Abstract
Strobilae from Taenia crassiceps (WFU strain) were obtained from outbred hamsters (Mesocricetus auratus) by feeding them viable metacestodes maintained by intraperitoneal passage in female Balb/c mice. Mature and gravid proglottids from strobilae were recovered from hamster intestines and fixed for light and electron microscopy. By light microscopy, the expected structure of taeniid proglottids was observed. Ultrastructural analysis of ten proglottids showed that testicular follicles and vas deferens contained filiform spermatids, with a single axoneme, and an elongated helicoidal nucleus inserted between the axoneme and the spiraled cortical microtubules. At the apical cone, a single crest-like body was found and mature spermatids also exhibited transverse intracytoplasmic walls. The morphology and characters of the spermatids in T. crassiceps conform to type III spermiogenesis, which has been described in other taeniids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taenia crassiceps has been widely used as a model for studying various aspects of the host–parasite relationships at the metacestode stage in mice, where they characteristically develop by asexual budding in the peritoneal cavity of these intermediate hosts (Terrazas et al. 1998; Toenjes and Kuhn 2003). A large number of these studies have used the ORF strain maintained by peritoneal passage and first isolated by Freeman (1962), the strain having become sterile and lacking a scolex (Smith et al. 1972).
In recent years, several groups have isolated wild-type strains and successfully developed adult worms in experimental laboratory animals such as the golden hamster and gerbil (Kitaoka et al. 1990; Miyaji et al. 1990; Sato and Kamiya 1989, 1990; Sato et al. 1994). However, the fine structure of these adult worms has not been described. Of taxonomic interest are the ultrastructural characteristics of the maturing spermatid as well as the mature spermatozoon, characters which have been useful as markers for phylogenetic inference in parasitic Platyhelminthes (Euzet et al. 1981; Justine 1998) We describe here the fine structure of mature spermatids in mature and gravid strobila obtained from an experimental infection of golden hamsters with the Wake Forest University (WFU) strain of T. crassiceps.
Materials and methods
T. crassiceps strain WFU was isolated from a wild field mouse (Peromyscus sp.) captured in the state of Michigan in August 1999 and maintained in the laboratory by intraperitoneal passage in female Balb/c mice (Everhart et al. 2004).
Metacestodes were maintained in 6-week-old Balb/c female mice by insertion of 8–10 larvae into the peritoneal cavity through a 1-cm abdominal incision. Animals were anesthetized with ether, the abdomen shaved and sterilized. The incision was closed with one or two stitches of surgical nylon.
Mice were killed by ether or an intramuscular (i.m.) injection of sodium penthotal 3–12 months after infection. The cysticerci were recovered by cutting open the abdominal wall and washing them with a sterile solution of phosphate buffered saline (PBS) or RPMI 1640 medium and collecting them in sterile dishes. Viability was controlled by incubating them in 1% trypsin/PBS and testing for 90% evagination at 1–2 h.
Outbred golden hamsters (Mesocricetus auratus), 6–8 months of age, were treated for 5 days with an oral suspension of albendazole (40 mg/kg) (Smith-Kline Beecham, Mexico) 8 days before being fed 15 viable metacestodes. The animals were immune-suppressed by i.m. injections of 2 mg methyl-prednisolone acetate (Depo-medrol, Upjohn, Mexico) the day of infection and every 2 weeks for the duration of the experiment. All animals were maintained on commercial food pellets and water.
Infected hamsters were killed at various times post-infection by i.m. injection of sodium barbital (Anestesal, Q-0001-065 for veterinary use, Pfizer, Mexico), and the small intestine clamped off at the pylorus and the ileum. The intestine was immersed in PBS and cut open longitudinally with fine scissors, and the contents examined under a dissection microscope. Individual strobilae were removed with a fine brush, washed briefly in fresh PBS and immersed immediately in a 1,000 mosmol/kg solution of Karnovsky’s (Karnovsky 1965) fixative for 4 h at room temperature or for 4 h in 10% buffered formalin.
The infectivity rate of mouse cysticerci was monitored by dividing the number of strobilae recovered from a hamster lot through the number of cyticerci fed to the animals and expressed as a percentage.
For the examination of whole worms, the strobilae were compressed between two glass plates and photographed under a dissecting microscope. Whole formalin-fixed worms were stained with carminic acid (Lillie 1954) and photographed.
For the light and electron microscopic studies of individual proglottids, Karnovsky-fixed tissues were post-fixed in 1% osmium tetroxide and 1.5% potassium ferrous cyanide, dehydrated in alcohol and embedded in Spurr’s low-viscosity resin (Spurr 1969). Segments were oriented longitudinally and numbered serially from the scolex to the last proglottid.
Thick 1-µm sections were cut for light microscopic examination, stained with buffered 0.5% toluidine blue and photographed using a Nikon Optiphot light microscope. Thin sections, 40–80 nm thick, were obtained from trimmed selected areas, counterstained with 5% uranyl acetate and 0.25% lead citrate and examined in a JEOL 1200EMII electron microscope.
Results
A total of 113 viable strobilae were recovered from 203 hamsters infected with cysticerci obtained from eight different lots of Balb/c mice. The infectivity rate of cysticerci from the individual mice appeared to decrease over time, from an average of 33% (May 2000) for third generation cysticerci to 3% (June 2003) in ninth generation cysticerci. The differences in infection rate were not statistically significant.
Gravid worms were found as early as 30–34 days post-infection (dpi), usually in strobilae with 80 or more proglottids. Most of the specimens recovered in our experiments contained mature proglottids. Gravid proglottids were occasionally found and contained eggs at different stages of development. The length of the individual strobilae was quite variable. In infected animals with more than one strobila, one specimen was always longer than the others.
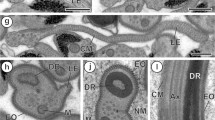
Light microscopic images were obtained from whole proglottids stained with carmine red illustrating the expected structure of a mature proglottid from a 54 dpi worm (Fig. 1A), in which gravid proglottids were also found (not shown). Longitudinal thick sections from ten strobilae (aged 20–54 dpi) were examined. The morphology of a mature proglottid is shown in Fig. 1B, in which the main structural features can be appreciated: genital pore, longitudinal excretory ducts, vas deferens, vaginal duct, ovaries, testicular follicles and the developing wall of the uterus. In Fig. 1C, a higher magnification of testicular follicles is shown, in which several beehive or crystalline structures as well as clusters of individual spermatocytes can be seen, all enclosed by a testicular epithelium.
Mature proglottids from Taenia crassiceps strobilae obtained from hamster intestine. A Whole proglottid from a 54-day infection, stained with carmine red in which the vas deferens (vd), genital pore (gp), ovaries (O), individual testicular follicles (T) and vaginal ducts (va) are shown. B Longitudinal section of a mature 35-day proglottid illustrating the genital pore (gp), longitudinal excretory duct (ed), cross-sectioned vas deferens (vd), vaginal ducts (va), spermatocyte follicles (sf), ovarian follicles (of) and the empty uterus (ut). C Thick section of testicular follicles from a 34-day proglottid showing three follicles with spermatids in different developmental stages. Spermatids (sp); axoneme bundles (ax)
In gravid proglottids, we found testicular lobules exhibiting all stages of spermiogenesis, from large cells with nuclei containing synaptolems, as well as cells with elongated nuclei containing dense fibers, areas of filiform spermatids, as well as the final stages of spermatid differentiation (Fig. 2A, B).
Electron micrographs of a spermatocyte follicle from a mature 34 day proglottid. A The follicle contains areas of spermatocytes in different stages of differentiation: in the center are a number of cells with elongated nuclei containing filamentous nuclear material (Nu and arrowheads); in the lower right corner, cells with whole nuclei, and cross-sections of filiform spermatids in the upper right-hand and lower left-hand corner (sp and arrows) are shown. B Higher magnification of elongated nuclei, as they are seen before migrating into the spermatid (arrowhead)
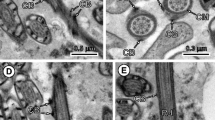
Figure 3 illustrates the individual characteristics of spermatid development: part A shows a longitudinal section of a spermatid with arched membranes in which part of the nucleus is inserted between the outer cortical microtubule layer and the central axoneme; in part B a longitudinal section of a whole spermatid, with the apical cone and the spiraled nucleus wrapped around the axoneme, is shown; and part C illustrates the twisted morphology of the cortical microtubules in spermatids found in a vas deferens.
Electron micrographs of maturing spermatids from a 40-dpi gravid proglottid. A Longitudinal view of the final stage of spermatid formation, with characteristic arched membranes (am), insertion of elongated nucleus (nu) between the axonemal sheath and layer of cortical microtubules. B Longitudinal view of a mature spermatid, with a nucleus (nu) wrapped helicoidally around the axoneme (arrowheads). C Section illustrating longitudinal views of spermatids in which the spiraled cortical microtubules (cmt) surrounding the axoneme are evident (arrows and arrowheads)
In a section from the lumen of a vas deferens, the presence of crest-like bodies at the apical end of mature spermatids is shown in Fig. 4A, as well as a higher magnification of segments of the spiral shaped nucleus encased between the cortical microtubular layer and the periaxonemal sheath (Fig. 4B), and a number of cross-sectioned spermatids containing annular or horseshoe-shaped nuclei, as well as transverse intracytoplasmic walls (Fig. 4C).
Electron micrographs of a T. crassiceps gravid proglottid illustrating spermatids in the lumen of a vas deferens. A Spermatids with crest bodies (cb) at the apical end. B Longitudinal section from caudal end of a spermatid showing segments of the helicoidal arrangement of the nucleus (Nu). Ac indicates apical cone. C Cross-sectioned bodies of spermatids. Transverse intra-cytoplasmic walls (tiw); axonemes (ax); nucleus (nu); vas deferens epithelium (vd)
Discussion
The T. crassiceps WFU strain used in the present experiments has been maintained in our laboratory since December 1999 by intraperitoneal passage in female Balb/c mice. The metacestodes from this strain are capable of producing adult strobilae in immune-suppressed golden hamsters in the laboratory, similar to those obtained in experimental models described by others (Sato and Kamiya 1989; Kitaoka et al. 1990; Sato et al. 1994).
We also noted that the infectivity rate of cysticerci from mice was ten times higher (33%) during the first months after the strain was isolated, compared to the infectivity rate found in hamsters fed cysticerci from 5–8 generations mice, in which only 3% of the cysticerci strobilated. A progressive loss of infectivity in experimental models has been reported for T. crassiceps by Freeman (1962), although the mechanisms for diminishing infectivity are not well understood. The lack of reproducibility in recovering consistent numbers of strobilae in the golden hamster has also been reported for experimental Taenia solium (Willms et al 2003).
The experimental model was very useful for the ultrastructural analysis of the T. crassiceps tapeworm, and the loss of infectivity was not reflected in morphological changes in the adult worms. The general morphology previously reported for taeniid proglottids was confirmed in the recovered strobilae, and it was possible to identify the structural characters of the spermatids, which are described here for the first time.
Our observations establish that T. crassiceps spermatozoa exhibit a number of morphological characters, which have been described for other cyclophyllideans and particularly the taeniids, namely: (1) a filiform shape, tapered at both ends; (2) a spiraled elongated and compressed nucleus situated between the cortical microtubules and the periaxonemal sheath, that exhibits a horseshoe or annular shape in cross-section; (3) spiral cortical microtubules underlying the external/outer membrane; (4) spermatids containing no mitochondria or typical acrosomes; (5) the presence of one crest-like body at the apical end; (6) the appearance of arched membranes in the final stage of differentiation, corresponding to the ring where the apical end is pinched off from the spermatocyte cell body; and (7) transverse intra-cytoplasmic walls in the mature spermatid.
Most of these characters are features of type III spermiogenesis as described by Justine (1998) and more recently by Ndiaye et al. (2002) in Taenia parva and Willms et al. (2003) in experimental T. solium strobilae. In T. solium the crest-like body was not observed, possibly because gravid proglottids, which support more advanced stages of differentiation, cannot be obtained from worms in experimental hosts. Similar ultrastructural characters have been described in T. mustelae (Miquel et al 2000) and Taenia hydatigena (Featherstone 1971). However, the crest-like bodies have so far been observed only in T. parva and in T. crassiceps (this paper). The absence of flagellar rotation has been noted in T. parva, T. mustelae and T. solium. An additional feature is the presence of transverse intracytoplasmic walls in the flagellae of the mature spermatozoa of T. mustelae (Miquel et al. 2000), T. parva and here in T. crassiceps.
According to a recent analysis of Ndiaye et al. (2003), several other cyclophyllidean cestodes also exhibit type III spermiogenesis: Mathevotaenia herpestis found in African hedgehogs (Ba and Marchand 1994b), Catenotenia pusilla infecting mice (Hidalgo et al 2000), Raillietina tunetensis found in turtle doves (Ba and Marchland 1994a), Dipylidium caninum, which infects domestic dogs (Canis familiaris) (Miquel et al. 1998) and Joyeuxiella echinorynchoides and J. pasqualei that infect foxes and wild cats (Ndiaye et al. 2003). Most of these species exhibit spermatozoa with one or two crest-like bodies and twisted cortical microtubules.
With the present observations on T. crassiceps, it seems that the spermatozoa of at least five taeniids— T. parva, T. mustelae, T. hydatigena, T. solium and now T. crassiceps—share the general ultrastructural characters of type III spermiogenesis, which displays growth of a single axoneme outside of the cytoplasmic mass, without flagellar rotation, striated roots and an intracentriolar body.
References
Ba CT, Marchand B (1994a) Ultrastructure of spermiogenesis and the spermatozoon of Raillietina (Raillietina) tunetensis (Cyclophyllidea, Davaineidae), intestinal parasite of turtle doves in Senegal. Int J Parasitol 24:237–248
Ba CT, Marchand B (1994b) Ultrastructure of spermiogenesis and the spermatozoon Mathevotaenia herpestis (Cestoda), intestinal parasite of Atelerix albiventrix in Senegal. Acta Zool (Stockh) 75:167–175
Euzet L, Swiderski Z, Mokhtar-Maamouri F (1981) Ultrastructure comparée du spermatozoïde des Cestodes. Relations avec la phylogénèse. Ann Parasitol Hum Comp 56:247–259
Everhart ME, Kuhn RE, Zelmer DA (2004) Intrapopulation dynamics of a wild strain of Taenia crassiceps (WFU) (Cestoda: Taeniidae) in Balb/cJ mice. J Parasitol (in press)
Featherstone DW (1971) Taenia hydatigena. III. Light and electron microscope study of spermatogenesis. Z Parasitenkd 37:148–168
Freeman RS (1962) Studies on the biology of Taenia crassiceps (Zeder, 1800) Rudolphi,1810 (Cestoda). Can J Zool 40:989–990
Hidalgo C, Miquel J, Torres J, Marchand B (2000) Ultrastructural study of spermiogenesis and the spermatozoon in Catenotaenia pusilla, an intestinal parasite of Mus musculus. J Helminthol 74:73–81
Justine JL (1998) Spermatozoa as phylogenetic characters for the Eucestoda. J Parasitol 84:385–408
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137A
Kitaoka M, Oku Y, Okamoto M, Kamiya M (1990) Development and sexual maturation of Taenia crassiceps (Cestoda) in golden hamster. J Parasitol 76:399–402
Lillie RD (1954) Histopathologic technic and practical histochemistry. McGraw-Hill, New York
Miquel J, Ba CT, Marchand B (1998) Ultrastructure of spermiogenesis of Dipylidium caninum (Cestoda, Cyclophyllidea, Dipylidiidae), an intestinal parasite of Canis familiaris. Int J Parasitol 28:1453–1458
Miquel J, Hidalgo C, Feliu C, Marchand B (2000) Spermatozoon ultrastructure of Taenia mustelae (Cestoda, Taeniidae), an intestinal parasite of the weasel, Mustela nivalis (Carnivora). Invert Reprod Dev 38:43–51
Miyaji S, Oku Y, Kamiya M, Okamoto M, Ohbayashi M, Uchida A, Rausch RL (1990) Growth of a Japanese isolate of Taenia crassiceps in intermediate and definitive hosts. Parasitol Res 76:351–354
Ndiaye PI, Miquel J, Marchand B (2002) Ultrastructure of spermiogenesis and spermatozoa of Taenia parva Baer, 1926 (Cestoda, Cyclophyllidea, Taeniidae), a parasite of the common genet. Parasitol Res 89:34–43
Ndiaye PI, Agostini S, Miquel J, Marchand B (2003) Ultrastructure of spermiogenesis and spermatozoon in the genus Joyeuxiella Fuhrmann, 1935 (Cestoda, Cyclophyllidea, Dipylidiidae): comparative analysis of J. echinorhynchoides (Sonsino, 1889) and J. pasqualei (Diamare, 1893). Parasitol Res 91:175–186
Sato H, Kamiya M (1989) Viable egg production of Taenia crassiceps developed in the intestine of prednisolone-treated golden hamsters. Jpn J Parasitol 38:46–53
Sato H, Kamiya M (1990) Establishment, development and fecundity of Taenia crassiceps in the intestine of prednisolone-treated Mongolian gerbils and inbred mice. J Helminthol 64:217–222
Sato H, Kamiya H, Oku Y, Kamiya M (1994). Infection course of the strobilar stage of Taenia crassiceps in golden hamster, with reference to host response. Parasitol Res. 80:99–103
Smith JK, Esch GW, Kuhn RE (1972) Growth and development of larval Taenia crassiceps (Cestoda). I. Aneuploidy in the anomalous ORF strain. Int J Parasitol 2:262–263
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31
Terrazas LI, Bojalil R, Govezensky T, Larralde C (1998) Shift from an early protective Th1-type immune response to a late permissive Th2-type response in murine cysticercosis (Taenia crassiceps). Parasitology 84:74–81
Toenjes SA, Kuhn RE (2003) The initial immune response during experimental cysticercosis is of the mixed Th1/Th2 type. Parasitol Res 89:407–412
Willms K, Caro JA, Robert L (2003) Ultrastructure of spermatogonia and spermatocyte lobules in Taenia solium strobilae (Cestoda, Cyclophyllidea, Taeniidae) from golden hamsters. Parasitol Res 90:479–488
Acknowledgements
The authors thank Laura Aguilar (M.Sc.) for her help in the preparation of whole mount specimens. This work was supported in part by funds from the Consejo Nacional de Ciencia y Tecnología, Mexico (no. L0026). The experiments described here comply with the current laws of Science and Technology in Mexico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willms, K., Robert, L., Jiménez, J.A. et al. Ultrastructure of spermiogenesis and the spermatozoon in Taenia crassiceps strobilae WFU strain (Cestoda, Cyclophyllidea, Taeniidae) from golden hamsters. Parasitol Res 93, 262–267 (2004). https://doi.org/10.1007/s00436-004-1125-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-004-1125-5