Abstract
The life cycle of Ixodes luciae was evaluated for five consecutive generations in the laboratory. Wild mice Calomys callosus and laboratory rats Rattus norvegicus were used as hosts for larvae and nymphs. For adult ticks, opossums Didelphis aurita were used as hosts. Off-host developmental periods were observed in an incubator at 27°C and 95% RH. The life cycle of I. luciae lasted 95–97 days, excluding prefeeding periods. C. callosus, one of the natural host species for I. luciae immature stages, was shown to be much more suitable than the artificial host R. norvegicus. Significantly (P < 0.05), more larvae and nymphs successfully fed on C. callosus than on R. norvegicus. When tick-naïve C. callosus were exposed to three consecutive larval infestations at 24-day intervals, recovery of engorged larvae were greater in the second and third infestations, indicating that previous infestations did not induce acquired resistance to ticks. Larval feeding period typically varied from 5 to 10 days on R. norvegicus, but was significantly (P < 0.05), longer on C. callosus (range, 7–34 days). The majority (71.7%) of I. luciae adult females successfully fed and oviposited after exposed to D. aurita. Mean engorged weight (581.9 mg; range, 237.1–796.0 mg) of these females were much higher than those previously reported for other New World Ixodes species. Our results are in accordance to the current literature that appoints opossums Didelphidae and small rodents (e.g., C. callosus) natural hosts for I. luciae immature and adult stages, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Brazilian tick fauna is currently represented by 61 species, of which only eight belong to the genus Ixodes (Dantas-Torres et al. 2009). At least four of these species, namely Ixodes amarali Fonseca, Ixodes schulzei Aragão and Fonseca, Ixodes loricatus Neumann, and Ixodes luciae Sénevet are morphologically close-related and have several ecological similarities; for this reason, they have been referred as the loricatus group (Barros-Battesti et al. 2007). Generally, immature stages of these four species feed primarily on small rodents (Sigmodontinae) and mouse opossums (Didelphidae), whereas the adult stage (except for I. schulzei) feed primarily on large opossums (Didelphidae; Labruna et al. 2005; Barros-Battesti et al. 2007).
I. luciae has been reported from Argentina to southern Mexico, with records in Argentina, Belize, Bolivia, Brazil, Colombia, Costa Rica, Ecuador, French Guiana, Guatemala, Mexico, Nicaragua, Panama, Peru, Surinam, Trinidad and Tobago, and Venezuela (Jones et al. 1972; Guglielmone et al. 2003). The vast majority of host records for I. luciae refer to adult ticks from large opossums, including species of the genera Didelphis and Philander. Host records for immature stages include the small rodents Calomys callosus (Rengger), Hylaeamys spp., Oecomys bicolor (Tomes), Oligorysomys spp., Orysomys spp., Zygodontomys microtinus (Lund), and the mouse opossums Marmosa sp., Micoureus spp., and Thylamys spp. (Fairchild et al. 1966; Labruna et al. 2005; Autino et al. 2006; Mónica-Díaz et al. 2007).
Among the eight Ixodes species occurring in Brazil, life cycle studies have been reported only for I. loricatus (Schumaker et al. 2000) and partially for I. schulzei (Labruna et al. 2003) and I. amarali (Faccini et al. 1999). Here, we evaluated the life cycle of I. luciae in the laboratory. For this purpose, we used both C. callosus and Rattus norvegicus Berkenhout as hosts for tick immature stages. For adult ticks, opossums Didelphis aurita Wied-Neuwied were used as hosts.
Materials and methods
Ticks used in this study were the progeny of two I. luciae-engorged females collected from an opossum Didelphis marsupialis Linnaeus in the headwaters of the Jamari River, Monte Negro, state of Rondônia, northern Brazil, during a previous study (Labruna et al. 2005). The engorged females were taken to the laboratory and maintained in an incubator at 27°C and 95% RH for egg laying. Egg masses of the two females were pooled, and the resultant hatched larvae were used to start laboratory infestations. We performed infestations with larvae, nymphs, and adults, approximately 30 days old, from five consecutive generations of the I. luciae population in the laboratory. Larval infestations were performed with 150 to 300 larvae per host, whereas nymphal infestations consisted of 12 to 50 ticks per host. Grouping the five generations, infestations by larvae were performed on 16 naive wild mice (C. callosus) and six naive wistar rats (R. norvegicus), and infestations by nymphs were performed on 11 naïve C. callosus and 11 naïve R. norvegicus. For each tick generation, 1 or 2 opossums (D. aurita) with previous natural contact (2 to 6 weeks before) with ticks I. loricatus were used. Infestations by adult ticks were performed with four to ten couples per opossum. Feeding periods of larvae, nymphs, and adults were observed by brushing ticks onto the host's nape around mid-day. All hosts were kept in individual wire cages large enough to allow for host grooming, and nothing was done to prevent selfgrooming. Water and commercial appropriate food were offered ad libitum. Each cage was kept over an aluminum table at least two times longer than the cage, with a double face-foam adhesive tape (19 mm wide, 3 M) attached to the borders to prevent engorged ticks from escaping. Detached engorged ticks were collected and counted daily. The duration of the feeding period was determined by the number of days from placement of the ticks on the hosts to the detachment. During tick feeding the hosts were maintained at a 12:12 photoperiod, using artificial cold light from 7:00a.m. to 7:00p.m. Detached ticks were immediately transferred to the incubator and kept in darkness at 27°C and RH 95%. Lengths of premolt period (number of days from detachment to ecdysis), preoviposition period (number of days from detachment to the beginning of oviposition), and incubation period (number of days from the beginning of oviposition to the hatching of the first larva) were observed daily. Each engorged female and its total egg mass were weighed on the day of detachment and on the day of the end of oviposition, respectively. For the measurement of the metabolic activity of females for the oviposition process, the index of egg production efficiency was determined using the formula: weight of eggs/weight of the engorged female × 100 (Bennett 1974). Percentage of hatching for each female egg mass was visually estimated according to Labruna et al. (2000).
Part of the forth laboratory generation of unfed larvae were used in a parallel trial that evaluated the possible occurrence of acquired resistance by C. callosus. For this purpose, three tick-naïve C. callosus (A, B, and C) were submitted to three consecutive infestations at 24-day intervals on days 0, 24, and 48. A second group of three tick-naïve C. callosus (D, E, and F) were infested on days 24 and 48; and a third group of three tick-naïve C. callosus (G, H, and I) were infested on day 48. In each infestation day (0, 24, or 48), care was taken to use the same batch of larvae to infest all C. callosus of that day. All infestations consisted of 300 larvae per animal. Procedures to perform infestations and recovery of engorged larvae were done as described earlier.
Immature feeding and premolt data were compared using the Student's t test between ticks fed on different host species, and between nymphs that molted to males or females. The numbers of ticks that engorged or successfully molted, after exposed to different host species, were analyzed by the chi-square distribution.
Results and discussion
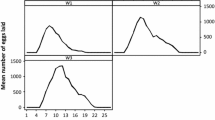
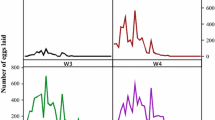
Feeding and premolt periods of larvae and nymphs as well as feeding and reproductive data of females from five consecutive generations of I. luciae were grouped and presented in Tables 1 and 2. Under laboratory conditions, the life cycle of I. luciae lasted 97 days, considering mean feeding periods of immature stages on C. callosus and adults on D. aurita, and excluding prefeeding periods. Using R. norvegicus as hosts for immature stages, life cycle length was 95 days. These values were very similar to previously reported data for I. loricatus under laboratory conditions, for which the life cycle was completed in 98 to 102 days (without prefeeding periods), considering feeding of immature ticks on C. callosus or R. norvegicus, adults on Didelphis spp, and off-host development at 27°C (Schumaker et al. 2000). Grouping the five generations, sex ratio of adult ticks was 1:1.3 (males/females). No significant difference (P > 0.05) was observed between premolt periods of male (mean, 18.9 ± 2.4 days; range, 16–30 days) and female (19.7 ± 2.3 days; range, 17–31 days) nymphs.
C. callosus, one of the natural host species for I. luciae immature stages (Autino et al. 2006), was shown to be much more suitable than the artificial host R. norvegicus. Significantly (P < 0.05), more larvae and nymphs successfully fed on C. callosus than on R. norvegicus. In addition, molting success was significantly (P < 0.05) greater for C. callosus- than R. norvegicus-exposed nymphs (Table 1). When tick-naïve C. callosus were exposed to three consecutive larval infestations at 24-day intervals, recovery of engorged larvae were greater in the second and third infestations (Table 3), indicating that previous infestations did not induce acquired resistance to ticks. Moreover, reinfested C. callosus became more susceptible to I. luciae larvae than tick-naïve animals, since in each set of infestations on days 24 and 48, significantly (P < 0.05) more engorged larvae were recovered from previously infested animals than to tick-naïve ones (Table 3). These findings reinforce the role of C. callosus as a natural host for I. luciae immature stages, and agree with several works reporting ticks to feed more successfully on their natural hosts than on unnatural hosts, even during successive infestations (Randolph 1979; Fielden et al. 1992; Rechav 1992; Labruna et al. 2000).
Many other studies related to biology of Ixodidae ticks, including the genus Ixodes, showed that larvae typically feed for 3 to 13 days on different host species (Neitz et al. 1971; Yonow 1995; Banks et al. 1998a, b; Jacobs et al. 2004; Labruna et al. 2000, 2004; Pinter et al. 2004). In the present study, larval feeding period typically varied from 5 to 10 days on R. norvegicus, but was significantly (P < 0.05) longer on C. callosus (Table 1). On this later host species, nearly 10% of the larvae took ≥20 days to complete feeding during five consecutive generations. Interestingly, longer feeding periods (up to 19 days) were previously observed for I. loricatus larvae exposed to C. callosus than those exposed to R. norvegicus (up to 9 days) (Schumaker et al. 2000). The mechanism and biological significance of such an unusual prolonged larval feeding period remain unknown. However, since C. callosus is one of the natural hosts for both I. luciae and I. loricatus (Nava et al. 2004; Autino et al. 2006), long feeding periods for some larvae on this host species is likely to have biological advantage for the tick. In addition, the longer the ticks remain feeding on a host, the longer they are exposed to pathogens that could potentially infect the vertebrate host.
After being exposed to I. loricatus-previously infested opossums, the majority (71.7%) of I. luciae adult females successfully fed and oviposited (Table 2). Mean engorged weight (581.9 mg) of these females were much higher than those previously reported for I. loricatus (<330 mg) after feeding on Didelphis spp. In fact, at least 14% of the I. luciae-engorged females weighed more than 700 mg, the heaviest female reaching 796 mg. To the best of our knowledge, this is the heaviest weight reported for a New World Ixodes species. Even though we cannot predict how the immune status of the previously infested D. aurita affected our results, indeed our results are in accordance with the current literature that appoints opossums Didelphidae natural hosts for adults of I. luciae (Fairchild et al. 1966; Jones et al. 1972; Labruna et al. 2005; Autino et al. 2006).
I. luciae has been reported to be morphologically and genetically close-related to I. loricatus. Our results bring biological evidence for this relatedness, since larvae, nymphs, and adults behaviored very similar to I. loricatus under laboratory conditions, notably by their similar feeding periods on C. callosus and Didelphis spp, and developmental periods at 27°C and 95% RH. While both species are ecologically similar regarding hosts usage (generally, small rodents for immature stages and opossums for adult ticks), they clearly differ in geographical distribution. For example, I. luciae prevails in the Amazonia (moist and warm throughout the year), whereas, I. loricatus prevails in the Brazilian Atlantic rainforest (moist and cool throughout the year; Labruna et al. 2005). In addition, I. luciae-engorged females are generally much heavier than I. loricatus.
References
Autino AG, Nava S, Venzal JM, Mangold AJ, Guglielmone AA (2006) La presencia de Ixodes luciae en el noroeste argentino y nuevos huéspedes para Ixodes pararicinus y algunas especies de Amblyomma (Acari: Ixodidae). Rev Soc Entomol Argent 65:27–32
Banks CW, Oliver JH Jr, Phillips JB, Clark KL (1998a) Life cycle of Ixodes minor (Acari: Ixodidae) in the laboratory. J Med Entomol 35:496–499
Banks CW, Oliver JH Jr, Hopla CE, Dotson EM (1998b) Laboratory life cycle of Ixodes woodi (Acari: Ixodidae). J Med Entomol 35:177–179
Barros-Battesti DM, Onofrio VC, Faccini JLH, Labruna MB, Arruda-Santos AD, Giacomin FG (2007) Description of the immature stages and redescription of the female of Ixodes schulzei Aragão & Fonseca, 1951 (Acari: Ixodidae), an endemic tick species of Brazil. Syst Parasit 68:157–166
Bennett GF (1974) Oviposition of Boophilus microplus (Canestrini) (Acarina: Ixodidae). Acarologia 16:1652–1661
Dantas-Torres F, Onofrio VC, Barros-Battesti DM (2009) The ticks (Acari: Ixodida: Argasidae, Ixodidae) of Brazil. Syst Appl Acarol 14:30–46
Faccini JLH, Prata MCA, Daemon E, Barros-Battesti DM (1999) Características biológicas da fase não parasitária do Ixodes amarali (Acari: Ixodidae) em gambá (Didelphis sp.) no Estado do Rio de Janeiro. Arq Bras Med Vet Zootec 51:267–270
Fairchild GB, Kohls GM, Tipton V (1966) The ticks of Panama. In: Wenzel RL, Tipton VJ (eds) Ectoparasites of Panama. Field Museum of Natural History, Chicago, pp 167–219
Fielden LJ, Rechav Y, Bryson NR (1992) Acquired immunity to larvae of Amblyomma marmoreum and A. hebraeum by tortoises, guinea pigs and guinea fowl. Med Vet Entomol 6:251–254
Guglielmone AA, Estrada-Peña A, Keirans JE, Robbins RG (2003) Ticks (Acari: Ixodida) of the Neotropical Zoogeographic Region. International Consortium on Ticks and Tick-borne Diseases, Atalanta, Houten, The Netherlands, 173 pp
Jacobs PAH, Fourie LJ, Horak IG (2004) A laboratory comparison of the life cycles of the dog ticks Haemaphysalis leachi and Rhipicephalus sanguineus. Onderstepoort J Vet Res 71:15–28
Jones EK, Clifford CM, Keirans JE, Kokls GM (1972) The ticks of Venezuela (Acarina: Ixodoidea) with a key to the species of Amblyomma in the Western Hemisphere. Brigham Young Univ Biol Ser Sci Bull Biol Ser 17:1–40
Labruna MB, Leite RC, Faccini JLH, Ferreira F (2000) Life-cycle of Haemaphysalis leporis-palustris (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 24:683–694
Labruna MB, Silva MJN, Oliveira MF, Barros-Battesti DM, Keirans JE (2003) New records and laboratory-rearing data for Ixodes schulzei (Acari: Ixodidae) in Brazil. J Med Entomol 40:116–118
Labruna MB, Pinter A, Teixeira RHF (2004) Life cycle of Amblyomma cooperi (Acari: Ixodidae) using capybaras (Hydrochaeris hydrochaeris) as hosts. Exp Appl Acarol 32:79–88
Labruna MB, Camargo LM, Terrassini FA, Ferreira F, Schumaker TTS, Camargo EP (2005) Ticks (Acari: Ixodidae) from the state of Rondonia, western Amazon, Brazil. Syst Appl Acarol 10:17–32
Mónica-Díaz M, Nava S, Venzal JM, Sánchez N, Guglielmone AA (2007) Tick collections from the Peruvian Amazon, with host records for species of Ixodes Latreille, 1795 (Acari: Ixodidae) and Ornithodoros Koch, 1844 (Acari: Ixodidae). Syst Appl Acarol 12:127–133
Nava S, Lareschi M, Beldomenico PM, Zerpa C, Venzal JM, Mangold AJ, Guglielmone AA (2004) Sigmodontinae rodents as hosts for larvae and nymphs of Ixodes loricatus Neumann, 1899 (Acari: Ixodidae). Parasite 11:411–414
Neitz WO, Boughton F, Walters HS (1971) Laboratory investigation on the life cycle of the Karoo Paralysis tick (Ixodes rubicundus Neumann, 1904). Onderstepoort J Vet Res 38:215–224
Pinter A, Dias RA, Gennari SM, Labruna MB (2004) Study of the seasonal dynamics, life cycle, and host specificity of Amblyomma aureolatum (Acari: Ixodidae). J Med Entomol 41:324–332
Randolph SE (1979) Population regulation in ticks: the role of acquired resistance in natural and unnatural hosts. Parasitology 79:141–156
Rechav Y (1992) Naturally acquired resistance to ticks. A global view. Insect Sci Appl 13:495–504
Schumaker TT, Labruna MB, Abel IS, Clerici PT (2000) Life cycle of Ixodes (Ixodes) loricatus (Acari: Ixodidae) under laboratory conditions. J Med Entomol 37:714–720
Yonow T (1995) The life-cycle of Amblyomma variegatum (Acari: Ixodidae): a literature synthesis with a view to modeling. Int J Parasitol 25:1023–1060
Acknowledgements
We thank Pedro Cesar Nogueira da Silva and Thomas Bronhall for technical assistance with animals. This work was supported by Fundação de Amparo a Pesquisa de São Paulo and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Labruna, M.B., Cabrera, R.R. & Pinter, A. Life cycle of Ixodes luciae (Acari: Ixodidae) in the laboratory. Parasitol Res 105, 1749–1753 (2009). https://doi.org/10.1007/s00436-009-1621-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1621-8