Abstract
Intracellular stages of Eimeria tenella reside within a membrane-bound parasitophorous vacuole (PV). PVs of apicomplexan parasites like E. tenella play important roles in nutrient acquisition, multiplication, and evasion of host immune responses. Different signal sequences from apicomplexan parasites were investigated in the transfected E. tenella for their functions in targeting yellow fluorescent protein (YFP) to subcompartments and the dynamic development of the PV of E. tenella was studied. Two 5′ terminal signal sequences derived from Toxoplasma gondii GRA8 protein and Plasmodium falciparum repetitive interspersed family protein, respectively, were confirmed to target YFP to the PVs of the transfected E. tenella, suggesting that signal sequences are functionally conserved among Apicomplexa. Three structurally different types of PVs were observed during the endogenous development of the transfected E. tenella in vitro. In addition, three subcompartments in the PV, namely, membranous extensions into the host cell cytosol, membranous extensions into the vacuolar lumen, and particle-like bodies, were detected during schizogony of the parasite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracellular stages of apicomplexan parasites reside within a membrane-bound parasitophorous vacuole (PV). The membrane of PVs derives from the plasma membrane of host cells and becomes non-fusigenic for lysosomes or any other vacuoles or vesicles as a result of elimination of host cell proteins and incorporation of parasite proteins (Beyer et al. 2002). Specialized secretory organelles, rhoptries, and dense granules of apicomplexan parasites are believed to be involved in the formation and maintenance of PVs soon after microneme-mediated entry into host cells (Carruthers and Sibley 1997; Baum et al. 2006). The maturation pattern of the PV is parasite-specific and differs within the same genera and species and even at different stages of endogenous development (Beyer et al. 2002). Among Apicomplexa, Plasmodium, Toxoplasma, and Eimeria represent three different types of PVs in terms of structure, transformation, and host cell specificity. Up to now, PVs of different parasite genera and species have been examined to a different extent. Most researches have been directed to the biogenesis, formation, and function of PV harboring Toxoplasma or Plasmodium because these parasites are important pathogens in humans as well as animals (Cesbron-Delauw et al. 2008; Ravindran and Boothroyd 2008; Saliba and Kirk 2001). However, rather little is known about the PV of Eimeria species.
In this study, we first investigated the dynamic development of PVs harboring transfected Eimeria tenella expressing a chimeric protein comprising an N-terminal signal peptide of the Toxoplasma gondii GRA8 protein appended to the yellow fluorescent protein (YFP). We showed cross-species conservation of T. gondii GRA8 signal peptide in the function of targeting this protein into the PV of E. tenella. We further illustrated this conserved protein-targeting machinery among Apicomplexa using another 5′ terminal signal sequence from Plasmodium falciparum repetitive interspersed family (RIFIN) protein.
Materials and methods
Plasmid constructs
Three plasmids, namely, pH−90-2E-A3′, pHgra8-2E-A3′, and pHrifin-2E-A3′, were constructed by polymerase chain reaction (PCR) using the plasmid pH-2E-A3′ (Fig. 1, B2) as the template, which was previously constructed in our laboratory and contains a tandem repeat yfp as the reporter gene flanked by a histone 4 promoter incorporating a 90-bp nucleus location signal sequence (NLS) and an actin 3′ untranslated region (Hao et al. 2007). Plasmid pH−90-2E-A3′ (Fig. 1, A2) was constructed by removing the 90-bp NLS from the promoter of the pH-2E-A3′ plasmid as follows. The histone 4 promoter without NLS was amplified by PCR from the pH-2E-A3′ plasmid with the primers 5′-CAGAGATCTAACCAGCAAAGGTAGCAAC-3′ and 5′-CTAGGTACCCATTTTGGTTTTCTATGGAAC-3′. The resulting fragment, bearing the BglII and KpnI restriction sites, was cloned into the pEASY-Blunt Simple Cloning Vector (TransGen Biotech, Beijing, China) and then placed upstream of the yfp gene in the pH-2E-A3′ plasmid.
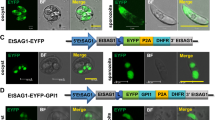
Different 5′ terminal signal sequences appending the histone 4 promoter-targeted YFP to different subcompartments of the transfected E. tenella. A1 Without additional signal sequence, YFP was expressed into cytosol of transfected E. tenella sporozoite in PCKCs. A2 Schematic construct of pH−90-2E-A3′ showing histone 4 (H4) promoter from E. tenella, tandem yfp genes, and an actin poly A tail from E. tenella. B1 NLS of E. tenella H4 gene-targeted YFP into the nucleus of the transfected E. tenella sporozoite in PCKCs. B2 Schematic construct of plasmid pH-2E-A3′ showing the H4 promoter from E. tenella appended by an NLS of E. tenella, tandem yfp genes, and an actin poly A tail from E. tenella (Actin ploy A). C1 5′ terminal signal sequence of T. gondii GRA8 gene-targeted YFP into PV of the transfected E. tenella sporozoite in PCKCs. C2 Schematic construct of pHgra8-2E-A3′ showing H4 promoter from E. tenella appended by a 5′ terminal signal sequence of T. gondii GRA8 gene (GRA8 SS), tandem yfp genes, and an actin poly A tail from E. tenella. D1 5′ terminal signal sequence of the P. falciparum RIFIN protein gene-targeted YFP into the PV of transfected E. tenella sporozoite in PCKCs. D2 Schematic construct of pHrifin-2E-A3′ showing H4 promoter from E. tenella appended by a 5′ terminal signal sequence of P. falciparum RIFIN gene (RIFIN SS), tandem yfp genes (YFP), and an actin poly A tail from E. tenella. Fluorescent fluorescent field image, Bright bright field image, Merged merged image. Bar = 20 μm
Plasmids, pHgra8-2E-A3′ (Fig. 1, C2) and pHrifin-2E-A3′ (Fig. 1, D2), were constructed, respectively, by replacing the NLS with the 84- or 153-bp 5′ terminal portion (including the signal sequence) of the GRA8 gene of T. gondii or the RIFIN gene of P. falciparum. The signal sequence of the T. gondii GRA8 gene was fused to the histone 4 promoter without NLS by PCR with an upper primer (5′-CAGAGATCTAACCAGCAAAGGTAGCAAC-3′) and two overlapping lower primers (5′-GCGAAGACCACGAACACCGTGGCCGAAACACGCAATGGTAAAGCCATTTTGGTTTTCTATGGAACAG-3′ and 5′-CTAGGTACCCAAAGGACCGTTCATGGCGCGAGCTACACCAAAGACAGCGAAGACCACGAACACCGTGGC-3′). The signal sequence of the RIFIN gene was fused to histone 4 promoter without NLS by PCR with an upper primer (5′-CAGAGATCTAACCAGCAAAGGTAGCAAC-3′) and two overlapping lower primers (5′-TATATAATGGAAAGAAAAATAATAATATTT TAGTGTAGTGCAGTTTCATTTTGGTTTTCTATGGAAC-3′ and 5′-CTAGGTACCGTCACATTCACATAATGATCTATTGGTTTGCACCAATATATATAATGGAAAGAAAAATA-3′). The resulting fragments, bearing the BglII and KpnI restriction sites, were cloned into the pEASY-Blunt simple cloning vector and then placed upstream of the yfp gene in the pH-2E-A3′ plasmid. All the inserts above were sequenced in both directions using the fluorescent dideoxynucleotide termination method. Plasmids, pH-2E-A3′ and pH−90-2E-A3′, were used as controls in the study.
Parasites
Oocysts of E. tenella BJ strain were maintained, isolated, and sporulated according to the method of Long (1982). Sporozoites were prepared from purified oocysts by grinding and then excystation from sporocytes using a trypsin–bile solution (10% (v/v) chicken bile and 0.75% (m/v) trypsin in PBS, pH 7.4) (Schmatz et al. 1984). The released sporozoites were purified by DE-52 anion exchange chromatography and then resuspended in a complete cytomix buffer (10 mM K2HPO4:KH2PO4, pH 7.6; 120 mM KCl; 0.15 mM CaCl2; 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; 2 mM ethylene glycol tetraacetic acid; 5 mM MgCl2; 2 mM adenosine triphosphate; and 5 mM glutathione).
Transfection
The restriction enzyme-mediated integration (REMI) method was used to transfect E. tenella sporozoites with the constructed plasmids (Liu et al. 2008). Briefly, a 800-μL complete cytomix mixture consisting of 1 × 107 freshly purified sporozoites, 50 μg plasmid pH−90-2E-A3′, pHgra8-2E-A3′, pHrifin-2E-A3′, or pH-2E-A3′ linearized by the restriction enzyme BglII (100 IU) was electroporated using a Gene Pulser Xcell™ Electroporation System (BioRad, Hercules, USA) at 2.0 kV and 25 μF. The electroporated sporozoites were allowed to stand for 20 min at room temperature before cultivation in primary chicken kidney cells (PCKCs).
Cultivation and investigation of transfectants
The PCKCs were prepared from 2-week-old chickens 3 days before transfection according to the method of Taylor and Baker (1978) with minor modification. The PCKCs were firstly grown in RPMI1640 (Gibco, Grand Island, USA) supplemented with 10% (v/v) fetal bovine serum (FBS), 200 U mL−1 penicillin, and 20 mg mL−1 streptomycin for 24 h at 41°C in an atmosphere containing 5% CO2. Afterwards, the cells were cultured in the same medium but with 5% FBS for 2 days before incubation with the transfected sporozoites.
The transfected E. tenella was investigated using a fluorescence microscope (Olympus, Tokyo, Japan) with 488-nm excitation and 508-nm emission filters, under which the expressed fluorescence appeared green (Hao et al. 2007).
Results and discussion
The histone 4 promoter of E. tenella was demonstrated as a powerful constitutively expressing promoter, which facilitated the expression of YFP throughout the life cycle of the transfected E. tenella (Fig. 2) (Shi et al. 2008). In the current study, we firstly showed that 5′ terminal signal sequences played essential roles in sorting proteins to different subcompartments of the transfected E. tenella with the histone 4 promoter. In E. tenella transfected with plasmid pH−90-2E-A3′ without any additional signal sequence, the expressed YFP was confined in the cytosol (Fig. 1, A1). In parasites transfected with plasmid pH-2E-A3′ containing a NLS between the histone 4 promoter and yfp, YFP was targeted into the nucleus of E. tenella (Fig. 1, B1). However, when the 5′ terminal signal sequences from T. gondii GRA8 or P. falciparum RIFIN protein genes were introduced replacing the NLS as in plasmids pHgra8-2E-A3′ or pHrifin-2E-A3′, YFP was expressed into the PV (Fig. 1, C1 and D1). As secretory proteins, GRA8 and RIFIN proteins were expressed into or beyond PVs of T. gondii and P. falciparum, respectively (Carey et al. 2000; Marti et al. 2004). Targeting of YFP into PVs of E. tenella by the two signal sequences from GRA8 and RIFIN proteins suggests that these sequences are functionally conserved across Apicomplexa parasites.
Under the promoting E. tenella histone 4 promoter, YFP was constitutively expressed throughout the life cycle of the transfected E. tenella in vitro. Plasmid pH-2E-A3′ was used in the transfection of E. tenella (Shi et al. 2008)
On the basis of the results above, we further investigated the dynamic development of the PV harboring E. tenella transfected with the GRA8 signal sequence and YFP. Three structurally different PVs were observed during the endogenous development of the parasite in vitro. The PV of sporozoites was characterized by a narrow lumen and few visible subcompartments (Fig. 1, C1). It was only detected 3 days post-inoculation despite that PV could actually be formed soon after entry of the sporozoite into the host cells within the first few hours after infection. This probably was because of delayed or lower YFP expression into the nascent PV. As the parasite developed into schizogony, three subcompartments were observed in the PVs around schizonts. The first subcompartment was thin membranous extensions of PV into the host cytosol (MEHC) (Fig. 3a,b). Same structures, called duct-like structures, were observed in stages of sporozoites and immature schizonts but not mature schizonts (first and second generation) or gametogony by Zgrzebski et al. (1993), who described that the duct-like structures always extend from the posterior pole of the sporozoite and the PV, marking the pathway of the parasite after host cell invasion. We observed the same phenomena in our study. We postulate that MEHC provides a trafficking pathway between the parasite and the host cell via which macromolecules can passively diffuse into or out of the parasite. Similar structures were also observed in T. gondii and P. falciparum (Magno et al. 2005; Schatten and Ris 2004; Adisa et al. 2003). The second compartment was membranous extensions into the vacuolar lumen of the PV (MEVL) (Fig. 3d). This was the first time that existence of numerous MEVL in E. tenella was observed. The third subcompartment observed in schizogony of the parasite was round, particle-like bodies (PLB) in the lumen of PV (Fig. 3c,e). As the parasite developed into gametogony, the lumen of the PV enlarged, MEVL seemed to disappear, and the MEHC were seldom visible under fluorescence microscopy (Fig. 3f,g). PVs harboring zygotes or oocysts were not detected in our study probably because of very low YFP expression into the degraded PV. In addition, no fluorescent zoites was found outside the PCKCs throughout all the developmental stages of the transfected E. tenella, indicating that YFP is only targeted to the PVs. For the parasites within the PCKCs, fluorescence labeling of dense granules was not observed despite that YFP was obviously detected in the PVs of the transfected parasites. In T. gondii, any soluble protein (including GFP or YFP) recombinantly fused to a signal peptide is first delivered to dense granules and then targeted to distinct subcompartments of the PVs (Joiner and Roos 2002). It was believed that a default route was involved in delivering soluble proteins into dense granules and subsequently targeting them into the PV for T. gondii (Kaasch et al. 2000). The pathway for the GRA8 signal peptide fused YFP targeting to PVs in transfected E. tenella is to be determined in a future study.
Dynamic development of the PV of E. tenella transfected with plasmid pHgra8-2E-A3′ carrying a 5′ terminal signal sequence of T. gondii GRA8 gene. a, b PVs harboring immature first-generation schizonts, showing MEHC. c PV of first-generation schizonts, showing PLB and numerous MEVL. d PV of a schizont, showing complicated MEVL within the PV. e PV of merozoites of a second-generation schizont, showing profuse MEVL around merozoites. f, e Enlarged and matrix-filling PVs harboring gamonts (asterisk). Fluorescent fluorescent field image, Bright bright field image, Merged merged image. Bar = 20 μm
Our study firstly provided an insight into the dynamic development of the PV harboring E. tenella and proved that N-terminal signal sequences from secretory proteins of apicomplexan parasites were determinant in protein trafficking to the PV. In addition, our results suggested that these signal sequences are conserved across species of Apicomplexa in the post-secretory targeting of proteins to the PV. Our findings are valuable in studying protein trafficking in apicomplexan parasites and developing E. tenella as a convenient vaccine vector delivering viral and/or bacterial antigens.
References
Adisa A, Rug M, Klonis N, Foley M, Cowman AF, Tilley L (2003) The signal sequence of exported protein-1 directs the green fluorescent protein to the parasitophorous vacuole of transfected malaria parasites. J Biol Chem 278(8):6532–6542
Baum J, Papenfuss AT, Baum B, Speed TP, Cowman AF (2006) Regulation of apicomplexan actin-based motility. Nat Rev Microbiol 4(8):621–628
Beyer TV, Svezhova NV, Radchenko AI, Sidorenko NV (2002) Parasitophorous vacuole: morphofunctional diversity in different coccidian genera (a short insight into the problem). Cell Biol Int 26(10):861–871
Carey KL, Donahue CG, Ward GE (2000) Identification and molecular characterization of GRA8, a novel, proline-rich, dense granule protein of Toxoplasma gondii. Mol Biochem Parasitol 105(1):25–37
Carruthers VB, Sibley LD (1997) Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol 73:114–1123
Cesbron-Delauw MF, Gendrin C, Travier L, Ruffiot P, Mercier C (2008) Apicomplexa in mammalian cells: trafficking to the parasitophorous vacuole. Traffic 9(5):657–664
Hao L, Liu X, Zhou X, Li J, Suo X (2007) Transient transfection of Eimeria tenella using yellow or red fluorescent protein as a marker. Mol Biochem Parasitol 153(2):213–215
Joiner KA, Roos DS (2002) Secretory traffic in the eukaryotic parasite Toxoplasma gondii: less is more. J Cell Biol 157(4):557–563
Kaasch AJ, Joiner KA (2000) Protein-targeting determinants in the secretory pathway of apicomplexan parasites. Curr Opin Microbiol 3(4):422–428
Liu X, Shi T, Ren H, Su H, Yan W, Suo X (2008) Restriction enzyme-mediated transfection improved transfection efficiency in vitro in apicomplexan parasite Eimeria tenella. Mol Biochem Parasitol 161(1):72–75
Long PL (1982) The biology of the coccidian. Part 4. Ultrastructure. University Park Press, Baltimore, pp 117–155
Magno RC, Straker LC, de Souza W, Attias M (2005) Interrelations between the parasitophorous vacuole of Toxoplasma gondii and host cell organelles. Microsc Microanal 11(2):166–174
Marti M, Good RT, Rug M, Knuepfer E, Cowman AF (2004) Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306(5703):1930–1933
Ravindran S, Boothroyd JC (2008) Secretion of proteins into host cells by apicomplexan parasites. Traffic 9(5):647–656
Saliba KJ, Kirk K (2001) Nutrient acquisition by intracellular apicomplexan parasites: staying in for dinner. Int J Parasitol 31(12):1321–1330
Schatten H, Ris H (2004) Three-dimensional imaging of Toxoplasma gondii—host cell interactions within the parasitophorous vacuole. Microsc Microanal 10(5):580–585
Schmatz DM, Crane MS, Murray PK (1984) Purification of Eimeria sporozoites by DE-52 anion exchange chromatography. J Protozool 31:181–183
Shi T, Liu X, Hao L, Li J, Abdel G, Abdille M, Suo X (2008) Transfected Eimeria tenella could complete its endogenous development in vitro. J Parasitol 11:1
Taylor AER, Baker JR (1978) Methods of cultivating parasites in vitro. Academic, London, pp 118–121
Zgrzebski G, Raether W, Hofmann J, Entzeroth R (1993) Secretion of an Eimeria tenella sporozoite antigen during host-cell invasion: visualization of the parasitophorous vacuole membrane and parasitophorous duct-like structures. Parasitol Res 79(1):77–79
Acknowledgement
This study was supported by the National Natural Science Foundation of China (project numbers 30471298 and 30540003), the National High Technology Research and Development Program of China (2006AA02Z458), and a grant from the Division of Science and Technology of China Agricultural University. We thank Dr. Jin Zhu of the Therapeutic Goods Administration, Australia, for his assistance in the preparation of the manuscript. We are grateful to Huali Su, Yonggen Jia, and Abdel Nabi Gh in our laboratory for their generous assistance during the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, T., Yan, W., Ren, H. et al. Dynamic development of parasitophorous vacuole of Eimeria tenella transfected with the yellow fluorescent protein gene fused to different signal sequences from apicomplexan parasites. Parasitol Res 104, 315–320 (2009). https://doi.org/10.1007/s00436-008-1194-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1194-y