Abstract
This study was conducted to isolate and identify natural entomopathogenic fungi from female Culex quinquefasciatus and to test their adulticidal activity. Field-collected female C. quinquefasciatus died early and were placed on a Saboraud’s dextrose agar plates for growth and isolation of natural entomopathogenic fungi. The plates were maintained in an incubator at 24 ± 2°C for 3 days. Four fungal species were isolated in two genera namely, Aspergillus and Fusarium. The identified fungal species were A. niger, A. flavus, A. nidulans var acristatus (ITCC-6327.04), and F. pallidoroseum (ITCC-6324.06). Adult bioassays were carried out using spore-impregnated paper in WHO-holding tubes. F. pallidoroseum was found to be more effective than the others. Exposure of C. quinquefasciatus to spores of A. flavus and A. niger for 4 h caused 5.53% and 5.51% mortality in the mosquitoes within a week, respectively. All the female C. quinquefasciatus were killed within 4 days of exposure to F. pallidoroseum at a concentration of 1.11 × 1010 conidia per m2. Significant difference of longevity was observed between the F. pallidoroseum-treated C. quinquefasciatus and control mosquitoes. The LT50 of F. pallidoroseum was 2.08 days for 4 h exposure to C. quinquefasciatus. Results of the present study confirm that F. pallidoroseum is one of the alternative biological control agents of adult mosquitoes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Culex quinquefasciatus is one of the most annoying vectors and important man-biting mosquitoes. It works as the vector of lymphatic filariasis and Japanese encephalitis in India (Mourya et al. 1989; Das et al. 2002). The period between when a mosquito blood feeds on an infected host and when it is able to transmit the infection is termed as the extrinsic incubation period (EIP). The EIP is estimated to be between 13 and 22 for lymphatic filariasis and 7 to 10 days for Japanese encephalitis (Muangman et al. 1972; Paily et al. 2006). Not all female mosquitoes start feeding immediately after they eclose; therefore, a vector must live longer than the duration of the initial nonfeeding period plus the EIP of the pathogen to contribute to disease transmission. Residual insecticides reduce the longevity of the insect disease vector and stop the disease transmission. However, the continuous use of insecticides in public health and agricultural practices has led to a substantial increase in physiological resistance in mosquitoes (Hargreaves et al. 2000, 2003). The problem of insecticide resistance has led to the search for alternative vector control methods including biological control agents. Historically, both environmental and biological controls of mosquitoes were exclusively aimed at larval stages and as such have been successful in a variety of geographical and ecological settings (Scholte et al. 2007). Among them, Metarhizium anisopliae, Beauveria tenella, Lagenidium giganteum, and Chrysosprium lobatum are studied widely for use against mosquito larvae (Balaraman et al. 1979; Federici 1981; Lacey et al. 1988; Vyas et al. 2007; Mohanty and Prakash 2008). Earlier, Fusarium oxysporum and F. pallidoroseum were isolated from mosquito larvae, and their efficacies were evaluated against the larval stages (Balaraman 1979; Ravindranath and Kapadnis 1991). However, a strain of F. pallidoroseum was recently isolated in India from forest pest Lymantria obfuscate and was efficacious against the same pest (Munshi et al. 2008).
Recently, insect pathogenic deuteromycetes fungi were tested in the laboratory as control agents of the adults of Anopheles and Culex spp., and the effective one was M. anisopliae (Clark et al. 1968; Scholte et al. 2003). Spores of M. anisopliae are currently tested in the field for the control of the malaria transmission as it is effectively killing the vector (Scholte et al. 2003; Blanford et al. 2005). The exploration of new biological agents that will be specific for the adult mosquitoes is the motive behind the present study. This is a first report of F. pallidoroseum isolation from adult C. quinquefasciatus mosquitoes. In the present investigation, we have evaluated the adulticide activity of spores of F. pallidoroseum against C. quinquefasciatus after exposure for 1, 2, and 4 h.
Materials and methods
Isolation of fungal strains
A survey was conducted in February and March, 2006, for the collection of adult female C. quinquefasciatus mosquitoes from households of the periphery of Delhi, India. Mosquitoes were collected by using an aspirator and flash light. The mosquitoes were placed in a cage and kept in the laboratory, maintained at 30 ± 1°C and relative humidity 60 ± 5%. Glucose solution of 10% in cotton was placed in the top of cage for nutrition. Those adults that died within 5 days of collection from the households were searched for entomopathogenic fungi. These dead mosquitoes were placed in three Saboraud dextrose agar (SDA) plates supplemented with chloramphenicol 25 μg/mL as a bacteriostatic agent. The plates were placed in an incubator and maintained at 24 ± 2°C for 5 days and were kept under observation. Fungi colonies grown on the mosquito were isolated on new agar plates. Fungi colonies were identified in the laboratory by the evaluation of the macromorphological aspects of the colonies, such as diameter, color, aspect, and mycelial texture. The micromorphological characteristics of conidia were observed under an optical microscope. Two slants of fungal colony were sent to Indian Type Culture Collection (ITCC), Indian Agricultural Research Institute, New Delhi-12, for identification and deposition.
Conidia impregnated on sheets
The conidia of four fungi were inoculated on SDA and placed in an incubator at 24 ± 2°C to grow for 2 weeks, after which fresh conidia were harvested using a 0.05% Triton-X solution and a glass rod. The solvent containing conidia was concentrated by removing the supernatant after centrifuging for 3 min at 5,000 × g. Just before impregnation, sunflower oil was added, resulting in an 8% oil formulation. Impregnation was done by shaking the flask containing the conidial oil-formulated suspension vigorously and then spreading it carefully on the sheets. Spreading was done manually using brushes and quickly before the suspension was absorbed by the sheet. This was done in a room maintained at 65 ± 5% humidity where the Whattman sheets were left to dry slowly for 4 h. The control sheets were treated similarly but were without conidia. Four dilutions were made for the range-finding assays. The range-finding assays for all the fungi was done in four different concentrations, 102, 105, 1010, and 1015 conidia per m2 in triplicates. The stock solution of F. pallidoroseum in 108 conidia per milliliter was used for the test experiment. Whattman no. 1 sheets of 0.018 m2 (15 × 12 cm) were impregnated with the stock solution described above. Two milliliters of this stock solution was used to impregnate one sheet, resulting in a conidial density of 1.11 × 1010 conidia per m2.
Bioassays
The bioassays were carried out on laboratory-reared C. quinquefasciatus adults. Freshly emerged 3-day-old sugar-fed adults were used for the assays. A total of 25 adults were used for each assay. Before exposure, the mosquitoes were kept in the holding tube and transferred to the exposure tube by blowing. These adults were exposed for 1, 2, and 4 h to the conidia-impregnated sheets and control sheets. Each exposure was done on separate batches of adults. After exposure, the adults were transferred to cages and provided 10% sugar as nutrition until death. The exposed mosquitoes were kept under observation, and dead mosquitoes were discarded daily. Each bioassay including the control was conducted in triplicate on different days. Daily mortality counts were done, and dead mosquitoes were scored for mortality. The bioassays were done at room temperature with 75 ± 5% humidity. The experiment was discarded when mortality of the mosquitoes exposed to the control sheets occurred more than 20%.
Statistics
Standard deviations were calculated and t test was done with the Microsoft Excel software. The LT50 and 95% fiducial limits were calculated by the probit analysis by SPSS.
Results and discussion
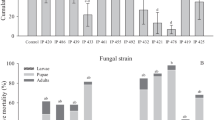
A total of 74 mosquitoes were collected during the survey from houses of peripheral Delhi. Among them, two adult female mosquitoes died after 1 day of collection, and one died after 2 days of collection in the insectary. Four fungal species were isolated and identified as Aspergillus niger, A. flavus, A. nidulans var acristatus (ITCC-6327.04) and F. pallidoroseum (ITCC-6324.06). A. nidulans var acristatus was common on all the three mosquitoes, whereas F. pallidoroseum and A. niger was grown only on one mosquito. A. flavus was isolated from two mosquitoes. During the range-finding assays of the spore-impregnated paper against C. quinquefasciatus, we did not observe any substantial differences of mortality between A. nidulans var acristatus, A. flavus, and A. niger spore-impregnated papers and the control paper (without spore). The adulticide activity of A. flavus and A. niger against C. quinquefasciatus was 5.53% and 5.51%, respectively; therefore, they were not selected for the experiment. The difference in mortality in C. quinquefasciatus against 1010 and 1015 conidia per m2 was less than 2% and was not significantly different (t test, P = 0.08) in mortality (Table 1). Therefore, the adulticide activity was evaluated in the concentration 1.11 × 1010 conidia per m2. Female C. quinquefasciatus were susceptible to spore-impregnated paper of F. pallidoroseum (Table 2). A significant difference (analysis of variance; df = 2, 9; F = 13.87, P = 0.001) in mortality in C. quinquefasciatus was observed between 1, 2, and 4 h of exposure to spores of F. pallidoroseum. A significant (t test; P = 0.001) higher mortality of C. quinquefasciatus was recorded after 2 h exposure than that of 1 h of exposure. When the duration of exposure of F. pallidoroseum was increased from 1 to 4 h, a significant 5.65-fold lower (t test, P = 0.008) LT50 was recorded (Table 2). All the infected C. quinquefasciatus of 4 h exposure to F. pallidoroseum spores died within 4 days. When C. quinquefasciatus control group was exposed for 1, 2, and 4 h to the control paper (conidia-free), the LT50 values were recorded as 20.82, 18.60, and 16.35 days, respectively. However, the LT50 for C. quinquefasciatus was 2.08, 6.34, and 11.76 days after an exposure to F. pallidoroseum-impregnated paper for 4, 2, and 1 h, respectively (Table 2). When the LT50 values of the control and F. pallidoroseum-infected group of C. quinquefasciatus were compared, a significant (t test, P < 0.0001) reduction in longevity in the F. pallidoroseum-infected mosquitoes was recorded.
A notable reduction in the lifespan of female C. quinquefasciatus will decrease the transmission risk of filariasis and Japanese encephalitis. The results from the current study show that longevity of F. pallidoroseum-infected C. quinquefasciatus is significantly lower than that of control mosquitoes. The length of EIP for filariasis is 13 to 22 days and is higher than the LT50 of F. pallidoroseum for 1 h exposure time. However, the EIP of dengue virus is 7 to 10 days, and therefore the higher exposure time of F. pallidoroseum is required to stop the transmission. Continuous exposure of female C. quinquefasciatus to M. anisopliae spore-impregnated paper at a concentration of 1.6 × 1010 conidia per m2 for 3.88 days resulted in a decline of 50% population (Scholte et al. 2003). The LT50 of female Aedes aegypti was 4.1 days when treated for 24 h to spores at the above concentration (Scholte et al. 2007). In the present study, LT50 for female C. quinquefasciatus was lower than the above two observations. Therefore, it can be concluded that spores of F. pallidoroseum are more effective than the spores of M. anisopliae against C. quinquefasciatus. Scholte et al. (2005) reduced the longevity of adult female Anopheles gambiae mosquitoes to 3.49 days from 9.30 days by applying the spores of M. anisopliae, which is similar to the present study. Blanford et al. (2005) for the first time used the impregnated spores of M. anisopliae for interrupting the malaria transmission in Tanzania and reduced the transmission by a factor of 80. Therefore, the application of F. pallidoroseum against Culex spp. will reduce the burden of filariasis and Japanese encephalitis in the tropical countries.
References
Balaraman K, Bheema Rao US, Rajagopalan PK (1979) Isolation of Metarrhizium anisopliae, Beauveria tenella and Fusarium oxysporum (Deuteromycetes) and their pathogenicity to Culex fatigans and Anopheles stephensi. Indian J Med Res 70:718–722
Blanford S, Chan BHK, Jenkins N, Sim D, Turner RJ, Read AF, Thomas MB (2005) Fungal pathogen reduces potential for malaria transmission. Science 308:1638–1641
Clark TB, Kellen WR, Fukuda T, Lindegren JE (1968) Field and laboratory studies on the pathogenicity of the fungus Beauveria bassiana to three genera of mosquito. J Invertebr Pathol 11:1–7
Das PK, Pani SP, Krishnamoorthy K (2002) Prospects of elimination of lymphatic filariasis in India. ICMR Bull 32:41–54
Federici BA (1981) Mosquito control by the fungi Culicinomyces, Lagenidium and Coelomomyces. In: Burges, HD (eds) Microbial control of pests and plant diseases 1970–1980. Academic, New York, pp 555–572
Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M (2000) Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol 14:181–189
Hargreaves K, Hunt RH, Brooke BD, Mthembu J, Weeto MM, Awolola TS, Coetzee M (2003) Anopheles arabiensis and An. quadriannulatus resistance to DDT in South Africa. Med Vet Entomol 17:417–422
Lacey CM, Lacey LA, Roberts DR (1988) Route of invasion and histopathology of Metarhizium anisopliae in Culex quinquefasciatus. J Invertebr Pathol 52:108–118
Mohanty SS, Prakash S (2008) Laboratory and field evaluation of the fungus Chrysosporium lobatum against the larvae of the mosquito Culex quinquefasciatus. Parasitol Res (in press), DOI 10.1007/s00436-007-0843-x
Mourya DT, Iikal MA, Mishra AC, Jacob PG, Pant U, Ramanujam S, Mavale MS, Bhat HR, Dhanda V (1989) Isolation of Japanese encephalitis virus from mosquitoes collected in Karnataka state, India from 1985 to 1987. Trans R Soc Trop Med Hyg 83:550–552
Muangman D, Edelman R, Sullivan MJ, Gould DJ (1972) Experimental transmission of Japanese encephalitis virus by Culex fuscocephala. Am J Trop Med Hyg 21:482–486
Munshi NA, Hussain B, Malik GN, Yousuf M, Fatima N (2008) Efficacy of entomopathogenic fungus Fusarium pallidoroseum (Cooke) Sacc. against gypsy moth (Lymantria obfuscata Walker). J Entomol 5:59–61
Paily KP, Hoti SL, Balaraman K (2006) Development of lymphatic filarial parasite Wuchereria bancrofti (Spirurida: Onchocercidae) in mosquito species (Diptera: Culicidae) fed artificially on microfilaremic blood. J Med Entomol 43:1222–1226
Ravindranath G, Kapadnis BP (1991) Isolation and extraction of Trichodermin from Fusarium pallidoroseum, a fungal pathogen of Anopheles stephensi. Indian J Microbiol 31:267–269
Scholte EJ, Njiru BN, Smallegange RC, Takken W, Knols BGJ (2003) Infection of adult malaria (Anopheles gambiae s.s.) and filariasis (Culex quinquefasciatus) vectors with the entomopathogenic fungus Metarhizium anisopliae. Malar J 2:29
Scholte EJ, Nghabi K, Kihonda J, Takken W, Paaijmans K, Abdulla S, Killeen GF, Knols BGJ (2005) An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308:1641–1642
Scholte EJ, Takken W, Knols BGJ (2007) Infection of adult Aedes aegypti and Ae. albopictus mosquitoes with entomopathogenic fungus Metarhizium anisopliae. Acta Trop 102:151–158
Vyas N, Dua K, Prakash S (2007) Efficacy of Lagenidium giganteum metabolites on mosquito larvae with reference to nontarget organisms. Parasitol Res 101:385–390
Acknowledgments
The first author acknowledges the Council of Scientific and Industrial Research, New Delhi, for financial support in the form of Research Associateship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohanty, S.S., Raghavendra, K., Rai, U. et al. Efficacy of female Culex quinquefasciatus with entomopathogenic fungus Fusarium pallidoroseum . Parasitol Res 103, 171–174 (2008). https://doi.org/10.1007/s00436-008-0946-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-0946-z