Abstract
Isolates of Dicrocoelium dendriticum (n = 150) from sheep and cattle bred in southern Italy and isolates (n = 5) of D. hospes from a Bos indicus from Senegal were characterized genetically. The 28S region and the second internal transcribed spacer (ITS-2) plus flanking 5.8S and 28S sequences (ITS-2+) of ribosomal DNA (rDNA) were amplified by polymerase chain reaction and sequenced from individual flukes. Regarding the 28S rDNA, sequences of 568 and 581 bp were obtained for D. dendriticum and D. hospes, respectively. No intraspecific variation was observed between the 28S rDNA of all the D. dendriticum specimens studied and the D. dendriticum 28S rDNA sequence present in GenBank™. However, intraspecific variation was observed in the 28S rDNA of the D. hospes specimens compared to the sequence present in GenBank™. Regarding the ITS2+ rDNA, sequences of 402 and 428 bp were obtained for D. dendriticum and D. hospes, respectively; both sequences were deposited in GenBank™. Variations intra- and interpopulation were observed for D. dendriticum, whereas 100% identity was observed in all the ITS2+ sequences of D. hospes. With respect to the interspecific variations, the ITS-2+ of D. dendriticum and D. hospes differed in 33 positions. The findings of the present study showed an ITS2+ sequence variability (8.2–8.5%) between D. dendriticum and D. hospes, thus demonstrating the utility of this sequence to discriminate the two species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dicrocoeliosis is caused by several species of Dicrocoelium Dujardin, 1845 (Trematoda, Digenea), which live in the bile ducts and gall bladder of domestic and wild ruminants (sheep, goats, cattle, buffaloes, roedeer, and camels) and occasionally affect rabbits, pigs, dogs, horses, and humans (Otranto and Traversa 2003). The most important species of this genus are the following: Dicrocoelium dendriticum Rudolphi, 1819, D. hospes Loss, 1907, D. chinensis Tang and Tang, 1978, and D. suppereri Hinaiday, 1983 (syn. D. orientalis Sudarikov and Ryjikiov, 1951). D. dendriticum is found in America, Asia, North Africa, and Europe; D. hospes is mainly found in Africa; D. chinensis is found in Asia, and D. suppereri is found in the old USRR and Austria (Manga-González et al. 2001; Otranto and Traversa 2002).
D. dendriticum is the most widespread liver fluke found in cattle and sheep in southern Italy (Cringoli et al. 2002), as well as in other Mediterranean countries; its occurrence is related to dry and calcareous or alkaline soils, which represent favorable biotopes for its intermediate hosts (Manga-González et al. 2001). In fact, it has a very complex life cycle because it involves numerous species of land mollusks and ants as first and second intermediate hosts, respectively. The economic and health significance of dicrocoeliosis is due to the direct losses occasioned by the confiscation of altered livers and also to the indirect ones caused by the digestive disorders derived from the hepatobiliary alterations, such as decreased animal weight, growth delay, and reduced milk production (for a review, see Manga-González et al. 2001). Diagnosis of dicrocoeliosis is usually based on egg detection in feces of live infected animals or on adult detection in liver on postmortem examination (Campo et al. 2000).
Several authors have made studies on the variability range and frequency of occurrence of different morphological types of D. dendriticum (Macko and Birova 1989), concluding on the possibility of interpretation of D. hospes as an intrapopulation of D. dendriticum. In addition, genetic variability of adult D. dendriticum specimens has been observed using random amplified polymorphic DNA (Sandoval et al. 1999; Manga-González and Gonzalez-Lanza 2005).
However, the findings of a recent study showed marked differences in the ultrastructure of the spermatozoon of D. dendriticum and D. hospes, constituting additional data supporting the specific identity of these two species (Agostini et al. 2005).
Based on the above debates, the aim of the present paper was to perform molecular studies on two regions of the ribosomal DNA (rDNA) of several D. dendriticum specimens from various hosts and locations in southern Italy. In particular, the second internal transcribed spacer (ITS-2) rDNA—a reliable genetic marker already used for molecular systematic studies of platyhelminthes (Adlard et al. 1993; Bowles et al. 1995; Gasser and Chilton 1995; Jousson et al. 1998; Rinaldi et al. 2005)—and the 28S rDNA were utilized. In addition, molecular differences (based on 28S rDNA and ITS-2) between several specimens of D. dendriticum and five specimens of D. hospes were also studied.
Materials and methods
Dicrocoelium collection
Adults of D. dendriticum (n = 150) were collected from livers of naturally infected ruminants, namely, sheep (n = 26) and cattle (n = 10), slaughtered at abattoirs located in the Campania and Calabria regions of southern Italy. Five adults of D. hospes from a Bos indicus from Senegal were kindly provided by the University of Dakar. The flukes were washed with physiological saline solution and stored in 70% ethanol solution before further DNA extraction.
Genomic DNA extraction
DNA was extracted from individual adult worms using spin columns of QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) as specified by the manufacturer. This method has been found to be the best among five methods for DNA isolation from adult D. dendriticum (Capuano et al. 2007). DNA concentration and purity were determined spectrophotometrically by measuring their absorbance at 260 and 280 nm. DNA samples were then kept at −20°C until used for polymerase chain reaction (PCR).
DNA amplification by PCR
Two different sets of 50 μl PCR mixes were prepared to amplify a 650-base-pair target of the 28S rDNA and the ITS-2+ rDNA regions, respectively, for both the Dicrocoelium species.
Each mix of PCR was made up using 15 mM of Tris-HCl (pH 8.0), 50 mM of KCl, 6–12 ng of adult fluke DNA, 0.2 mM of the four nucleoside triphosphate (dNTPs, Takara, Japan), and 2.0 mM of MgCl2. The partial region of the 28S rDNA was amplified using 0.2 μM of each primer (Sigma, USA) described by Marcilla et al. (2002) and 1.5 U of TaqGold DNA Polymerase (Applied Biosystems, USA), performing a hotstart PCR in a GeneAmp PCR System 2700 (Applied Biosystems, USA) under the following conditions: after an initial step at 95°C for 10 min, the mixture was subjected to amplification cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 60 s, followed by a final extension at 72°C for 5 min. ITS-2, plus the partial flanking rDNA regions 5.8S and 28S (a total region also known as ITS-2+), was amplified using 12.5 pmol of each primer (MWG Biotech, Germany) described by Itagaki et al. (2003) and 2.5 U of TaqGold DNA Polymerase under the following conditions: 95°C for 10 min, 35 cycles at 94°C for 1 min, 53°C for 90 s, 72°C for 1 min, and finally, 72°C for 10 min. Eight microliters of PCR products were electrophoresed on a 1% agarose gel containing 1× tris borate ethylenediamine tetraacetic acid (EDTA; 100 mM Tris-HCl, pH 8.0; 90 mM boric acid, 1.0 mM EDTA, Invitrogen, USA) and ethidium bromide (0.5 μg/ml), visualized and photographed under UV, then analyzed with the GelPro 3.1 software (MediaCybernetics, USA).
Nucleotide sequences analysis
For each marker analyzed, the 150 amplicons of D. dendriticum and the five amplicons of D. hospes were purified using QIAquick PCR Purification Kit (QIAGEN, Germany) as specified by the manufacturer. Sequencing reactions were performed with both primers used for the PCR on a GeneAmp 2700 using the BigDye Terminator v1.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems, USA), and the results were analyzed on a 310 automated DNA sequencer (Applied Biosystems, USA).
The obtained 28S rDNA sequences were compared with those of D. dendriticum and D. hospes already present in the GenBank™ database (accession no. AF 151939 and AY 251233, respectively). In addition, the ITS2+ sequences of D. dendriticum and D. hospes were compared. All the comparison and alignments were conducted using BLAST system (basic local alignment tool) and ClustalW (http://www.ebi.ac.uk/clustalw/).
Results
28S
DNA amplification of the 28S rDNA produced a 650-bp fragment for all the Dicrocoelium specimens, although different numbers of nucleotides were defined in both amplicons for each species.
From each 28S rDNA amplicon of D. dendriticum, a sequence of 568 bp was obtained; whereas after sequencing of the 28S rDNA of D. hospes, a total of 581 nucleotides were obtained. All the 28S rDNA partial sequences of D. dendriticum showed a total (100%) identity with the 28S sequence already deposited in GenBank™ (accession no. AF 151939). However, an intraspecific variation was observed in the 28S rDNA of the five D. hospes specimens compared to the sequence already deposited in GenBank™ (accession no. AY 251233). Specifically, four D. hospes specimens showed 11 single base substitutions (point mutations), whereas one specimen showed five single base substitutions (Table 1).
The 28S rDNA sequences of D. hospes described in this study are now available from the GenBank™ database under the following accession nos. EF102024 and EF102025.
Interspecific variations were observed between the 28S of D. dendriticum and either the 28S of D. hospes EF102024 (nine variant nucleotides) and the 28S of D. hospes EF102025 (three variant nucleotides; Table 2).
ITS2+
DNA amplification of the ITS2+ rDNA produced a 450-bp fragment for all the Dicrocoelium specimens; however, after sequencing the ITS2+ region of D. dendriticum, a total of 402 nucleotides were obtained; whereas a total of 428 nucleotides were obtained for the ITS2+ region of D. hospes.
Variations of intra- and interpopulation were observed for D. dendriticum analyzing the ITS-2+ sequences. In particular, 15 samples showed a T/A substitution in position 215 and a A/G substitution in position 300; four samples showed only a T/A substitution in position 215; nine samples showed a C/A substitution in position 267.
The ITS2+ sequences of D. hospes were confirmed in all the analyzed samples (100% identity).
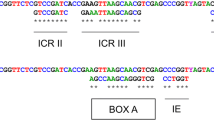
The ITS2+ sequences described in this study are now available from the GenBankTM database under the following accession nos. DQ 379986 and EF102026, for D. dendriticum and D. hospes, respectively. With respect to interspecific variations, the ITS2+ sequences of D. dendriticum and D. hospes differed in 33 positions: 26 single substitutions and seven single base deletions. The point mutations were either purine substitutions (A by G and G by A; n = 11), or pyrimidine substitution (C by T and T by C; n = 8), or purine/pyrimidine substitutions (G by T, A by T; n = 5), or pyrimidine/purine (T by G, T by A, C by G, C by A; n = 2; Fig. 1).
Interspecific variations were also observed between the D. dendriticum variants (isolate groups 2, 3, 4) and D. hospes, as summarized in Table 3.
Discussion
Molecular tools, usually DNA sequencing, might provide an alternative approach to identify closely related parasites (reviewed in Nolan and Cribb 2005). Among all target sequences used for phylogenetic studies and polymorphism analyses, rDNA is a powerful tool to demonstrate substantial intra- and interspecific variability of distinct parasite species because it includes regions with varying rates of evolution from highly conserved (18S, 5.8S, and 28S) to highly variable (transcribed and nontranscribed or intergenic spacer regions), such as the ITS-1 and the ITS-2 regions, by far the most frequently studied regions for the exploration of species boundaries in digeneans (Hillis and Dixon 1991).
In the present study, the DNA technology was used to characterize two regions of the rDNA, namely, the ITS-2 and the 28S, from several D. dendriticum specimens from various hosts and locations in southern Italy and from five D. hospes specimens from Africa. Although previous papers observed a high variability in the parasitic species of D. dendriticum, both upon genetic (Sandoval et al. 1999) and morphoanatomic parameters (Birova and Macko 1987; Macko and Birova 1989), the ITS-2 intraspecific variation was less than 1.0% (ranging from 0.25 to 0.50%) in the D. dendriticum specimens studied.
A limitation of this study is the small number of samples used for D. hospes; however, although we could not rigorously argue on rDNA variation for this parasite and on sequence divergence between it and D. dendriticum, we tried to compare the sequences obtained. This comparison showed an interspecific variation of the ITS-2+ nucleotidic composition ranging between 8.2 and 8.5% between D. dendriticum and D. hospes, thus demonstrating an interesting variability in the ITS-2 region.
Similar studies performed on other digenean species have showed similar results: the ITS-2 sequence divergence between Fasciola hepatica and F. gigantica was 2.8% and between F. hepatica and Fascioloides magna was 13.2% (Adlard et al. 1993); in addition, ITS-2 sequence divergence between Calicophoron daubneyi and C. calicophorum was 2.8% and between C. daubneyi and C. microbothrioides was 2.6% (Rinaldi et al. 2005).
Even if it would be strengthened if we would be able to obtain other samples of D. dendriticum and mostly of D. hospes from other sources from other parts of the world, we believe that our findings will be useful for the identification of these liver flukes in Italy, Africa, and elsewhere.
References
Adlard RD, Barker SC, Blair D, Cribb TH (1993) Comparison of the second internal transcribed spacer (ribosomal DNA) from populations and species of Fasciolidae (Digenea). Int J Parasitol 23:422–425
Agostini S, Miquel J, Ndiaye PI, Marchand B (2005) Dicrocoelium hospes Loos, 1907 (Digenea, Dicrocoeliidae): spermiogenesis, mature spermatozoon and ultrastructural comparative study. Parasitol Res 96:38–48
Birova V, Macko JK (1987) On the variability of Dicrocoelium dendriticum (Rudolphi, 1819) in domestic and free-living animals. III. On variability of organophenotes from sheep and cattle in East Slovakia. Helminthologia 24:197–208
Bowles J, Blair D, McManus DP (1995) A molecular phylogeny of the genus Echinococcus. Parasitology 110:317–328
Campo R, Manga-González MY, González-Lanza C (2000) Relationship between egg output and parasitic burden in lambs experimentally infected with different doses of Dicrocoelium dendriticum (Digenea). Vet Parasitol 87:139–149
Capuano F, Rinaldi L, Maurelli MP, Perugini AG, Musella V, Cringoli G (2007) A comparison of five methods for DNA isolation from liver and rumen flukes to perform ITS-2+ amplification. Parassitologia (in press)
Cringoli G, Rinaldi L, Veneziano V, Capelli G, Malone JB (2002) A cross-sectional coprological survey of liver flukes in cattle and sheep from an area of the southern Italian Apennines. Vet Parasitol 108:137–143
Gasser RB, Chilton NB (1995) Characterization of taeniid cestode species by PCR-RFLP of ITS-2 ribosomal DNA. Acta Trop 59:31–40
Hillis DM, Dixon MT (1991) Ribosomal DNA: molecular evolution and phylogenetic inference. Q J Biol 66:411–453
Jousson O, Bartoli P, Zaninetti L, Pawlowski J (1998) Use of the ITS rDNA for elucidation of some life-cycles of Mesometridae (Trematoda: Digenea). Int J Parasitol 28:1403–1411
Itagaki T, Tsumagari N, Tsutsumi K, Chinone S (2003) Discrimination of three amphistome species by PCR-RFLP based on rDNA ITS2 markers. J Vet Med Sci 65:931–933
Macko JK, Birova V (1989) On the variability of Dicrocoelium dendriticum (Rudolphi, 1819) in domestic and free-living animals. V. On the variability of hostophenates from free-living Artiodactyla in Slovakia (Czechoslovakia). Helminthologia 26:177–186
Manga-González MY, Gonzalez-Lanza C (2005) Field and experimental studies on Dicrocoelium dendriticum and dicrocoeliasis in northern Spain. J Helminthol 79:291–302
Manga-González MY, González-Lanza C, Cabanas E, Campo R (2001) Contributions to and review of dicrocoeliosis, with special reference to the intermediate hosts of Dicrocoelium dendriticum. Parasitology 123:S91–S114
Marcilla A, Bargues MD, Mas-Coma S (2002) A PCR-RFLP assay for the distinction between Fasciola hepatica and Fasciola gigantica. Mol Cell Prob 16:327–333
Nolan M, Cribb T (2005) The use and implications of ribosomal DNA sequencing for the discrimination of digenean species. Adv Parasitol 60:101–163
Otranto D, Traversa D (2002) A review of dicrocoeliosis of ruminants including recent advances in the diagnosis and treatment. Vet Parasitol 107:317–355
Otranto D, Traversa D (2003) Dicrocoeliosis of ruminants: a little known fluke disease. Trends Parasitol 19:12–15
Rinaldi L, Perugini AG, Capuano F, Fenizia D, Musella V, Veneziano V, Cringoli G (2005) Characterization of the second internal transcribed spacer of ribosomal DNA of Calicophoron daubneyi from various hosts and locations in southern Italy. Vet Parasitol 131:247–253
Sandoval H, Manga-González Y, Campo R, García P, Castro JM, Pérez de la Vega M (1999) Preliminary study on genetic variability of Dicrocoelium dendriticum determined by random amplified polymorphic DNA. Parasitol Int 48:21–26
Acknowledgments
We wish to thank Prof. Bâ Cheikh (University of Dakar, Senegal) and Dr. Silvia Agostini, Prof. Carlos Feliu, and Dr. Jordi Miquel (University of Barcelona, Spain) for helping us in obtaining the D. hospes specimens.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maurelli, M.P., Rinaldi, L., Capuano, F. et al. Characterization of the 28S and the second internal transcribed spacer of ribosomal DNA of Dicrocoelium dendriticum and Dicrocoelium hospes . Parasitol Res 101, 1251–1255 (2007). https://doi.org/10.1007/s00436-007-0629-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0629-1