Abstract
Tungiasis is endemic in many countries in Latin America, the Caribbean and sub-Saharan Africa, and it is associated with severe morbidity. The pathophysiological and immunological characteristics of the ectoparasitosis are not well understood, and no effective therapy is currently available. The aim of this study was to describe the natural history of tungiasis in laboratory-raised Wistar rats. The rats were exposed in the laboratory to the parasite or were kept in a natural environment with an intense transmission of Tunga penetrans. The time course of the infestation was determined, and lesions were photographed, described clinically in detail and biopsied. Biopsies were examined histopathologically and by light and scanning electron microscopy. Based on these findings, the natural history of tungiasis in Wistar rats was described and divided in five stages. Our data show that the natural history of tungiasis in Wistar rats and humans is almost identical, except that in the animals, the basement membrane disrupts 5 days after penetration and provokes an intense infiltration of the dermis, while in humans, the basement membrane remains intact. The study indicates that the Wistar rat is an appropriate model for the study of clinical and pathological aspects of tungiasis. Using this model should enable a better understanding of the pathophysiology and immunology of the ectoparasitosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tungiasis is a neglected parasitic skin disease widespread in resource-poor communities in sub-Saharan Africa, South America and the Caribbean (Ibanez-Bernal and Velasco-Castrejon 1996; Heukelbach et al. 2001; Njeumi et al. 2002; Franck et al. 2003; Heukelbach 2005; Joseph et al. 2006; Ugbomoiko et al. 2007). The parasite causes considerable morbidity and is a health threat for many people living in extreme poverty (Heukelbach et al. 2001; Muehlen et al. 2003; Feldmeier et al. 2003a; Joseph et al. 2006). Individuals living in impoverished settings are also prone to infestation with other ectoparasites and frequently show co-infestation with scabies mites, head lice and animal hookworm larvae, causing cutaneous larva migrans (Heukelbach et al. 2003; Heukelbach et al. 2005). This makes the identification of parasite–host interactions specific for Tunga penetrans in humans very difficult. Responses of the human host could have been enhanced or modulated by the simultaneous or previous presence of other ectoparasites also eliciting local inflammation of the skin (Feldmeier et al. 2002; Feldmeier et al. 2003b). An animal model in which the natural history of the infestation is similar to the course of the disease in humans will allow studying host–parasite interactions without these confounding factors. Such a model will also allow to evaluate new therapeutic and prophylactic approaches and to test pharmacological substances that could interrupt the in vivo development of T. penetrans or mitigate the development of inflammation.
Based on the observation that in an endemic area, Rattus rattus is frequently infested with T. penetrans and that in this rodent species, sand fleas penetrate almost exclusively at the feet (Heukelbach et al. 2004), we decided to scrutinize the suitability of laboratory-raised Wistar rats as a model for human tungiasis. We have previously shown that Wistar rats can be easily infested experimentally and that animals exposed in natural environments can be used as sentinels to assess local transmission dynamics (Feldmeier et al. 2004b; Nagy et al. 2007; Witt et al. 2007). Besides, it has been demonstrated that in Wistar rats, the topographic distribution of lesions closely resembles that in humans with virtually all lesions located either at the distal end of the digits or at the foot pad (Heukelbach, unpublished observation).
In this paper, we describe in depth the natural history of tungiasis in the Wistar rat based on a time series of macroscopic, microscopic and histopathological examinations beginning with the penetration of the female sand flea and ending with the elimination of the remains of the chitinous exoskeleton of the parasite from the epidermis. It is concluded that the natural history of tungiasis in the Wistar rat reflects the course of the infestation in humans very closely and that this animal is an optimal model to study the kinetics of parasite development and involution as well as the inflammatory responses of the host.
Materials and methods
Host animals
Wistar rats (4 weeks of age) were obtained from the Department of Pharmacology of the Universidade Federal do Ceará, Fortaleza. Rats weighted 180–200 g and were kept in standard cages (60 × 45 × 15 cm) on saw dust. They were fed with commercial dog food. Food and water were provided ad libitum.
In total, 80 rats were exposed to T. penetrans in two different ways:
-
1.
In the laboratory, the tray of a cage was covered with sand and the cage tightly enfolded in a cotton sheet. Between 40 and 60 free-running adults of T. penetrans, previously collected from the skin of the inhabitants of an endemic area, were placed on the tray. Then, three to four rats were placed in the cage and examined every 3 h for the next 24 h.
-
2.
For exposure in a natural environment, four to six rats were put in cages. These cages were placed on the ground close to dwellings in an endemic community where at least one member of the household was affected by tungiasis at the time of investigation.
Exposure sites were usually resting places of dogs and cats, situated in shadow or in half-shadow during the day. The rats were examined at the following points in time: 3, 6, 8, 12 and 18 h after penetration and again 1, 2, 3, 5, 7, 9, 11,12, 15, 16, 17, 18, 20, 21 and 28 days after penetration.
Macroscopic examination
Because in Wistar rats, T. penetrans usually penetrates at the foot pad or the digits (Heukelbach unpublished observation), these areas were examined carefully. If penetration was supposed to have occurred, the corresponding area was cleaned with water and examined with a magnifying glass. If rats were very excited, they were anaesthetized with ether. For this purpose, a cotton pad was impregnated with ether 50% (v/v) and placed into a glass container until the rat did not longer react to external stimuli.
Time of penetration, number and topographic localization of penetrated fleas were noted. The macroscopic appearance of each lesion was described according to the guidelines previously established (Eisele et al. 2003). Lesions were photographed with a Dental Eye II camera (Yashica, Hamburg, Germany), equipped with a macro-objective and a close-up lens.
Microscopic investigation
For light microscopy, fleas were taken from the skin using a dissecting forceps immediately after the animal had been killed with ether. Embedded fleas were taken out by biopsy postmortem. Tunga specimens were placed into a tube containing aqueous formaldehyde (10% v/v) and kept at ambient temperature. Fleas were examined by means of an inverted microscope (Olympus SZ-PT, Tokio, Japan) to determine the morphological characteristics of the parasite. T. penetrans was identified according to the criteria established by Linardi (2000). To assess the kinetics of the hypertrophy, the morphological change of each abdominal segment was noted at defined intervals. In some cases, fleas were dissected under the inverted microscope with a fine needle.
For scanning electron microscopy (SEM), extracted fleas were placed in phosphate-buffered glutaraldehyde (5% v/v) and kept at 4°C. Before samples were dehydrated in ascending concentrations of ethanol, they were rinsed in distilled water for 1 h. Critical point drying was performed using a Critical Point Dryer (Polaron, Uckfield, Great Britain) by replacing cellular water with carbondioxide in a step-wise manner. Samples were glued to specimen mounts with graphite adhesive Leit-C (Plano, Marburg, Germany). Coating with gold particles of 20–30 nm in diameter was performed using the SEM Coating Unit E5100 (Polaron). SEM was performed with an Amray 1610 Turbo electron microscope (Amray, Bedford, MA).
Histopathology
Formalin-fixed (formaldehyde 10% v/v) skin biopsy specimens were embedded in paraffin in a routine manner, sectioned at 5 μm and stained with haematoxylin–eosin.
Results
Based on clinical and histopathological findings, together with morphological characteristics detectable by light microscopy and SEM, the natural history of tungiasis in Wistar rats was divided into five stages.
Stage 1: penetration phase
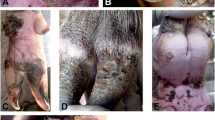
This stage began when a female flea had identified a suitable place for penetration—either at the foot pad or at the distal part of a digit. Within minutes the flea took up a characteristic position with its head close to the skin surface. It placed both anterior pairs of legs alongside the body and used the posterior legs to push the rear end up to an angle of approximately 45°. In this position, the head started to burrow into the epidermis (Fig. 1). During the penetration process, the angle to the skin surface changed continuously and eventually reached 90° after 7 h. When burrowing came to an end after 12 h, the female flea had completely disappeared in the epidermis except its distal abdominal segments, which remained visible above the skin surface. Already 3 h after the start of penetration, the anterior abdominal segments started to enlarge.
Rarely, a slight erythema became visible immediately after penetration had started. However, an erythema was always present after 8 h. At this stage, no histopathological alteration could be detected in the epidermis or in the dermis. The macroscopic and microscopic characteristics of stage 1 are summarized in Table 1.
Stage 2: phase of rapidly increasing hypertrophy
Eighteen hours after onset of penetration, the hypertrophy zone between abdominal segments 2 and 3 became clearly visible (Figs. 2 and 3). Six hours later, the hypertrophy zone had the appearance of a small life-belt. The last three abdominal segments still protruded the surface of the skin (Fig. 4) and formed a kind of crater in which the four stigmata, the anus and the genital opening could be seen.
Macroscopically, the penetration site became obvious as a black dot surrounded by a pale halo. The erythema enlarged and intensified, while the skin around the embedded flea became slightly oedematous. When soft pressure was exerted onto the lesion with a dull instrument, the animal immediately retracted the foot, while uninfected rats showed a less distinctive reaction to the same stimulus.
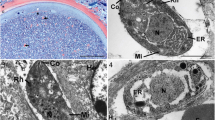
Histological sections showed that the parasite was confined to the epidermis and that only its mouth parts had penetrated the basement membrane (Fig. 5). In the dermis, a cellular infiltrate developed containing mainly eosinophilic and neutrophilic granulocytes (Fig. 6). The characteristics of stage 2 are summarized in Table 2.
Stage 3: phase of white halo
Based on macroscopic and morphological characteristics, stage 3 was divided into two sub-stages: sub-stage 3a, characterized by a continuous increase in size of the hypertrophy zone until a sphere with a diameter of up to 10 mm was formed, and sub-stage 3b with no longer increasing size of the sphere or change of appearance of the flea.
Sub-stage 3a: phase of maximal hypertrophy
Three days after the beginning of the penetration, the hypertrophy zone developed into a sphere. The tergits and the sternits of abdominal segments 2, 3 and 4 stretched more and more, bent apart and were surrounded by newly built, crescent-shaped chitinous clasps (Fig. 7). The thoraxic segments did not change their size but were bent towards the head by the bulging neosome. The head of the flea remained unchanged (Fig. 7). Eggs were expelled from the rear cone, and faecal material was excreted intermittently as helical coils.
As a characteristic phenomenon of sub-stage 3a, pulsations appeared as roughly 1-mm-thick darkish strings visible below the surface of the sphere showing peristaltic-like movements. The strings changed their localizations within the sphere continuously, and the pulsations occurred intermittently. There was no temporal relation between pulsations and the release of faeces or expulsion of eggs. A watery secretion was observed intermittently.
At this time, the bulging hypertrophy zone became macroscopically apparent as a white halo surrounding the brown dot of the rear cone. It stretched the epidermis of the phalanges in a way that the lesion took the appearance of a dome. Dome formation was less obvious for lesions located at the foot pad. Erythema, oedema and signs of pain were constantly present.
Histological sections showed that the parasite had disrupted the basement membrane and entered the dermis (Fig. 8). In the dermis, an intense cellular infiltrate was present, which extended to the vicinity of the periosteum, when lesions were located at a phalanx. Eosinophils and neutrophils dominated the dermal infiltrate. Neovascularization was seen next to the proboscis. At the end of sub-stage 3a, the composition of the cellular infiltrate changed. In addition to granulocytes, lymphocytes had migrated into the area, and occasionally, foreign-body giant cells were seen.
Sub-stage 3b: formation of mini-caldera and change of consistency
With the beginning of sub-stage 3b, the macroscopic appearance of the lesions indicated that the development of the neosome had reached its maximum. The hypertrophy zone had increased its volume so that it towered above the rear cone formed by the last three abdominal segments. Macroscopically, the lesion took the appearance of a mini-caldera. Subsequently, the white halo turned brownish, and the surface of the lesion lost its bulging aspect and became slightly wrinkled (Fig. 9). The shrinking was also visible microscopically (Fig. 10). Expulsion of eggs, watery secretion, excretion of faecal coils and signs of inflammation persisted.
Histologically, sub-stage 3b was characterized by a further augmentation of the intensity of the dermal infiltrate, at the phalanges almost reaching the digital bones (Fig. 11). Mononuclear cells became more and more frequent and signs of sac formation appeared such as an increase in intradermal connective tissue. Fifteen days after penetration, the epidermis showed hyperkeratosis and parakeratosis.
The characteristics of sub-stages 3a and 3b are summarized in Tables 3 and 4.
Stage 4: involution phase
After expelling the majority of eggs, the hyperthrophy zone began to involute (Fig. 12). Involution was a continuous process starting at the beginning of the third week and ending with the death of the parasite approximately 20 to 30 days after penetration (Fig. 13). Remnants of the parasite were sloughed off the epidermis by days 28 to 30.
Macroscopically, the beginning of stage 4 was heralded by signs indicating a loss of viability: The watery secretion stopped, the excretion of faeces came to an end and the pulsation phenomenon was no longer observed. The sphere continued to shrink, and its surface became more and more wrinkled. Simultaneously, the lesion darkened and eventually turned black (Figs. 14 and 15). The epidermis surrounding the dead parasite became necrotic (Fig. 15); however, signs of intense local inflammation persisted (Fig. 14).
Involution of the parasite was also obvious in histological sections. Simultaneously, the destroyed epidermis around the remnants of the parasite started to regenerate, appeared thickened and turned more and more hyperkeratotic (Fig. 16). The destroyed basement membrane was rebuilt in some cases. Granulocytes disappeared from the infiltrate and were replaced by lymphocytes, plasma cells and foreign-body giant cells (Fig. 17). The latter closely encircled the disintegrating chitinous skeleton of the parasite.
Stage 4, 28 days after penetration (200×). Magnification of the dermal cellular infiltrate seen in Fig. 16
After the remains of the dead parasite had spontaneously fallen off or were taken out actively by the rat itself during scratching or gnawing, a crater-like hollow remained in the epidermis containing occasionally eggs (Fig. 18). Table 5 summarizes the main characteristics of stage 4.
Stage 5: residue stage
Within a couple of days, the crater-like hollow in the epidermis healed and developed into a slight round depression. As the re-organization of the epidermis continued, the site previously occupied by the parasite was flattened and eventually became indistinguishable from normal skin.
A comparison of stages between Wistar rats and humans is presented in Table 6.
Discussion
T. penetrans (L., 1758) is a fascinating example of an arthropod that has adapted to a peculiar form of endoparasitism for the reproductive phase of its life cycle. Many generic names were given to the sand flea since its first scientific description: Pulex (Linnaeus 1758), Rhynchoprion (Oken 1815), Dermatophilus (Guerin 1836), Sarcopsylla (Westwood 1837, Karsten 1865).
In addition, the long list of local designations in use in the Americas—nigua (Venezuela, Mexico, Paraguay), pique (Uruguay, Paraguay, Argentina, Bolivian lowlands), pico (Peru), manta blanca (Peru), suthi (Bolivian highlands), chica (Columbia), piroque (Ecuador), puce chique/puce pénétrante (French Guayana, French Antilles), chigoe (British Colonies), chigger/jigger flea (West Indies), chik (Haiti), tungay (Mexico), bicho do pé/bicho do porco/jatecuba/migór (Brazil) and ogri eye/aagrani (indigenous populations of Surinam)—indicates that tungiasis has been a well-recognized health problem in many regions, occurred in different environments and has been associated with various animal reservoirs. In fact, books of scientific travelers to the neotropics abound with notes on the presence of T. penetrans in native populations and the morbidity it caused (Burmeister 1853; Up der Graff 1923; von Humboldt 1981).
Once the female flea starts to penetrate into the epidermis of a host, a complex sequence of structural and morphological changes is initiated. A phase of astounding abdominal hypertrophy is followed by involution of the neosome; at the end, the parasite dies, and its remains are sloughed off the epidermis by repair mechanisms of the skin, as it occurs with foreign bodies (Eisele et al. 2003). Thus, in the vertebrate host, the natural history of T. penetrans is a dynamic, albeit self-limiting process.
Surprisingly, tungiasis has not elicited much scientific interest during the last decades and until recently has been almost totally neglected as a health problem of people living in extreme poverty (Heukelbach et al. 2001). The pathophysiological basis of the severe inflammation is not understood, immunological host–parasite interactions have not been studied systematically and effective chemotherapy against embedded sand fleas is not at hand. The availability of an animal model, in which the natural course of disease in humans can be reproduced, would greatly facilitate to address these issues. This study was designed to investigate whether the Wistar rat is a suitable model for human tungiasis.
After a free-running flea has identified a suitable place for penetration, it starts to burrow into the epidermis. In contrast to male fleas, which blood feed only transiently on the host (Bonnet 1880; Witt et al. 2004), in the 140 females monitored in this study, the characteristic abdominal hypertrophy developed after penetration, and eggs were expelled. This indicates that once a female has started to penetrate, the programmed sequence of altered feeding behavior and morphological changes begins, and the natural history only comes to an end with the death of the parasite 3 to 4 weeks later.
According to Geigy and colleagues (Geigy and Herbig 1949; Geigy 1953; Geigy and Suter 1960), females are fertilized only after they are embedded in the epidermis. During copulation, the male sand flea takes up an upright position and puts its protudable copulatory organ into direct contact with the rear cone of the embedded female. Similarly, in tungiasis caused by T. monositus, a species that penetrates in the pinna of the ear of small rodents, mating also occurs after embedding and fertilization is only possible 2 weeks after penetration (Lavoipierre et al. 1979). It has been suggested that faecal material released from embedded females and spread in the papillae of the skin attract males; the presence of embedded females in clusters is a biological advantage, as a single male could inseminate more females per unit of time, than if they were spread over a wide surface of the host (Lavoipierre et al. 1979; Eisele et al. 2003). The fact that lesions are virtually confined to the toes and other parts of the foot and that in humans ectopic localizations account for less than 6% of all lesions also may be explained by higher chances for successful copulation of embedded females by free-running males (Heukelbach et al. 2002).
Fleas started to penetrate the epidermis at an angle of 45° (Fig. 1). However, during the penetration process, this angle changed continuously until the flea became oriented perpendicular to the surface of the skin. Penetration is probably a mainly physical process during which the flea uses its saw-like mouthparts to drill a tunnel and enlarges it with his head so that its body can move forward. As the cells of the stratum corneum are tightly overlapped, penetration may be easier if started at a small angle rather than vertically. However, once the stratum granulosum is reached, where cells are organized in columns, a vertical position seems to be more appropriate, as the distance to the dermis becomes considerably shorter than if penetration would be continued at 45°.
Already 3 h after the start of penetration, the abdomen of the female flea starts to enlarge. The rapid increase in size of the anterior abdominal segments is identical to that in human infestations (Eisele et al. 2003). Conceivably, this enlargement is related to an increased metabolic activity. As Geigy and Herbig (1949) have pointed out, the flea already feeds on blood when penetrating. This was confirmed in human tungiasis, where the presence of intact or partially digested erythrocytes in the intestine of the flea was demonstrated soon after the beginning of penetration (Eisele et al. 2003).
The time a female sand flea needed to penetrate into the epidermis of Wistar rats was approximately 12 h. In comparison, T. monositus, an ectoparasite of critecid rodents of the genus Peromyscus, needs 24 h to complete penetration (Lavoipierre et al. 1979). When carefully observing cases in animals and humans in French Guyana, Bonnet (1880) found that penetration lasted 24 to 36 h. This period is considerably longer than the 3–7 h previously estimated for humans (Bruce et al. 1942; Eisele et al. 2003). The epidemis of the rat consists only of four layers (stratum basale, stratum spinosum, stratum granulosum, stratum corneum), while in humans, the epidermis has at least palmar and plantar an additional fifth layer—the stratum lucidum. On the other hand, the stratum corneum of the toes and the heel is considerably thicker in inhabitants of the endemic area, who frequently walk barefooted or only wear slippers, than in 4-week-old laboratory-raised Wistar rats. Thus, penetration may be more rapid in the Wistar rat than in humans.
Furthermore, in humans exposed in a natural environment, the beginning and the end of penetration cannot be investigated with the same precision as in experimentally infested animals. It seems therefore reasonable that because of experimental constraints, the duration of the penetration into the human skin has been underestimated and that 12 h reflect the time a sand flea needs to complete penetration. Bonnet (1880) suggested that different daytimes and the topographic localization influence the duration of the penetration time. Whereas in humans, penetration presumably starts at daytime, in naturally exposed Wistar rats, infestation mainly occurred during the night (Witt, unpublished observation). Bonnet (1880) also observed that penetration time depended on the activity of the host. For example, when the female flea was disturbed during penetration, e.g. by rapid movements of the affected limb, the penetration time was prolonged.
The only clinical sign during the penetration phase was the development of a slight erythema. Because the animals used were immunologically naive, the dilation of small blood vessels in the dermis leading to a macroscopically visible erythema cannot be explained by an immediate-type hypersensitivity reaction. However, nociceptors of pain fibres, when activated by an appropriate stimulus, can induce local vasodilatation through synapses with autonomic fibres. As the presence of a continuously enlarging foreign body in the epidermis increases local pressure, local nociceptors are expected to be stimulated, which in turn would trigger vasodilatation. Alternatively, vasoactive substances secreted by the parasite might result in vasodilatation.
In contrast to human infestation, no signs of itching were observed in animals at stage 1. In humans, itching occurs promptly and is explained by previous sensitization during earlier infestations (Eisele et al. 2003). Whereas in the rat, no histological changes occurred during the penetration phase, in humans, already in stage 1 a mild inflammatory infiltrate consisting of neutrophils and eosinophils was seen (Feldmeier et al. 2004a). In analogy to itching, the presence of eosinophils at this early stage in biopsies taken from patients points to previous sensitization of the individual.
The beginning of the separation of the abdominal segments 2 and 3 and the generation of new inter-segmental skin characterizes the start of stage 2 and coincides with a visible enlargement of the abdomen, the so-called neosome (Fig. 2). Initially, the hypertrophy zone forms a bulge, which when seen transversally, looks like a miniature life-belt but then rapidly transforms into a sphere. Macroscopically, the new inter-segmental skin appeared like a tiny halo around the dark dot representing the rear cone of the flea. At this stage, the gut of the flea contained many erythrocytes and leucocytes, the latter probably ingested from the cellular infiltrate that has formed around the proboscis (Table 2; Geigy and Herbig 1949).
The existence of local inflammation is reflected by the appearance of indicators of pain. The rat retracts the affected foot, if the lesion is touched with an instrument.
In stage 3, the morphological changes become impressive. In sub-stage 3a, the spherical hypertrophy zone reached a diameter of 10 mm (Fig. 7). Simultaneously, the two tergits and the sternit of abdominal segment 2 were stretched and bent apart, so that, when looked at from cranial to caudal, the flea looks like a three-leafed clover (Fig. 10), the margins of which consisted of bulging chitinous clasps. According to Fülleborn (1908), the chitinous clasps are insertion sites for muscles (Fülleborn 1908). Geigy and Herbing (1949), however, clearly showed that the clasps are newly built and emerged from inter-segmental skin.
Macroscopically, the enlarging balloon-like inter-segmental skin shimmers through the thinned keratin layer as a white circular halo, which continuously increases in size. Especially, when a lesion is located at the phalanx, the neosome pushes the stretched stratum corneum upwards so that the lesions appeared like a dome. Apparently, the firm consistency of the dome-like protrusion reflects the pressure that the increasing hypertrophy of the ovarian ducts, the gut, the Malpighian ductules and other organs exert against the inter-segmental skin (Geigy 1953). In fact, at this stage, many eggs were seen in the ovarian ducts.
In this stage, a high metabolic activity is also indicated by the intermittent excretion of faeces, the expulsion of eggs, a brownish-watery secretion and a pulsation phenomenon probably reflecting peristaltic movements of the gut (Bonnet 1880; Eisele et al. 2003).
According to our definition, in sub-stage 3b, the hypertrophy zone has reached its climax (Fig. 10). As the stock of eggs is becoming depleted, the pressure exerted against the inter-segmental skin decreases. The loss of firm consistency of the dome-shaped lesion and its subsequent wrinkled appearance reflect the functional and structural changes taking place in this sub-stage.
The functional and morphological changes of stage 3 were accompanied by an altered cellular pattern around the embedded flea. The cellular infiltrate in the dermis increased in size—sometimes it extended almost to the periosteum (Fig. 11)—and changed its composition. Mononuclear cells were seen together with granulocytes. Signs of fibrosation also occurred. The appearance of fibroblasts and the deposition of collagen have been noted in T. monositus at a similar stage of development (Lavoipierre et al. 1979).
It is interesting to note that the change in cellular composition of the infiltrate coincides with an altered pattern of circulating cytokines. Whereas from stages 1 to 3b, interleukin (IL)-1β and tumour necrosis factor-α increased, 6 days after penetration, the concentration of IL-10 augmented (Feldmeier et al. 2003b).
The observation that the inflammatory infiltrate is more intense in the rat than in humans may be explained by the fact that in the animal, the basal membrane is ruptured in sub-stage 3a and that an increasing portion of the parasite is lodged in the dermis. A similar observation was reported by Fülleborn (1908) in tungiasis of an armadillo (Fülleborn 1908). In contrast, in the human host, with the exception of the proboscis, the parasite remains confined to the epidermis.
The presence of newly formed blood vessels in the dermis is an intriguing observation and has also been observed in T. monositus (Lavoipierre et al. 1979). Assumingly, T. penetrans excretes a substance that induces angiogenesis with the aim to increase the number of small blood vessels and hence the quantity of blood accessible. Another possibility is that neo-vascularization is triggered by the host as a consequence of inflammation.
Stage 4 is a transition between a further involution of the neosome and the elimination of the remnants of the dead parasite by repair mechanisms of the skin. The neosome shrinks more and more. Macroscopically, the lesion appeared to dry out, with its colour changed from whitish-yellow to brown and eventually turned to black. However, not only the parasite was destroyed, but necrosis also occurred in the surrounding host tissues. This phenomenon has also been observed in human tungiasis, particularly when lesions occurred in clusters.
Histologically, stage 4 is reflected by signs of tissue re-organization. The basement membrane was reconstituted, and the remains of the chitinous skeleton were removed by phagocytic cells, among which foreign-body giant cells were frequent.
In single cases, eggs were observed, in the crater-like depression remaining after the remains of the dead parasite has fallen out. These eggs had been expelled at the end of phase 3 and became incrusted in the necrotic tissue surrounding the lesion. This finding explains the anecdotal observation of sores at the base of which eggs were present in patients supposed to have experienced tungiasis recently (Basler et al. 1988). Under very rare circumstances, eggs may develop into first instar larvae if wounds remain untreated (Faust and Maxwell 1930).
Our data show that the natural history of tungiasis in Wistar rats very closely resembles the course the infestation in humans. Only in two aspects, the animal model shows pecularities. First, in contrast to humans, the basement membrane is disrupted 5 days after penetration, and a continuously growing portion of the fleas is located in the dermis. This provokes an intense cellular infiltration the type of which changes as the course of the infestation progresses. Whereas in humans and dogs, parakeratosis and hyperkeratosis are very common (Rietschel 1989; Eisele et al. 2003; Feldmeier et al. 2004a), these alterations were only rarely observed in rats. Whether this is due to the peculiar epidermis of the rat or whether it is a result of the destruction of epidermis by the parasite remains to be investigated. Furthermore, bacterial superinfection, virtually almost constantly present in human tungiasis (Joyeux and Sicé 1937; Feldmeier et al. 2002), was not obvious in rats. This is puzzling, as pathogenic bacteria are expected to migrate along the outer surface of the exoskeleton through the epidermis via the disrupted basal membrane into the dermis.
In conclusion, the Wistar rat is an appropriate animal model to study the kinetics of host–parasite interactions in tungiasis. It seems also suitable for the evaluation of pharmacological approaches attempting to abrogate the in situ development of the flea and to modulate the intense inflammatory response of the host.
References
Basler EA, Stephens JH, Tschen JA (1988) Tunga penetrans. Cutis 42:47–48
Bonnet G (1880) Mémoire sur la puce pénétrante, ou chique. tungiasis
Bruce CO, Knigin TD, Yolles SF (1942) A discussion of the chigoe (Tunga penetrans) based on experiences in British Guiana. Military Surgeon 82:446–452
Burmeister H (1853) Reise nach Brasilien, durch die Provinzen von Rio de Janeiro und Minas geraës. Georg Reimer, Berlin
Eisele M, Heukelbach J, van Marck E et al (2003) Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: I. Natural history of tungiasis in man. Parasitol Res 90:87–99
Faust EC, Maxwell TA (1930) The findings of the larvae of the chigo, Tunga penetrans, in scrapings from the human skin. Arch Dermatol Syphilol 22:94–97
Feldmeier H, Heukelbach J, Eisele M et al (2002) Bacterial superinfection in human tungiasis. Trop Med Int Health 7:559–564
Feldmeier H, Eisele M, Saboia-Moura RC, Heukelbach J (2003a) Severe tungiasis in underprivileged communities: case series from Brazil. Emerg Infect Dis 9:949–955
Feldmeier H, Heukelbach J, Eisele M et al (2003b) Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: III. Cytokine levels in peripheral blood of infected humans. Parasitol Res 91:298–303
Feldmeier H, Eisele M, Van ME et al (2004a) Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: IV. Clinical and histopathology. Parasitol Res 94:275–282
Feldmeier H, Witt LH, Schwalfenberg S et al (2004b) Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil. V. Cytokine concentrations in experimentally infected Wistar rats. Parasitol Res 94:371–376
Franck S, Feldmeier H, Heukelbach J (2003) Tungiasis: more than an exotic nuisance. Travel Medicine and Infectious Disease 1:159–166
Fülleborn F (1908) Untersuchungen über den Sandfloh. Arch Schiffs Trop Hyg 6:269–273
Geigy R (1953) Sandfloh-Probleme. Naturwissenschaften 40:40–42
Geigy R, Herbig A (1949) Die Hypertrophie der Organe beim Weibchen von Tunga penetrans. Acta Trop 6:246–262
Geigy R, Suter P (1960) Zur Copulation der Flöhe. Rev Suisse Zool 67:206–210
Heukelbach J (2005a) Tungiasis. Rev Inst Med Trop São Paulo 47:307–313
Heukelbach J, de Oliveira FA, Hesse G, Feldmeier H (2001) Tungiasis: a neglected health problem of poor communities. Trop Med Int Health 6:267–272
Heukelbach J, Wilcke T, Eisele M, Feldmeier H (2002) Ectopic localization of tungiasis. Am J Trop Med Hyg 67:214–216
Heukelbach J, van Haeff E, Rump B et al (2003) Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health 8:368–373
Heukelbach J, Costa AM, Wilcke T, Mencke N, Feldmeier H (2004) The animal reservoir of Tunga penetrans in severely affected communities of north-east Brazil. Med Vet Entomol 18:329–335
Heukelbach J, Walton SF, Feldmeier H (2005) Ectoparasitic Infestations. Curr Infect Dis Rep 7:373–380
Ibanez-Bernal S, Velasco-Castrejon O (1996) New records of human tungiasis in Mexico (Siphonaptera:Tungidae). J Med Entomol 33:988–989
Joseph JK, Bazile J, Mutter J et al (2006) Tungiasis in rural Haiti: a community-based response. Trans R Soc Trop Med Hyg 100:970–974
Joyeux C, Sicé A (1937) Précis de médecine coloniale, 2nd edn. Masson et Cte, Paris, p 441
Lavoipierre MMJ, Radovsky FJ, Budwiser PD (1979) The feeding process of a tungid flea, Tunga monositus (Siphonaptera: Tungidae), and its relationship to the host inflammatory and repair response. J Med Entomol 15:187–217
Linardi PM (2000) Família tungidae. In: Linardi PM, Guimaraes LR (eds) Sifonápteros do Brasil, 1st edn. Museu de Zoologia da Universidade de São Paulo, São Paulo, pp 48–53
Muehlen M, Heukelbach J, Wilcke T et al (2003) Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil II. Prevalence, parasite load and topographic distribution of lesions in the population of a traditional fishing village. Parasitol Res 90:449–455
Nagy N, Abari E, Calheiros CM et al (2007) Investigations on the biology, epidemiology, pathology and control of Tunga pentrans in Brazil. VII Investigations on the life cycle and morphology. Parasitol Res (in press)
Njeumi F, Nsangou C, Ndjend AG et al (2002) Tunga penetrans au Cameroun. Rev Méd Vét 153:176–180
Rietschel W (1989) Beobachtungen zum Sandfloh (Tunga penetrans) bei Mensch und Hund in Französisch-Guayana. Tierärztl Prax 17:189–193
Ugbomoiko US, Ofoezie IE, Heukelbach J (2007) Tungiasis: high prevalence, parasite load and morbidity in a rural community in Lagos State, Nigeria. Int J Dermatol 46:475–481
Up der Graff FW (1923) Head hunters of the Amazon—seven years of exploration and adventure. Garden City Publication, New York
von Humboldt A (1981) Südamerikanische Reise. Ullstein GmbH, Berlin
Witt LH, Linardi PM, Meckes O et al (2004) Blood-feeding of Tunga penetrans males. Med Vet Entomol 18:439–441
Witt LH, Heukelbach J, Schwalfenberg S et al (2007) Infestation of Wistar rats with Tunga penetrans in different microenvironments. Am J Trop Med Hyg 76:666–668
Acknowledgements
The study was supported by the DAAD-CAPES German-Brazilian academic exchange programme (Bonn, Germany; Brasília, Brazil) and by Komittee Ärzte für die Dritte Welt (Frankfurt, Germany). PML and RAR are research fellows from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brasil). The excellent secretarial assistance of Michi Feldmeier is greatly appreciated. The data are part of a medical thesis by LW.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feldmeier, H., Witt, L., Schwalfenberg, S. et al. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil. VI. Natural history of the infestation in laboratory-raised Wistar rats. Parasitol Res 102, 1–13 (2007). https://doi.org/10.1007/s00436-007-0731-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0731-4