoxicity. Further experiments have indicated that adherence is also cell-specific and species-specific. T. vaginalis can adhere to human vaginal epithelial cells (hVECs) and produce cytotoxic effects, but neither adherence nor cytotoxicity has been observed when T. vaginalis is exposed to human vaginal fibroblasts or bovine vaginal epithelial cells. Similarly, the bovine parasite Tritrichomonas foetus had no cytotoxic effects on hVECs (Gilbert et al. 2000). Four proteins—AP65, AP51, AP23, and AP33—have been reported to be adhesins that bind T. vaginalis to its host cells. Antibodies against these adhesins can inhibit the binding of live parasites to vaginal epithelial cells and protect vaginal epithelial cells from contact-dependent cytotoxicity (Arroyo et al. 1992). As a result, subsequent research activities have mostly focused on AP65, which has been found to be a primary adhesin. Antisense silencing of the AP65 gene resulted in lower levels of adherence of parasitic cells to vaginal epithelial cells (Mundodi et al. 2004). In addition to the presence of the four adhesins, cysteine proteinase (CP) activity is also necessary for cytoadherence (Arroyo and Alderete 1989). TVCP30 and TVCP62, in particular, have been reported to be related to cytoadherence (Arroyo and Alderete 1995; Hernandez et al. 2004; Mendoza-Lopez et al. 2000).
There have been numerous studies about how T. vaginalis causes diseases, although little is clearly illustrated yet. T. vaginalis lives as a parasite and utilizes nutrients from its host for survival, which is considered to be the ultimate consequence of pathogenesis. It hydrolyzes organic components from the mucus and extracellular matrix of its host cells into glucose, which is then transported into the parasitic cells (Asami and Nakamura 1955; ter Kuile and Muller 1995) where nucleotides from the host are used for DNA synthesis (Gonzalez-Lazaro et al. 2005). We assume that cell surface proteins are probably critical for preliminary interactions between parasites and mucosa/extracellular matrices and thus may be related to subsequent cytotoxicity. In this study, we performed differential expression analyses on surface proteins from three major developmental stages of T. vaginalis—trophozoite, pseudocyst, and ameboid—and found that GP63 proteases appeared differentially expressed. As the largest surface protease family and the second largest surface protein family in T. vaginalis (Hirt et al. 2007), a TvGP63 has been previously noted as a possible factor (Carlton et al. 2007) and always participates in the pathogenesis of protozoan parasites.
GP63 proteases were first detected in Leishmania. According to crystal structure analyses of GP63 proteases in Leishmania major, they are zinc metallopeptidase with a zinc-binding motif of HEXXH (Joshi et al. 2002). The primary function of GP63 protease proteolytic activity is related to the cleavage of host cell macromolecules, either for self-protection or to provide nutrition for the parasite (Chaudhuri et al. 1989b). GP63 proteases are the major pathogenic agent of Leishmania spp. (Joshi et al. 2002) and play an important role in host–parasite interactions (Bouvier et al. 1995). Evidence has suggested that GP63 proteases facilitate the attachment of the promastigote form of the parasite to host cell surface receptors, such as the fibronectin receptor (Chang and Chang 1986; Russell and Wright 1988; Soteriadou et al. 1992). In addition, GP63 proteases interfere with the host complement system (Russell 1987) and facilitate the survival of the amastigote form inside macrophages (Chaudhuri et al. 1989b; McGwire and Chang 1994; Seay et al. 1996). GP63 proteases have also been reported to play a role in the pathogenicity of two other parasites, Trypanosome cruzi and Trypanosome brucei. T. brucei GP63 proteases are predominantly expressed in the bloodstream from of the parasite and may protect bloodstream trypanosomes against complement-mediated lysis by imparting protein-processing capabilities to T. brucei surface. Neutralization assays have indicated that anti-GP63 serum significantly inhibits infection by T. cruzi (El-Sayed and Donelson 1997; Cuevas et al. 2003; Kulkarni et al. 2009).
We performed protease domain comparisons among all related species, and the results indicated that TvGP63 is quite different from the GP63 of other species and has the shortest protease domain. It also lost the glycosylphosphatidylinositol (GPI) structure at its C-terminus, which attaches to cell surface proteins in organisms such as trypanosomatids (Englund 1993). However, the results of recent genome sequencing have demonstrated that T. vaginalis is a very special species where a great number of GP63 genes were found, which bear very little similarity to their orthologs (Carlton et al. 2007), although this does not necessarily mean that the functions of these genes have changed. We assume that the function of GP63 proteases in the cytotoxicity of T. vaginalis has been preserved and then performed the following assays. We used the specific inhibitor of GP63 protease, 1,10-phenanthroline (Chaudhuri et al. 1989a; Seay et al. 1996), in our series of experiments, hoping to collect useful information about the function of TvGP63. We discovered that GP63 protease participated in the destruction of HeLa cells, but found that the inhibition of GP63 protease did not affect the adherence of parasite cells to HeLa cells.
Materials and methods
Parasite culture
We obtained the T. vaginalis T016 isolate from Prof. J. F. Alderete and the T. vaginalis 30236 isolate from Chang Gung University. T. vaginalis T016 was cultured in Diamond’s trypticase–yeast extract maltose medium (TYM) with 10% heat-inactivated horse serum for 36 h at 37°C. T. vaginalis 30236 was cultured in TYM with 10% heat-inactivated horse serum for 24 h at 37°C. Cultures in the logarithmic-growth phase were used for all assays.
Annotation and classification of GP63 protease in T. vaginalis
We annotated 48 proteins to be GP63 proteases using HmmPfam (Krogh et al. 1994) based on the 69 proteins that have been previously identified as GP63 or leishmanolysin by TIGR. HmmerPfam compares one or more sequences to the pfam database of profile hidden Markov model of GP63 in order to identify known domains within the sequences. The gathering threshold of the local search model was used as the cutoff score.
We built a phylogenic tree from the 48 proteins using the neighbor-joining (NJ) method in Clustal W (http://www.ch.embnet.org/software/ClustalW.html). The robustness of the phylogenic inferences was tested via bootstrapping, using 100 replications of the data based on 50% majority rule consensus. Sequence identity values were calculated using the software MEGA 3.1.
Differential expression analysis
We used three expressed sequence tag (EST) libraries that represented the three main developmental stages of T. vaginalis—trophozoite, pseudocyst, and ameboid (http://tvxpress.cgu.edu.tw/)—for our differential expression analysis. Paired comparisons of gene expression levels were carried out between the three libraries using IDEG6 (Romualdi et al. 2003) and the standard p < 0.05. Because surface proteins are the most probable proteins to be involved in host–parasite interactions, we chose all potential cell surface proteins as candidates for analysis. Transmembrane proteins were annotated using TMHMM and Phobius (Kall et al. 2007). We sought to verify the differential expression results of TvGP63-b, but failed due to the fact that the EST level of TvGP63 was too low to be verifiable through RT-PCR.
Gene expression and antibody generation
The sequence of tvgp63 gene was obtained from isolate T016 based on PCR amplification (forward primer: ATGATATTGTCTCTACTCCT; reverse primer: CTAAATCGATAAATTTGCAT; designed according to the gene TVAG_367130 based on TIGR ID in isolate G3). The sequence was submitted to GenBank (GenBank ID: GU356538). As characterized by the result of Protscale antigen prediction (http://www.expasy.org/cgi-bin/protscale.pl), we used the hydrophilic segment of the coding sequence between positions 757 and 1749 to generate polyclonal antibodies. This segment was amplified using Pyrobest DNA Polymerase to avoid mutations and inserted between restriction sites NdeI and HindIII of pET-28b. The purified recombinant TvGP63 were injected into rabbits to generate polyclonal antibodies. The antibodies were purified from the anti-serum using ammonium sulfate.
Western blot assays
Approximately 4 × 107 mid-log phase T. vaginalis 30236 cells were collected, washed in PBS (pH 6.2), and lysed in 400 μl lysis buffer (8 M urea, 4% CHAPS) along with the protease inhibitor cocktail (Sigma). The supernatant was centrifuged at 13,000 rpm for 10 min at 4°C to remove insoluble material. After electrophoresis, proteins were transferred to a nitrocellulose membrane, blocked with 5% skim milk in PBS-0.1% Tween 20 (TBST) for 18 h at 4°C, and incubated with the anti-synthetic peptide antibody (anti-TvGP63, at 1:2,000 dilution) for 18 h at 4°C. Membranes were washed five times with PBST, incubated with peroxidase-conjugated secondary antibody (at 1:3,000 dilution; Amersham) for 2 h at 25°C, and developed using the chemiluminescence system (Sigma). Normal rabbit serum was used as negative control (at 1: 3,000 dilution).
Indirect immunofluorescence assays
For the indirect immunofluorescence assays, approximately 1 × 107 cells were fixed with 6 ml 3.5% of paraformaldehyde for 7 min at 37°C. We collected the parasites at low centrifugation speed and suspended the cells in 1 ml 3.5% paraformaldehyde. We added an aliquot of 200 μl onto a gradient microscope slide and air-dried the slides at room temperature for 15 min. The slides were washed in PBS (pH 7.4) and incubated with anti-TvGP63 antibodies (at 1:200 dilution) for 30–45 min at 37°C and washed in PBS again. The parasites were incubated with fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulins (at 1:100 dilution) for 30 min at 37°C and washed. Finally, the parasites were incubated with DAPI (1:2,500 dilution) for 10 min at 37°C and washed. Slides were examined using laser confocal microscopy (Olympus BX51) and photographed.
Antibody inhibition experiment
HeLa cells were cultured in 24-well plate with Dulbecco’s modified minimal essential medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin in 5% CO2 atmosphere at 37°C. Logarithmic growth phase T. vaginalis was collected and washed three times in PBS (pH 6.2). The parasites were divided into three groups. One group was incubated with anti-TvGP63 antibodies at concentrations of 250, 500, 1,000, and 2,000 ng/ml. The second group received the same treatment using unrelated antibodies. The third group was incubated without any antibodies. HeLa cell monolayers were washed with PBS (pH 7.4). Parasite cells were incubated with HeLa cell monolayers in the medium mixture (DMEM/TYM = 2:1) in 5% CO2 atmosphere at 37°C at a ratio of 5:1. The cells were examined 4 h after incubation.
Effect of 1,10-phenanthroline
The mid-log phase parasites were collected and washed three times in PBS (pH 6.2) and divided into three groups. One was treated with 1,10-phenanthroline ranging from 1 to 11 mM; a second was treated with EDTA and the same concentration of 1,10-phenanthroline; the control group was incubated in PBS (pH 6.2) without 1,10-phenanthroline or EDTA. All the incubations were performed at 37°C for 30 min. HeLa cell monolayers were washed with PBS (pH 7.4) and the parasite cells incubated with HeLa cell monolayers in the mixture medium (DMEM/TYM = 2:1) in 5% CO2 atmosphere at 37°C. In the adherence experiment, after 30 min of incubation with HeLa cell monolayers, the unbounded parasites were collected from each well. PBS (100 μl , pH 6.2) was added to each tube to resuspend the parasites and the cells were counted.
Results and discussion
Classification of T. vaginalis GP63
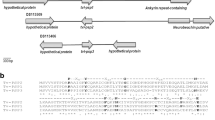
From the three EST libraries (trophozoite, pseudocyst, and ameboid stages), we identified TvGP63, one of the differentially expressed membrane proteins (data not shown). Only a segment of TvGP63, about 70 amino acids long, has the conserved protease motif when compared to GP63 proteases of non-pathogenic protists, pathogenic protists, animals, and plants (Electronic Supplementary Material (ESM) Online Resource 1). Unlike trypanosomatids, T. vaginalis TvGP63 has no GPI anchorage but a transmembrane domain at its C-terminus. In fact, T. vaginalis is special in that many of its genes have very little similarity with their orthologs (Carlton et al. 2007). We used the program HmmPfam to annotate all versions of TvGP63 and found 48 family members. Two newly duplicated groups were detected among TvGP63 proteases: TvGP63-b and TvGP63-c. The remaining sequences were grouped into TvGP63-a because its sequences were more primitive (Fig. 1).
Phylogenic tree of TvGP63. The tree was built using NJ method in clustal W. A sequence of Leishmania major was used as an outgroup. Two groups can be evidently detected, which we named as TvGP63-b and TvGP63-c. The remaining sequences were grouped into TvGP63-a to facilitate description. TvGP63-b can be further divided into two subgroups according to the phylogenic tree: TvGP63-bI and TvGP63-bII. Names for each sequence is formed by the accession number as “TVAG_xxxxxx” and the zinc-binding motif “HEXXH”, with “/” to separate the two parts. If there is no zinc-binding motif, “0” is used
According to the NJ tree of the 48 amino acid sequences, tvgp63 may have experienced five to eight duplication events and form three main groups. The three TvGP63 groups are conserved between amino acid positions 81 and 515 (the amino acid positions mentioned in our paper correspond to the sequence of TVAG_367130), and the conserved sites are mostly centered in the positions immediately behind the zinc atom-binding motif HEXXH (Fig. 2a). There are 90 highly conserved amino acid sites which appear in more than 75% of all TvGP63 sequences (Fig. 2b).
Sequence features of TvGP63 protease family. a General scheme of sequence conservation (heat scale) along the whole amino acid sequence over all TvGP63. The blue spot indicates the zinc-binding motif. The segment from 276AA to 345AA is conserved among the GP63 proteases of other species. b Detailed sequence information among all TvGP63 proteases. Signal peptide (1AA–15AA) and the transmembrane peptide (586AA–610AA) are shaded. The open box highlighted the active protease site, which contains a zinc-binding site. Asterisks indicate positions that are conserved among the three GP63 groups of T. vaginalis. Solid circles above the amino acid sites indicate the sites conserved among all species in our analysis. The underlined segment is what was used to prepare the polyclonal antibody
We investigated gene expression, degrees of conservation, and the potential cellular locations of protein in each group (Table 1 ). There were 11 members of TvGP63-a (out of 22) expressed according to the EST data. However, only six members (out of 13) of TvGP63-b and two members of TvGP63-c (out of 13) were expressed. Among these three groups, the members of TvGP63-b were the most conserved. They had the highest average amino acid identity value, 56.3%, and the lowest average ω value (ω = K a/K s), 0.1230. Also, all members of TvGP63-b had a uniform pattern for their zinc-binding motif HEIFH (Fig. 1), while the motifs of the other two groups varied greatly. Three amino acids in the motif, two H and one E, form catalytic sites. Although the remaining two sites, I and F, do not contribute significantly to protease function, their degree of variation is influenced by the adjacent active sites. In conclusion, TvGP63-b is the group that has evolved most recently and is also the group that has the highest degree of conservation. TvGP63-b also has the largest number of expressed ESTs, 46 in all, while TvGP63-a and TvGP63-c have only 26 and 5, respectively (ESM Online Resource 2). Coincidentally, the differentially expressed member of TvGP63 and TVAG_367130 also belong to TvGP63-b.
In order to study the function of TvGP63, we chose to use TVAG_367130 in the following experimental assays. The CDS sequence of TVAG_367130, taken from T016 isolate, was cloned and expressed for preparing antibodies. The cloned TVAG_367130 from T016 isolate resembles its homolog from G3 isolate (nucleotide acid identity value, 96.9%) more than the TvGP63-b sequences from isolate G3, and the observation suggests that TvGP63 homologs from different T. vaginalis isolates may also be quite conserved. We paid particular attention to TvGP63-b and further divided TvGP63-b into two subgroups according to the phylogenetic tree, TvGP63-bI and TvGP63-bII (Fig. 1). TVAG_367130 and six other members constituted TvGP63-bI, and the average amino acid identity value among the seven members was 75.6%. TVAG_367130 expressed the largest number of ESTs in the trophozoite form. Its potential full length is 630AA, and TMHMM and Phobius predicted it to be a transmembrane protein, with its C terminal embedded in the membrane (Fig. 2b). With a combination of differential expression, high mRNA levels, and high degrees of conservation, TVAG_367130 is qualified to be our antigen for antibody production to study cellular location and function, representing GP63 proteases of T. vaginalis.
Expression and localization of TvGP63
We used the specific rabbit polyclonal IgGs against TVAG_367130 to test GP63 protease expression. Before doing this, the sensitivity and specificity of the anti-TVAG_367130 serum was tested, and we observed a single major band in the blot (Fig. 3c and ESM Online Resource 3). The region cloned and expressed to prepare antibodies only has an average amino acid similarity value of 45.1% with the remaining 47 members (49.0% with members of TvGP63-bII and 79.3% with the remaining six members of TvGP63-bI). Therefore, the members of TvGP63-bI would be the most likely sequences to interfere in Western blotting. Although we saw other minor bands in the blot, they are most likely unprocessed and degraded forms of TVAG_367130.
Demonstration of the presence and localization of TvGP63. a Indirect immunofluorescence assays on the trophozoite form of isolate T016. The parasites were probed with antibody against TvGP63: FITC (a), DAPI (b), and FITC + DAPI (c). b Indirect immunofluorescence assays of the trophozoite form of isolate 30236. The phase contract images are shown in a–e and the indirect immunofluorescence on the right. The same antibody was used for T016. Scale bar, 100 μm. c Result of Western blotting. Polyacrylamide gel (15%) SDS-PAGE was used for electrophoresis. Total protein (10 μg) was loaded per lane. The lanes show (from the left): the blot, Tv protein extract, the size markers, and the extract stained with Coomassie brilliant blue
Proteins which are localized on the cell surface always have greater opportunities to participate in interactions between parasites and their hosts, and this is why we chose membrane proteins as the subjects of our differential expression analysis. We first used TMHMM and Phobius to predict transmembrane proteins and then chose those proteins that were differently expressed. TvGP63 was such a protein. To verify the cellular localization of TvGP63, we carried out indirect immunofluorescence assays on fixed parasites using the specific rabbit antibody against TVAG_367130. TvGP63 was detected on the plasma membranes of both the T016 (Fig. 3a) and 30236 isolates (Fig. 3b).
Patches of TvGP63 were clearly detected on the cell surface of isolate 30236, and a clear outer ring was detected in isolate T016. We used the trophozoite form of the two isolates, T016 and 30236, and both assays were able to detect the cell surface localization of TvGP63, and also discovered TvGP63 in the cytosol. We considered this result to be normal since all proteins are processed in the cytosol before moving to their final positions. In conclusion, the results presented here indicate that TvGP63 was expressed in both T016 and 30236 isolates and also localized on their cell surfaces.
Effect of 1,10-phenanthroline
In order to study the function of GP63 protease in T. vaginalis, we utilized antibodies against the highly expressed GP63 gene to inhibit trichomonal cytotoxicity. It was found that antibodies against TvGP63 and the control group that was given unrelated antibodies against a human protein can at most delay trichomonal cytotoxicity for about 2 h even at the highest concentration of 2,000 ng/ml in our assays. On the other hand, antibodies against TvGP63 failed to make any significant difference compared to the control group in time delay. This result was really discouraging, but may still have been acceptable considering the following reasons. First, there are 48 members of the GP63 protease family in T. vaginalis, so antibodies against only one TvGP63 may have very limited influence and may need to be present in very large quantities to have a significant inhibitory effect on T. vaginalis’ cytotoxicity. Second, there were two kinds of TvGP63 according to our predictions—some may have been located on cell membranes (37 members are potential membrane proteins) and others may have been secreted out of the parasite cells (11 members are potential secreted proteins). Experimental evidences have suggested that soluble extract antigen of T. vaginalis isolates from the symptomatic women have cytotoxic activity (Malla et al. 2008). We do not have enough knowledge concerning which kinds of GP63 are effectively involved in the parasite cytotoxicity, and since there are so many members of the GP63 family, studies based only on one specific TvGP63 may not really be able to generate any significant results. We changed our tactics and chose to try to study all members of TvGP63. A specific inhibitor of the GP63 family, which can inhibit all TvGP63, 1,10-phenanthroline (Chaudhuri et al. 1989a; Seay et al. 1996), was chosen as the most favorable reagent for studying the functions of the GP63 protease family in T. vaginalis.
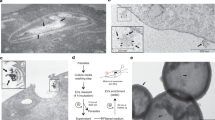
In the following experiments, we found that the use of the GP63 inhibitor 1,10-phenanthroline could prevent the destruction of HeLa cells by T. vaginalis to a significant degree (Fig. 4). We used different concentrations of 1,10-phenanthroline (ranging from 1 to 11 mM) and the same concentrations of a negative control (EDTA) to perform inhibition assays. We observed that the cytotoxicity of T. vaginalis could be inhibited by 1,10-phenanthroline at a concentration of 1 mM, while EDTA was not able to inhibit cytotoxicity, even at a concentration of 11 mM (data not shown). We continued observing the cells for more than 12 h and found that the inhibitory effect of 1,10-phenanthroline was sustained without killing the parasites. Our results suggested that GP63 may participate in the killing of HeLa cells.
Results from inhibition experiments. a Intact monolayer without T. vaginalis. b Group treated with the inhibitor 1,10-phenanthroline (5 mM). c The mock group. d Group treated with 5 mM EDTA. Arrowheads in b and c show the clumps formed by the parasites, and this is considered as the indicator of cytotoxicity. The images were captured 4 h after adding parasites to the HeLa cell monolayers; more than 50% of the HeLa monolayers were destructed (c, d)
Does GP63 facilitate the adherence of parasite cells to HeLa cells? To address this question, we incubated the parasites with 5 mM 1,10-phenanthroline. We did not observe differences between the test and control (ESM Online Resource 4; the parasite-to-Hela cell ratio is 5:1). The p value of a t test on adhered parasite number of the two groups was 0.978, which is >0.05 (Fig. 5). As a result of our experiments, we drew a preliminary conclusion that TvGP63 may not influence the adherence of the parasites to HeLa cells. We observed that T. vaginalis cells remained alive after both incubations, and because our adherence evaluation experiment was carried out in quite a short time, the results were unlikely to have been influenced by the growth of T. vaginalis.
Results from adherence assays. Two groups of parasitic cells with different treatments were used. The inhibited group was incubated with 5 mM 1,10-phenanthroline and the control group was mock-treated in PBS. The y-axis displays the number of T. vaginalis cells that adhered to HeLa monolayer. Eleven pairs of data were used. The detailed number for each group is supplied in ESM Online Resource 4
Based on previous studies, it was thought that the parasites need protease in order to hydrolyze the mucosa and extracellular matrix proteins of host cells. However, the mechanisms of how the protease actually works have remained unclear. Surface protease is thought to be the type of protease most likely to be involved in parasite–host interactions. Through our preliminary study of the function of TvGP63, we came to the conclusion that TvGP63 is likely necessary for the cytotoxicity of T. vaginalis, but may not influence cytoadherence. Whether TvGP63 has any relationship with mucus or extracellular matrix hydrolysis needs to be examined further. Many other questions need further investigation as well, such as how secreted GP63 protease and surface GP63 protease differ in function and how they may interact with other surface proteins in T. vaginalis or with a host cell’s surface coating. Because of the great variety of TvGP63 in existence, and the limitations of our experiments, we were not able to generate very elaborate findings. Nevertheless, this preliminary study furthers our understanding of the pathogenesis of T. vaginalis, and we hope that this will inspire more in-depth studies.
References
Arroyo R, Alderete JF (1989) Trichomonas vaginalis surface proteinase activity is necessary for parasite adherence to epithelial cells. Infect Immun 57:2991–2997
Arroyo R, Alderete JF (1995) Two Trichomonas vaginalis surface proteinases bind to host epithelial cells and are related to levels of cytoadherence and cytotoxicity. Arch Med Res 26:279–285
Arroyo R, Engbring J, Alderete JF (1992) Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol 6:853–862
Asami K, Nakamura M (1955) Experimental inoculation of bacteria-free Trichomonas vaginalis into human vaginae and its effect on the glycogen content of vaginal epithelia. Am J Trop Med Hyg 4:254–258
Bouvier J, Schneider P, Etges R (1995) Leishmanolysin: surface metalloproteinase of Leishmania. Methods Enzymol 248:614–633
Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UC, Besteiro S, Sicheritz-Ponten T, Noel CJ, Dacks JB, Foster PG, Simillion C, Van de Peer Y, Miranda-Saavedra D, Barton GJ, Westrop GD, Muller S, Dessi D, Fiori PL, Ren Q, Paulsen I, Zhang H, Bastida-Corcuera FD, Simoes-Barbosa A, Brown MT, Hayes RD, Mukherjee M, Okumura CY, Schneider R, Smith AJ, Vanacova S, Villalvazo M, Haas BJ, Pertea M, Feldblyum TV, Utterback TR, Shu CL, Osoegawa K, de Jong PJ, Hrdy I, Horvathova L, Zubacova Z, Dolezal P, Malik SB, Logsdon JM Jr, Henze K, Gupta A, Wang CC, Dunne RL, Upcroft JA, Upcroft P, White O, Salzberg SL, Tang P, Chiu CH, Lee YS, Embley TM, Coombs GH, Mottram JC, Tachezy J, Fraser-Liggett CM, Johnson PJ (2007) Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315:207–212
Chang CS, Chang KP (1986) Monoclonal antibody affinity purification of a Leishmania membrane glycoprotein and its inhibition of Leishmania-macrophage binding. Proc Natl Acad Sci USA 83:100–104
Chaudhuri G, Chaudhuri M, Pan A, Chang KP (1989) Surface acid proteinase (gp63) of Leishmania mexicana. A metalloenzyme capable of protecting liposome-encapsulated proteins from phagolysosomal degradation by macrophages. J Biol Chem 264:7483–7489
Cotch MF, Pastorek JG 2nd, Nugent RP, Hillier SL, Gibbs RS, Martin DH, Eschenbach DA, Edelman R, Carey JC, Regan JA, Krohn MA, Klebanoff MA, Rao AV, Rhoads GG (1997) Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis 24:353–360
Cuevas IC, Cazzulo JJ, Sanchez DO (2003) gp63 Homologues in Trypanosoma cruzi: surface antigens with metalloprotease activity and a possible role in host cell infection. Infect Immun 71:5739–5749
El-Sayed NM, Donelson JE (1997) African trypanosomes have differentially expressed genes encoding homologues of the Leishmania GP63 surface protease. J Biol Chem 272:26742–26748
Englund PT (1993) The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem 62:121–138
Gilbert RO, Elia G, Beach DH, Klaessig S, Singh BN (2000) Cytopathogenic effect of Trichomonas vaginalis on human vaginal epithelial cells cultured in vitro. Infect Immun 68:4200–4206
Gonzalez-Lazaro M, Gonzalez-Robles A, Hernandez-Gutierrez R, Arroyo R (2005) HeLa cell nucleus, a source of thymidine for Trichomonas vaginalis growing in vitro. Int J Biochem Cell Biol 37:166–176
Grodstein F, Goldman MB, Cramer DW (1993) Relation of tubal infertility to history of sexually transmitted diseases. Am J Epidemiol 137:577–584
Hernandez H, Sariego I, Garber G, Delgado R, Lopez O, Sarracent J (2004) Monoclonal antibodies against a 62 kDa proteinase of Trichomonas vaginalis decrease parasite cytoadherence to epithelial cells and confer protection in mice. Parasite Immunol 26:119–125
Hirt RP, Noel CJ, Sicheritz-Ponten T, Tachezy J, Fiori PL (2007) Trichomonas vaginalis surface proteins: a view from the genome. Trends Parasitol 23:540–547
Joshi PB, Kelly BL, Kamhawi S, Sacks DL, McMaster WR (2002) Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol Biochem Parasitol 120:33–40
Kall L, Krogh A, Sonnhammer EL (2007) Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res 35:W429–432
Kharsany AB, Hoosen AA, Moodley J, Bagaratee J, Gouws E (1993) The association between sexually transmitted pathogens and cervical intra-epithelial neoplasia in a developing community. Genitourin Med 69:357–360
Krieger JN, Ravdin JI, Rein MF (1985) Contact-dependent cytopathogenic mechanisms of Trichomonas vaginalis. Infect Immun 50:778–786
Krogh A, Brown M, Mian IS, Sjolander K, Haussler D (1994) Hidden Markov models in computational biology. Applications to protein modeling. J Mol Biol 235:1501–1531
Kulkarni MM, Olson CL, Engman DM, McGwire BS (2009) Trypanosoma cruzi GP63 proteins undergo stage-specific differential posttranslational modification and are important for host cell infection. Infect Immun 77:2193–2200
Malla N, Kaul P, Sehgal R, Gupta I (2008) In vitro haemolytic and cytotoxic activity of soluble extract antigen of T. vaginalis isolates from symptomatic and asymptomatic women. Parasitol Res 102:1375–1378
McGwire B, Chang KP (1994) Genetic rescue of surface metalloproteinase (gp63)-deficiency in Leishmania amazonensis variants increases their infection of macrophages at the early phase. Mol Biochem Parasitol 66:345–347
Mendoza-Lopez MR, Becerril-Garcia C, Fattel-Facenda LV, Avila-Gonzalez L, Ruiz-Tachiquin ME, Ortega-Lopez J, Arroyo R (2000) CP30, a cysteine proteinase involved in Trichomonas vaginalis cytoadherence. Infect Immun 68:4907–4912
Minkoff H, Grunebaum AN, Schwarz RH, Feldman J, Cummings M, Crombleholme W, Clark L, Pringle G, McCormack WM (1984) Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol 150:965–972
Mundodi V, Kucknoor AS, Klumpp DJ, Chang TH, Alderete JF (2004) Silencing the ap65 gene reduces adherence to vaginal epithelial cells by Trichomonas vaginalis. Mol Microbiol 53:1099–1108
Romualdi C, Bortoluzzi S, D’Alessi F, Danieli GA (2003) IDEG6: a web tool for detection of differentially expressed genes in multiple tag sampling experiments. Physiol Genomics 12:159–162
Russell DG (1987) The macrophage-attachment glycoprotein gp63 is the predominant C3-acceptor site on Leishmania mexicana promastigotes. Eur J Biochem 164:213–221
Russell DG, Wright SD (1988) Complement receptor type 3 (CR3) binds to an Arg-Gly-Asp-containing region of the major surface glycoprotein, gp63, of Leishmania promastigotes. J Exp Med 168:279–292
Seay MB, Heard PL, Chaudhuri G (1996) Surface Zn-proteinase as a molecule for defense of Leishmania mexicana amazonensis promastigotes against cytolysis inside macrophage phagolysosomes. Infect Immun 64:5129–5137
Soper D (2004) Trichomoniasis: under control or undercontrolled? Am J Obstet Gynecol 190:281–290
Sorvillo F, Kerndt P (1998) Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet 351:213–214
Sorvillo F, Smith L, Kerndt P, Ash L (2001) Trichomonas vaginalis, HIV, and African-Americans. Emerg Infect Dis 7:927–932
Soteriadou KP, Remoundos MS, Katsikas MC, Tzinia AK, Tsikaris V, Sakarellos C, Tzartos SJ (1992) The Ser-Arg-Tyr-Asp region of the major surface glycoprotein of Leishmania mimics the Arg-Gly-Asp-Ser cell attachment region of fibronectin. J Biol Chem 267:13980–13985
ter Kuile BH, Muller M (1995) Maltose utilization by extracellular hydrolysis followed by glucose transport in Trichomonas vaginalis. Parasitology 110(Pt 1):37–44
Yap EH, Ho TH, Chan YC, Thong TW, Ng GC, Ho LC, Singh M (1995) Serum antibodies to Trichomonas vaginalis in invasive cervical cancer patients. Genitourin Med 71:402–404
Zhang ZF, Begg CB (1994) Is Trichomonas vaginalis a cause of cervical neoplasia? Results from a combined analysis of 24 studies. Int J Epidemiol 23:682–690
Acknowledgments
We would like to acknowledge Prof. J. F. Alderete for supplying T016 isolate. We thank Prof. Changqing Zeng, Prof. Zhiyang Dong, Dr. Jie Feng, Mr. Xumin Wang, and Mr. Yanqiang Wang for their help in carrying out certain experiments. We are also grateful to Mr. Joe Yu for his revision of the manuscript. This work is supported by grants from National Science and Technology Key Project (2008ZX1004-013) and 863 Program (2009AA01A130) from the Ministry of Science and Technology of the People’s Republic of China.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Online Resource 1
HmmPfam annotation results. The segment that is annotated as the part of GP63 protease domain is listed as following (TVAG_271800 has reached the cutoff score, but has no annotation of the protease domain because of less similarity to the GP63 protease domain in pfam) (DOC 32 kb)
Online Resource 2
Basic information of TvGP63 (DOC 104 kb)
Online Resource 3
Antiserum test (DOC 69 kb)
Online Resource 4
Adhered T. vaginalis number in the adherence experiment. The initial number of parasite cell adding to each well is 1.2 × 106 (DOC 34 kb)
Rights and permissions
About this article
Cite this article
Ma, L., Meng, Q., Cheng, W. et al. Involvement of the GP63 protease in infection of Trichomonas vaginalis . Parasitol Res 109, 71–79 (2011). https://doi.org/10.1007/s00436-010-2222-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2222-2